Abstract
Purpose: The early and long-term outcomes of bony chest wall reconstruction with expanded polytetrafluoroethylene (Gore-Tex) soft tissue patch remain a concern. No clinical study has reported the shrinkage of Gore-Tex following reconstruction to date.
Methods: Thirty-seven patients who underwent bony chest wall reconstruction from 1994 to 2012 were retrospectively reviewed. Postoperative chest computed tomography images of 17 patients were examined, and shrinkage of reconstruction materials was measured and compared.
Results: Gore-Tex was used for reconstruction in 18 patients, autologous materials were used in 14, Marlex mesh was used in four, and Medifit felt was used in one. No surgery-related deaths were observed. Twenty patients experienced early postoperative complications. Four patients experienced local infection. One patient with Marlex-mesh experienced empyema 33 days postoperatively. Chest drainage time in the Gore-Tex patients was significantly lower than in patients with other types of prosthetic reconstruction. No dislocation or dehiscence was found. Shrinkage of Gore-Tex was absent in 4 patients and acceptable in seven patients. No granulation formation was evident around the Gore-Tex, No significant difference in shrinkage was seen between the different materials used.
Conclusion: Chest wall reconstruction with Gore-Tex was feasible with favorable early and long-term results.
Keywords: chest wall reconstruction, tissue prosthesis, outcomes, long-term observation, computed tomography
Introduction
Stability and integrity of the bony chest wall are important for maintaining normal cardiopulmonary function. Bony chest wall reconstruction is necessary in the case of chest wall resection as resection will affect the stability and integrity of the wall. In the past, autologous materials such as fascia lata and muscle flaps were mostly used for chest wall reconstruction.1,2) However, recent developments have led to new medical materials and surgical techniques, and several kinds of prosthetic materials including polypropylene and polytetrafluoroethylene have shown satisfactory results and are in widespread use.3)
The Gore-Tex soft tissue patch is one kind of prosthesis employed for bony chest wall reconstruction. Composed of the inert biomaterial expanded polytetrafluoroethylene (ePTFE), Gore-Tex features a microporous structure that enables host tissue incorporation. It is convenient for clinical application due to its wide availability, ease of handling, guaranteed source, and quality. However, early complications, such as prosthesis infection, and long-term outcomes, especially the possible shrinkage of the prosthesis, remain a concern to many doctors. To date, no studies have focused on the shrinkage of chest wall reconstruction prostheses or on the changes in the defect areas in the bony chest wall that may result in chest pain and chest wall deformity. Therefore, we conducted a retrospective analysis of 37 cases of bony chest wall reconstruction in patients who underwent the procedure from 1994 to 2012 at our hospital, and we evaluated the early and long-term outcomes of such reconstructions with a Gore-Tex soft tissue patch.
Materials and Methods
From January 1994 to December 2012, 37 patients underwent bony chest wall reconstruction with different materials at our hospital. After obtaining approval from the Institutional Review Board, who waived the requirement for patient or family consent, medical records were reviewed.
Three kinds of prosthesis were used. A Gore-Tex soft tissue patch (W.L. Gore and Associates, Inc., Flagstaff, Arizona, USA), 2-mm thick, was used in 18 patients. Marlex mesh (C.R. Bard Inc. Murray Hill, New Jersey, USA), a kind of knitted polypropylene mesh, was used in four patients. Medifit Felt (JMS Co. Hiroshima, Japan), a kind of absorbable pledget, was used in one patient. Fourteen patients underwent reconstruction with autologous materials (eight with fascia lata free grafts, four with latissimus dorsi muscle flaps, one with a pectoralis major muscle flap, and one with a rectus abdominis flap).
Patients were divided into the following three groups according to the reconstruction material used: autologous group, Gore-Tex group, and other prosthesis group. Clinical characteristics, operation time, operative blood loss, extent of surgical resection, mechanical ventilation time, intensive care unit (ICU) stay, drainage time, postoperative hospital stay, early complications, long-term complications, and follow-up computed tomography (CT) images of the three groups were analyzed retrospectively and compared.
