Abstract
Objectives: We aimed to identify high-resolution computed tomography (HRCT) features useful to distinguish the anaplastic lymphoma kinase gene (ALK) fusion-positive and negative lung adenocarcinomas.
Methods: We included 236 surgically resected adenocarcinoma lesions, which included 27 consecutive ALK fusion-positive (AP) lesions, 115 epidermal growth factor receptor mutation-positive lesions, and 94 double-negative lesions. HRCT parameters including size, air bronchograms, pleural indentation, spiculation, and tumor disappearance rate (TDR) were compared. In addition, prevalence of small lesions (≤20 mm) and solid lesions (TDR ≤20%) were compared.
Results: AP lesions were significantly smaller and had lower TDR (%) than ALK fusion-negative (AN) lesions (tumor diameter: 20.7 mm ± 14.1 mm vs. 27.4 mm ± 13.8 mm, respectively, p <0.01; TDR: 22.8% ± 24.8% vs. 44.8% ± 33.2%, respectively, p <0.01). All AP lesions >20 mm (n = 7, 25.9%) showed a solid pattern. Among all small lesions, AP lesions had lower TDR and more frequent spiculation than AN lesions (p <0.01). Among solid lesions, AP lesions were smaller than AN lesions (p = 0.01).
Conclusion: AP lung lesions were significantly smaller and had a lower TDR than AN lesions. Spiculation was more frequent in small lesions. Non-solid >20 mm lesions may be ALK fusion-negative.
Keywords: ALK fusion-positive, lung cancer, adenocarcinoma, computed tomography, imaging
Introduction
Lung cancer is the most common cause of cancer deaths worldwide, with >1 million deaths occurring each year. Adenocarcinoma is the most common lung cancer type in both non-smokers and smokers. In recent years, a novel transforming fusion gene joining the anaplastic lymphoma kinase gene (ALK) has been detected in lung carcinomas.1) The ALK fusion-type tyrosine kinase is an oncoprotein found in 4%–5% of non-small cell lung cancers (NSCLC), and clinical trials of specific inhibitors of ALK for the treatment of such tumors are currently underway.2–5) Other investigations have compared the clinicopathological features of ALK fusion-positive and epidermal growth factor receptor (EGFR) mutation-positive lung carcinomas, indicating that non-exposure to cigarette smoke or light smoking exposure is a common clinical feature in both groups.
Recent advances in high-resolution computed tomography (HRCT) screening has improved the detection rates of small lung cancers, especially adenocarcinomas. In this study, we compared the CT appearance of adenocarcinoma lesions positive for ALK fusion-positive (AP) and negative (AN) lung adenocarcinomas.
Materials and Methods
Patients
This retrospective study was performed after approval from our institutional review board, which waived the need for obtaining consent. A total of 27 patients undergoing surgical resection of AP lesions were found on record from October 2004 to December 2010 at the Cancer Institute Hospital, Japanese Foundation for Cancer Research, Tokyo, Japan. The pathological stage of each lesion was evaluated according to the current international TNM staging system and the World Health Organization classification. Smokers and non-smokers were categorized on the basis of the smoking history questionnaire. Smokers were further classified into three subgroups on the basis of the smoking index (SI), which was calculated as the product of the number of cigarettes smoked and inhaled years. Subgroups of patients included non-smokers (SI = 0), light smokers (SI <600), and heavy smokers (SI ≥600). We compared the surgically resected AN adenocarcinomas, which included 115 adenocarcinomas positive for EGFR mutations and 94 adenocarcinomas that were negative for both (wild-type; WT) from January 2009 to December 2010 in our hospital.
Detection of ALK and EGFR mutations
A highly-sensitive immunohistochemical method, the intercalated antibody-enhanced polymer (iAEP) method, has been developed that enables reliable immunohistochemistry-based detection of ALK fusion products. This technique can be applied to small biopsy samples, as those collected by endobronchial ultrasound-guided transbronchial needle aspiration.6) We analyzed mutations in EGFR (exons 18–21) using the PCR-single strand conformational polymorphism technique.
Evaluation of tumor disappearance rate using multidetector CT
The CT images were retrieved on our Picture Archiving and Communication System (FUJIFILM Synapse 3.2.1 SR-356; FUJIFILM Corporation, Tokyo, Japan) and reviewed by members of our thoracic surgical oncology department in consensus. Chest images were acquired using multidetector CT with 5-mm slices. High-resolution images of targeted tumors were acquired using a 1.25-mm section thickness. Images on each sheet of film were photographed using mediastinal (level, 50 Hounsfield units [HU]; width, 400 HU) and lung (level, 600 HU; width, 1800 HU) window settings. Radiological parameters including size, air bronchograms, pleural indentation, spiculation, and tumor disappearance rate (TDR) on HRCT were analyzed for each patient. TDR ratios were defined as the ratio of the tumor area of the mediastinal window to that of the lung window on HRCT. TDR (%) was calculated as [1 – (maximum dimension of tumor in the mediastinal windows/ maximum dimension of tumor in the lung windows)] × 100.7–10) We classified the pulmonary lesions into one of the three categories on the basis of TDR as follows: TDR ≥75%, least solid lesion; TDR ≤20%, solid lesion; and all others, partially solid lesions. Herein, we showed representative CT images of AP lesions in Fig. 1. We also examined small lesions (≤20 mm) and solid lesions (TDR ≤20%) with respect to radiological parameters including size, air bronchograms, pleural indentation, spiculation, and TDR. We further examined differences in imaging features by gene variations compared anaplastic lymphoma kinase with EGFR tyrosine kinase.
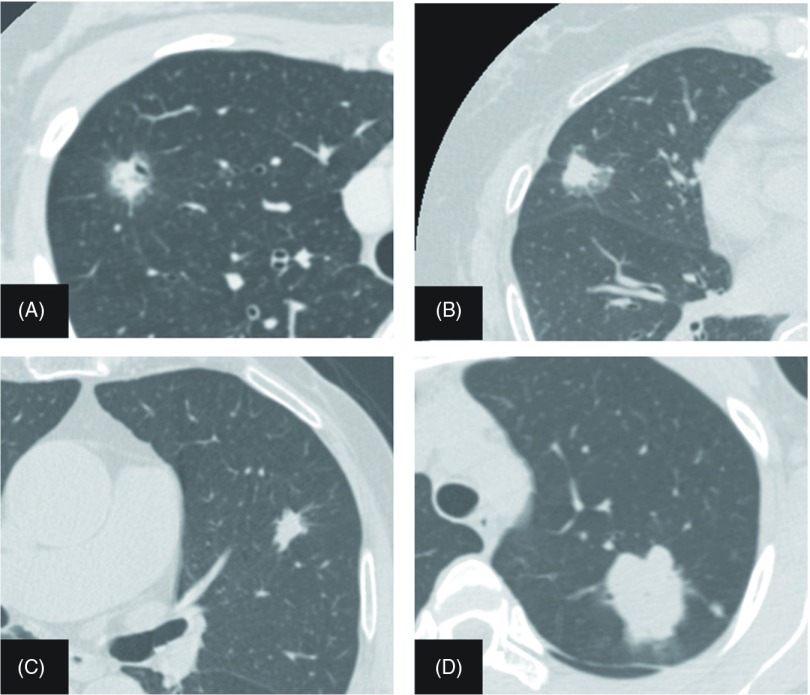
Fig. 1.
High-resolution computed tomography images of anaplastic lymphoma kinase (ALK) fusion-positive lung cancer. (A) A 12-mm lesion with a tumor disappearance rate (TDR) of 25%, spiculation and air bronchogram. (B) A 17-mm lesion with a TDR of 35.3% and pleural indentation. (C) A 16-mm lesion with a TDR of 12.5% and spiculation. (D) A 42-mm lesion with a TDR of 4.8%.
Statistical analysis
All data were analyzed using SPSS software (SPSS version 17.0J; SPSS Inc., Chicago, Illinois, USA). Comparisons between two groups were made using the Mann–Whitney U-test. Comparisons between ≥3 groups were made using the Kruskal–Wallis test. The Cox proportional hazards model was used for multivariate analyses. The effect of each variable in the model was expressed by the hazard ratio with a 95% confidence interval. p values <0.05 were considered significant.