Postoperative complications that occurred within 30 days were considered early complications, and those which occurred after 30 days were considered long-term complications. Pneumonia was defined as localized pulmonary infiltrates on chest X-ray or chest CT. Local infection was defined as culture-positive infection within the cavity adjacent to the reconstructed chest wall, including both the thoracic cavity and the wound cavity. Wound seroma was defined as a detectable local effusion layer over the reconstructed chest wall without fever and without culture-positive identification of a pathogenic organism.
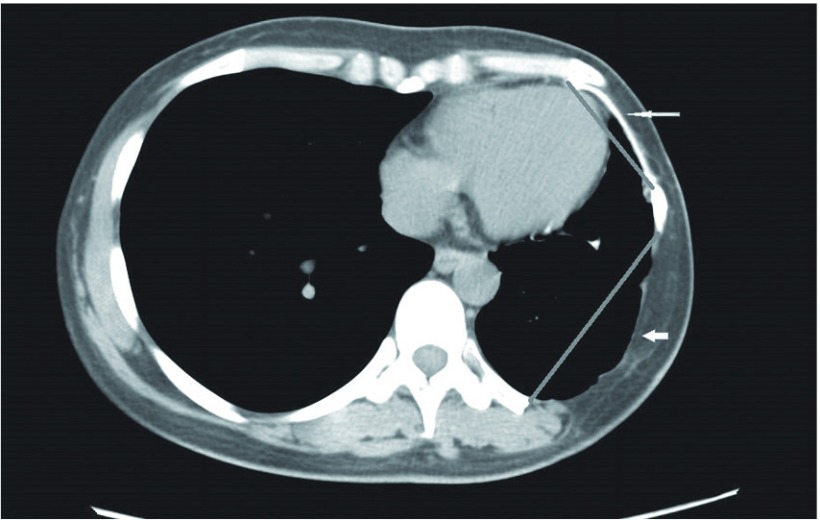
To ascertain the durability of the prostheses, the following up imaging data were collected and reviewed. We measured chronological changes in the size of the Gore-Tex patch and its maximal transverse diameter on follow-up CT images. Because the autologous materials and other prostheses are not visible on chest CT, we observed the same layer on consecutive CT images and measure the transverse distance of the two-sided bony defect as the size of the reconstruction materials (Fig. 1).
Fig. 1.
Measurement parameters on computed tomography (CT). Thin white arrow shows the Gore-Tex patch, which is visible on chest CT. Thick white arrow shows the bony chest wall defect reconstructed using a local muscle flap. Straight gray lines within the CT image show the measurement parameters.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS 19.0 Inc. Chicago, Illinois) for Windows. Numerical data comparisons between groups were analyzed using the one-way analysis of variance and least significant difference test, and categorical data comparisons between groups were analyzed using Fisher’s exact test. Statistical significance was set at p <0.05 both sides.
Results
Twenty-eight male and nine female patients (median age, 57 years; age range, 20–78 years) were eligible for the study. Bony chest wall resection was performed in five patients with primary malignancy (2 chondrosarcomas, 1 Ewing sarcoma, 1 pleomorphic sarcoma, and 1 malignant fibrous histiocytoma), 20 with metastatic malignancy (four cases of lung cancer, 3 thyroid carcinomas, 2 leiomyosarcomas, 2 renal carcinomas, 2 hepatomas, 1 parotid carcinoma, 1 osteosarcoma, 1 gastric carcinoma, 1 rectal carcinoma, 1 pancreatic carcinoma, 1 esophageal carcinoma, and 1 of skin cancer), 10 with local malignancy invasion (9 of lung cancer invasion and 1 of breast cancer recurrence invasion), and 2 with primary benign tumor (1 desmoid tumor and 1 schwannoma).