Results
Patient characteristics
The mean ages for each group in years were as follows: AP, 57.7; EP, 70; WT, 64.3. The gender ratio (M:F) in each group was as follows: AP, 8:19 (29.6% vs. 70.4%); EP, 28:87 (24.3% vs. 75.7%); WT, 55:39 females (58.5% vs. 41.5%). EP group mutations were in exon 18 (n = 3), exon 19 (n = 44), and exon 21 (n = 63). There were 5 patients with duplicative exons: 18/21 (n = 2), 19/20 (n = 1), 19/21 (n = 1), and 20/21 (n = 1). Of the 236 resected adenocarcinomas, 27 were AP lesions. The clinicopathological features of these lesions are summarized in Table 1. There were no EGFR mutation-positive AP lesions. AP lesions were more likely to be moderately/poorly differentiated and have positive lymph node metastasis than AN lesions (p <0.01; p = 0.01). AP lesions tended to occur in patients who were younger (p = 0.03), had lighter smoking exposure (p <0.01), and in women (p <0.01) compared with WT lesions. Age (p <0.01), smoking exposure (p <0.56), and gender (p <0.57) were the only variables that differed significantly between AP and EP lesions.
Table 1.
Clinicopathological characteristics of lung adenocarcinomas
| ALK | EGFR | WT | |
|---|---|---|---|
| Number | 27 | 115 | 94 |
| Age (yr; mean ± SD) | 57.7 ± 14.4 | 70.0 ± 10.1 | 64.3 ± 10.2 |
| Sex | |||
| Male | 8 (29.6%) | 28 (24.3%) | 55 (58.5%) |
| Female | 19 (70.4%) | 87 (75.7%) | 39 (41.5%) |
| Smoking index | |||
| Never (SI = 0) | 21 (77.8%) | 85 (74.0%) | 41 (44.6%) |
| Light (SI <600) | 4 (14.8%) | 17 (14.7%) | 20 (21.3%) |
| Heavy (SI ≥600) | 2 (7.4%) | 13 (11.3%) | 33 (35.1%) |
| Differentiation | |||
| Well | 5 (18.5%) | 74 (64.3%) | 51 (56.7%) |
| Moderate/Poor | 22 (81.5%) | 41 (35.7%) | 39 (43.3%) |
| f-stage | |||
| IA | 14 (51.9%) | 69 (60.0%) | 53 (56.4%) |
| IB | 1 (3.7%) | 19 (16.5%) | 23 (24.5%) |
| II–IV | 12 (44.4%) | 27 (23.5%) | 18 (18.9%) |
Smoking index = (number of cigarettes smoked per day) × (number of years smoked). ALK: anaplastic lymphoma kinase; EGFR: epidermal growth factor receptor; WT: wild-type; yr: year old; SD: standard deviation; SI: smoking index; f-stage: final stage
Radiological analysis of ALK fusion-positives
Table 2 shows the radiological features of pulmonary nodules from AP and AN lesions acquired using HRCT. The mean size was significantly lower in AP lesions (0.76-fold, 20.7 mm ± 14.1 mm) than in AN lesions (27.4 mm ± 13.8 mm) (p <0.01). AP lesion types comprised 15 (55.6%) solid, 10 (37.0%) partial solid, and five (7.4%) least solid lesions. AN lesion types comprised 64 (30.6%) solid, 93 (44.5%) partial solid, and 52 (24.9%) least solid lesions. All >20-mm AP nodules (n = 7, 25.9%) were of solid type. The mean TDR score was significantly higher (1.96-fold) in AN lesions (44.8 ± 33.2) than in AP lesions (22.8 ± 24.8) (p <0.01). The prevalence of air bronchograms, pleural indentation, and spiculation did not differ significantly between AN and AP lesions (p = 0.374, p = 0.487, p = 0.941, respectively).
Table 2.
Imaging characteristics of ALK fusion-positive and negative nodules on HRCT
| ALK fusion-positive | ALK fusion-negative | p-value | |
|---|---|---|---|
| Total number | 27 | 209 | |
| Size (mm), (range) | 20.7 ± 14.1 (6–71) | 27.4 ± 13.8 (6–100) | p <0.01 |
| ≤20 | 20 (74.1%) | 76 (36.4%) | p <0.01 |
| >20 | 7(25.9%) | 133(63.6%) | p <0.01 |
| Air bronchogram | 19 (70.4%) | 156 (74.6%) | p = 0.374 |
| Pleural indentation | 19 (70.4%) | 141 (67.5%) | p = 0.487 |
| Spiculation | 22 (81.5%) | 156 (74.6%) | p = 0.941 |
| TDR (%) | 22.8 ± 24.8 | 44.8 ± 33.2 | p <0.01 |
| Solid (TDR ≤20%) | 15 (55.6%) | 64 (30.6%) | p = 0.01 |
| Partial solid (20< TDR <75%) | 10 (37.0%) | 93 (44.5%) | p = 0.46 |
| Least solid (TDR ≥75%) | 2 (7.4%) | 52 (24.9%) | p = 0.04 |
TDR (%) = [1 – (maximum dimension of tumor in the mediastinal windows/ maximum dimension of tumor in the lung windows)] × 100. ALK: anaplastic lymphoma kinase; HRCT: high-resolution computed tomography; TDR: tumor disappearance rate
Subtype categories (small size and solid-type pattern)
Small lung nodules (≤20 mm in the maximum dimension) comprised 74.1% (20/27) AP lesions and 36.3% (76/209) AN lesions; this difference was significant (p <0.01). No significant difference was observed in the mean lesion size (AP, 13.9 mm ± 3.8 mm; AN, 14.6 mm ± 3.5 mm; p = 0.47). The mean TDR was significantly lower in AP lesions than in AN lesions (AP, 29.4% ± 25.6%; AN, 58.7% ± 33.8%; p <0.01). Spiculation was the only radiological feature that differed significantly between AP and AN lesions, occurring in 18 (90.0%) small AP nodules (p <0.01). Among sold type lesions, 15 were AP (55.6%) and 69 were AN (33%) lesions. AP lesions were significantly smaller than AN lesions (AP, 23.5 ± 11.4 mm; AN, 34.4 ± 12.8 mm; p = 0.01). The prevalence of air bronchograms (p = 0.32), pleural indentation (p = 0.84) and spiculation (p = 0.33) did not differ significantly between AP and AN lesions (Table 3).
Table 3.
Imaging characteristics of ALK fusion-positive and negative nodules on HRCT (small size and solid-type pattern)
| ALK fusion-positive | ALK fusion-negative | p-value | |
|---|---|---|---|
| Size ≤20 mm | |||
| Total number | 20 (74.1%) | 76 (36.3%) | p <0.01 |
| Size (mm) | 13.9 ± 3.8 | 14.6 ± 3.5 | p = 0.47 |
| Air bronchogram | 13 (65.0%) | 41 (53.9%) | p = 0.38 |
| Pleural indentation | 13 (65.0%) | 37 (48.7%) | p = 0.20 |
| Spiculation | 18 (90.0%) | 41 (53.9%) | p <0.01 |
| TDR (%) | 29.4 ± 25.6 | 58.7 ± 33.8 | p <0.01 |
| Solid (TDR ≤20%) | 8 (40.0%) | 12 (15.8%) | p = 0.02 |
| Partial solid (20< TDR <75%) | 10 (50.0%) | 34 (44.7%) | p = 0.68 |
| Least solid (TDR ≥75%) | 2 (10.0%) | 30 (39.5%) | p = 0.01 |
| TDR ≤20% | |||
| Total number | 15 (55.6%) | 66 (33%) | p <0.01 |
| Size (mm) | 23.5 ± 11.4 | 34.4 ± 12.8 | p = 0.01 |
| Air bronchogram | 10 (66.7%) | 52 (78.9%) | p = 0.32 |
| Pleural indentation | 12 (80.0%) | 58 (87.9%) | p = 0.84 |
| Spiculation | 12 (80.0%) | 54 (81.2%) | p = 0.33 |
TDR (%) = [1 – (maximum dimension of tumor in the mediastinal windows/ maximum dimension of tumor in the lung windows)] × 100. ALK: anaplastic lymphoma kinase; HRCT: high-resolution computed tomography; TDR: tumor disappearance rate
ALK fusion-positive and EGFR mutation subtype
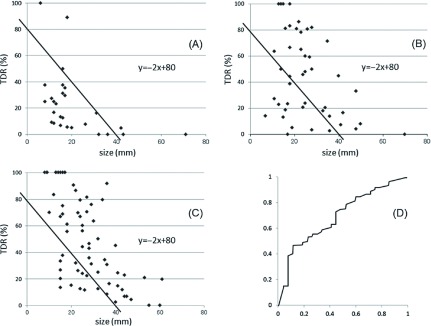