The clinical characteristics of the patients are shown in Table 1. Patients in all three groups were similar with regard to age, sex, diagnosis, preoperative comorbidity, smoking habit, and preoperative treatment. The bony chest wall resection included ribs, costal cartilage, sternum, clavicle, and vertebral transverse process. Median number of resected ribs was 3 (range, 2–5). Manubrium with adjacent clavicle or costal cartilage resection was performed in four patients. Sternum body resection was performed in 1 patient. Vertebral transverse process resection was performed in 1 patient. Median chest wall defect size was 116 cm2 (range, 36–330 cm2). Three patients underwent full-thickness chest wall resection, and the bony chest wall defect and soft tissue defect were reconstructed at that time using local musculocutaneous flaps.
Table 1.
Characteristics of the patients in the three study groups
| Autologous group | Gore-Tex group | Other prosthesis group | p value | |
|---|---|---|---|---|
| Case number | 14 | 18 | 5 | |
| Age (year) | 52.5 ± 16.5 | 57.4 ± 13.4 | 65.0 ± 9.1 | 0.24 |
| Gender | 0.88 | |||
| Male | 10 | 14 | 4 | |
| Female | 4 | 4 | 1 | |
| Diagnosis | 0.07 | |||
| Primary malignancy | 2 | 3 | 0 | |
| Primary benign | 0 | 2 | 0 | |
| Metastatic malignancy | 5 | 12 | 3 | |
| Local malignancy invasion | 7 | 1 | 2 | |
| Hypertension (%) | 2 (14.3) | 3 (16.7) | 3 (60.0) | 0.11 |
| Diabetes mellitus (%) | 0 (0.0) | 3 (16.7) | 1 (20.0) | 0.23 |
| Smoking habit (%) | 10 (71.4) | 13 (72.2) | 5 (100) | 0.66 |
| Preoperative treatmenta(%) | 5 (35.7) | 6 (33.3) | 3 (60.0) | 0.58 |
aPreoperative treatment included chemotherapy and radiation or both.
No surgery-related deaths occurred. Two patients (5.4%) needed postoperative mechanical ventilation (1 in the autologous group and 1 in the Gore-Tex group), 20 patients (54.1%) experienced early postoperative complications; 4 patients (10.8%) had local infection (two in the autologous group and two in the Gore-Tex group). One patient (2.7%) with Marlex-mesh experienced empyema 33 days after the operation. The prosthesis was completely removed if an infection was confirmed. One patient who had undergone reconstruction of the manubrium with Gore-Tex developed a prosthetic infection, which was controlled after he underwent re-reconstruction with a pectoralis major muscle flap. Another two patients underwent complete removal of the infected prosthesis without reconstruction because the chest wall was stable, and no evident lung collapse occurred due to the adhesion caused by the infection. The infections of the two patients in the autologous group were controlled conservatively, and all patients with a postoperative chest wall infection were treated successfully. Six patients (16.2%) had complications of wound seroma that eventually resolved.
Median postoperative hospital stay was 18 days (range, 8–130 days). Follow-up data were available for 34 patients; 3 patients were lost to follow up. Median follow-up time was 31.6 months (range, 3–197 months).
Intraoperative, postoperative, and follow-up data are shown in Table 2. One case in other prosthesis group suffered from long term complication, but no long term complication occurred in the other two groups. The drainage time of the other prosthesis group was significantly longer than Gore-Tex group. No significant differences were noted between the three groups for ICU stay, postoperative hospital stay, or incidence of other early complications.
Table 2.