When the cutoff level of our graphic assay, which showed TDR on the y-axis and size on x-axis, was set as y = −2x + 80 according to the proportional formula, the proportion of AP lesions falling under this formula line was 88.9% (24/27) (Fig. 2A), whereas that for exon 19 EGFR mutation-positive lesions was 29.5% (13/44) (Fig. 2B) and for exon 21 EGFR mutation-positive lesions was 19.0% (12/63) (Fig. 2C); these differences were significant (exon 19 vs. AP, p <0.01; exon 21 vs. AP, p <0.01). The receiver operating characteristic curve was shown in Fig. 2D. The area under the receiver operating characteristic curve for TDR in AP was 0.70.
Fig. 2.
When the cutoff level of our graphic assay was set as y = −2x + 80 according to the proportional formula, the number of anaplastic lymphoma kinase fusion-positive (AP) lesions falling under this formula line was 88.9% (24/27) (A), whereas that for exon 19 was 29.5% (13/44) (B) and for exon 21 was 19.0% (12/63) (C). Significant differences were found between exon 19 and AP lesions (p <0.01) and between exon 21 and AP lesions (p <0.01). The area under the receiver operating characteristic curve for tumor disappearance rate (TDR) in AP lesions was 0.70 (D).
Discussion
In an effort to develop new treatment strategies, several molecular-targeted drugs have recently been developed. Crizotinib was reported to cause tumor shrinkage or stabilization in about 90% patients positive for ALK fusion gene.11) A phase 3 trial, PROFILE 1007, that compares crizotinib to standard second-line chemotherapy for ALK-positive NSCLC is presently underway. In addition, a phase 2 trial, PROFILE 1005, that studies patients meeting similar criteria who have received more than one line of previous chemotherapy is also in progress. To treat ALK-positive lung cancers, detection of this newly recognized fusion gene and its associated characteristics is important.
Several authors investigating ALK fusion-positive lung cancer reported the clinicopathological features such as lighter smoking exposure, younger age of onset, and no or rare mutations in EGFR, KRAS, and TP53. EGFR mutations have been reported more frequently in women, non-smokers, and Asian patients.12–16) Common characteristics of our patients with ALK fusion-positive tumors included lighter smoking exposure, younger age, and female gender. Although light smoking exposure and female gender were common to both ALK fusion-positive and EGFR mutation-positive lesions in this study, AP lesions were associated with a lower patient age than EP lesions (p <0.01). Pathologically, the presence of the solid signet-ring cell pattern or mucinous cribriform pattern can strongly predict ALK rearrangement status.2–5) In this study, moderate to poorly differentiated adenocarcinoma was more strongly associated with ALK fusion-positive tumors than the others (p <0.01).
To the best of our knowledge, this is the first report to study HRCT futures between the ALK fusion-positive and negative lung adenocarcinomas. We observed several significant differences between ALK fusion-positive tumors and the others. First, AP lesions were significantly smaller than AN lesions. Among AP tumors, 74.1% were ≤20 mm in size. Second, AP tumors showed a tendency toward the solid pattern because of their low TDRs. Radiological parameters including air bronchograms, pleural indentation, and spiculation did not differ significantly between tumor types. In this study, a 74-year-old female with a TDR of 100% and a 46-year-old male with a TDR of 88.9% were the cases with very high TDR, which was rare in the study population.
We categorized lesions by size (small size, ≤20 mm) and type (solid pattern, TDR ≤20%) on the basis of CT images. The majority of the tumors in the AP group were small. These small tumors had a solid pattern, which correlated with the fact that TDR was lowest in AP lesions. Spiculation was significantly more frequent in AP lesions than in AN lesions (p <0.01). Among solid-type tumors, AP lesions were significantly smaller than AN lesions (p = 0.01).
Overall, AP lesions were associated with a small tumor size and a solid tumor type with spiculation. In analysis comprising only solitary lesions, spiculation was not found to be significant predictive factor, and only small size remained a factor predictive of ALK fusion-positive lesions.
All seven AP lesions >20-mm diameter had a solid pattern. This subgroup had a significantly lower TDR than the group with smaller tumors (≤20 mm, p <0.01). Thus, our results suggest that lesions >20 mm diameter without a solid pattern may be negative for ALK rearrangement.
We further examined differences in imaging features by gene variations. In accordance with the fact that the EGFR tyrosine kinase and anaplastic lymphoma kinase have different characteristics, imaging of AP lesions was showed a limited distribution within the lung fields. In contrast, EP lesions showed extensively and diffuse distribution. So the limited part which its TDR were low and it was small width, was distributed over the relatively small zone.
If ALK mutation is predicted by CT findings before treatment, this may allow medical oncologists to consider the use of targeted therapies sooner in patients with advanced stage, tumor-related inadequate general condition or relapsed disease. In this study, we could detect several significant differences between tumors and the others by using HRCT to help suggest the ALK fusion-positive status. However, we could not found perfect CT characteristics. At this time we should investigate any pathological specimen according to the guideline to make a definite diagnosis.
Conclusion
ALK fusion-positive lung lesions were significantly smaller and had a lower TDR than ALK fusion-negative lesions. Spiculation was more likely to be present in small lesions. Lesions >20 mm without a solid pattern may be negative for ALK rearrangement.
Disclosure Statement
The authors declare that they have no conflict of interests.
References
- 1).Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561-6. [DOI] [PubMed] [Google Scholar]
- 2).Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011; 35: 1226-34. [DOI] [PubMed] [Google Scholar]
- 3).Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009; 22: 508-15. [DOI] [PubMed] [Google Scholar]
- 4).Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol 2010; 17: 889-97. [DOI] [PubMed] [Google Scholar]
- 5).Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010; 363: 1734-9. [DOI] [PubMed] [Google Scholar]
- 6).Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res 2010; 16: 4938-45. [DOI] [PubMed] [Google Scholar]
- 7).Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006; 132: 769-75. [DOI] [PubMed] [Google Scholar]
- 8).Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 2007; 84: 1675-9. [DOI] [PubMed] [Google Scholar]
- 9).Shimizu K, Yamada K, Saito H, et al. Surgically curable peripheral lung carcinoma: correlation of thin-section CT findings with histologic prognostic factors and survival. Chest 2005; 127: 871-8. [DOI] [PubMed] [Google Scholar]
- 10).Nakayama H, Okumura S, Daisaki H, et al. Value of integrated positron emission tomography revised using a phantom study to evaluate malignancy grade of lung adenocarcinoma: a multicenter study. Cancer 2010; 116: 3170-7. [DOI] [PubMed] [Google Scholar]
- 11).Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010; 363: 1693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97: 339-46. [DOI] [PubMed] [Google Scholar]
- 13).Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129-39. [DOI] [PubMed] [Google Scholar]
- 14).Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J Clin Oncol 2005; 23: 3227-34. [DOI] [PubMed] [Google Scholar]
- 15).Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 2005; 11: 1167-73. [PubMed] [Google Scholar]
- 16).Sugio K, Uramoto H, Ono K, et al. Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking. Br J Cancer 2006; 94: 896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]