Operative and postoperative patient characteristics in the three groups
| Autologous group | Gore-Tex group | Other prosthesis group | p value | |
|---|---|---|---|---|
| Defect size (cm2) | 111 ± 60 | 127 ± 90 | 89 ± 31 | 0.58 |
| Operation time (min) | 323 ± 141 | 265 ± 187 | 266 ± 132 | 0.59 |
| Operative blood loss (ml) | 768 ± 601 | 396 ± 499 | 581 ± 606 | 0.19 |
| Mechanical ventilation (%) | 1 (7.1) | 1 (5.6) | 0 (0.0) | 1.00 |
| ICU stay (days) | 1.9 ± 2.6 | 1.2 ± 0.6 | 1.2 ± 0.4 | 0.48 |
| Drainage time (days) | 8.1 ± 6.9 | 5.2 ± 2.8 | 11.6 ± 9.5 | 0.04a |
| Early complications (%) | 6 (42.9)b | 12 (66.7) | 2 (40.0) | 0.35 |
| Wound seroma | 1 | 5 | 0 | 0.26 |
| Pneumonia | 1 | 2 | 1 | 0.62 |
| Local infection | 2 | 2 | 0 | 1.00 |
| Liver function abnormal | 2 | 0 | 0 | 0.39 |
| Respiratory deficiency | 1 | 0 | 0 | 0.51 |
| Paradoxical respiration | 0 | 1 | 0 | 1.00 |
| Arrhythmia | 0 | 2 | 0 | 0.62 |
| Chylothorax | 0 | 0 | 1 | 0.14 |
| Transient upper limb dysfunction | 1 | 0 | 0 | 0.51 |
| Long-term complication (%) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0.14 |
| Postoperative hospital stay (days) | 26 ± 14 | 29 ± 34 | 29 ± 15 | 0.96 |
| Follow up (months) | 73.9 ± 70.3 | 37.8 ± 37.5 | 37.1 ± 35.3 | 0.14 |
ap value of the comparison between the Gore-Tex group and other prosthetic group.
bAmong the six patients,one patient complicated with both respiratory deficiency and liver function abnormal, another patient complicated with both pneumonia and local infection
Seventeen patients underwent at least two chest CT sessions during follow up, including 11 patients with Gore-Tex patch, four patients with autologous materials and two patients with Marlex mesh. The median time interval between the CT sessions that we used for measurement was 48.5 months (range, 6.4–120.7 months). Of the 11 patients with Gore-Tex, four showed no change in the size of the prosthesis, 5 showed minimal shrinkage (<5%), and two showed moderate shrinkage (5%–10.2%). Of the four patients with autologous reconstruction, one showed no shrinkage, one showed minimal shrinkage, and the other two showed moderate shrinkage. Two patients with Marlex reconstruction both had minimal shrinkage. The degree of prosthetic shrinkage did not differ significantly between the three groups (Table 3). In the Gore-Tex group, the shrinkage of the prosthesis did not correlate with CT time interval (Fig. 2). Other 18 patients underwent one time chest CT or not during follow up. For these patients, the CT images or chest radiographs were reviewed and no evident deformity and no dehiscence were found.
Table 3.
Shrinkage of different reconstruction materials in the three patient groups
| Autologous group | Gore-Tex group | Other prosthesis group | p value | |
|---|---|---|---|---|
| Case number | 4 | 11 | 2 | |
| Shrinkage(mm) | 4.5 ± 5.0 | 2.8 ± 3.7 | 1.8 ± 2.6 | 0.69 |
| Without shrinkage | 1 | 4 | 0 | 1.00 |
| Shrinkage ≤ 5% | 1 | 5 | 2 | 0.32 |
| Shrinkage > 5% | 2 | 2 | 0 | 0.54 |
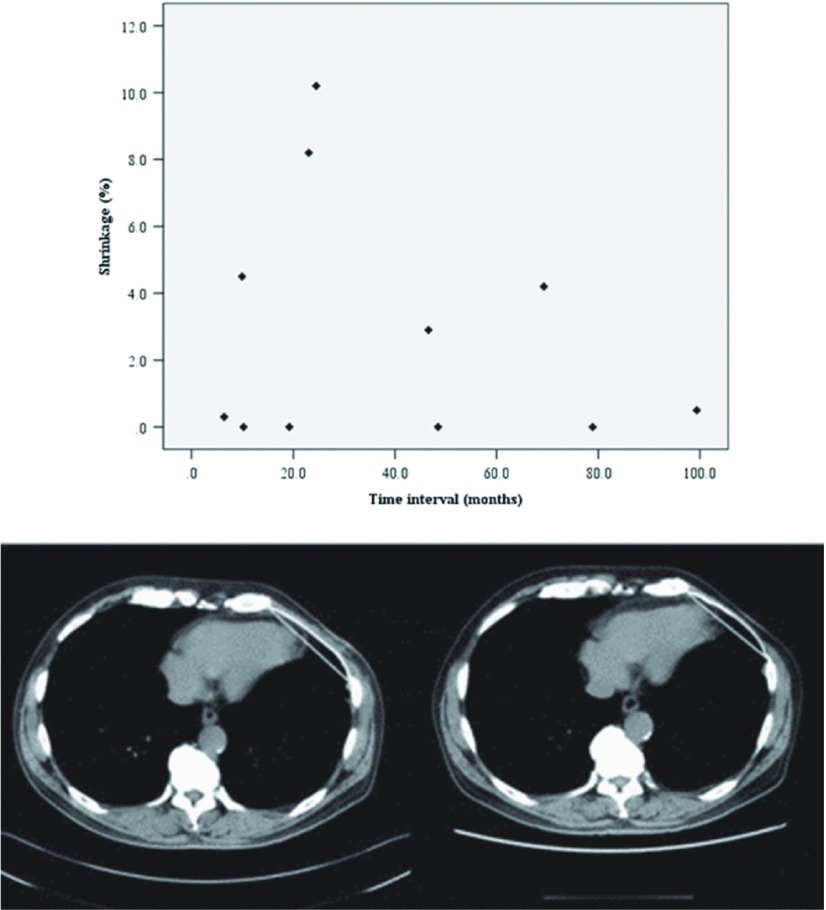
Fig. 2.
(Above) Correlation of Gore-Tex prosthesis shrinkage over time in relation to the interval of two computed tomography examinations. (Below) Computed tomography (CT) images of a patient with Gore-Tex shrinkage being less than 5%. Left panel shows a CT image taken 3 months after the operation. Right panel shows a CT image taken 6 years after operation. Straight lines in the figure show the measurement parameters, the values of which were determined by the CT software.
Discussion
A chest wall defect following chest wall resection for a neoplasm (primary, locally invasion, or metastasis), infection, radiation injury, or trauma was once a considerable challenge to thoracic surgeons. Although the materials for reconstruction of the bony chest wall are still controversial,4) it is accepted that large defects should be reconstructed to avoid paradoxical respiration.
Chest wall reconstruction was introduced in the 1940s when Watson and James first described chest wall reconstruction using an autologous fascia lata free graft.5) Autologous materials have the advantage of self-compatibility but the disadvantage of another incision having to be made to harvest the graft. In this study, 1 patient with a latissimus dorsi muscle reconstruction experienced transient donor side upper limb movement disorder. Prosthetic chest wall reconstruction was first reported in the 1950s.6) In our department, we preferred to use autologous materials before 2000.2) However, with the development of medical materials, we gradually turned to use prostheses for chest wall reconstruction. We formerly used Marlex mesh for bony chest wall reconstruction. However, Marlex mesh is rigid in only one direction when under tension. It is also permeable to water and air; in other words, it cannot function as a watertight seal for the pleural space. Gore-Tex, on the other hand, is made of a carbon and fluorine based synthetic polymer (ePTFE), is biologically inert, and is rigid in two directions. It is also microporous, which enables penetration by fibrous tissue. However, it is not permeable to fluid or air so it can fulfill closure of the thoracic cavity. Its special ability to be radiolucent, while being detectable on CT, makes it suitable for follow up. All these characteristics were in accord with the ideal characteristics of prostheses, as set out by LeRoux and Shama.7) Therefore, we gradually turned to choose Gore-Tex for chest wall reconstruction from 2002 onwards.
Major early complications related to chest wall prostheses are wound seroma and local infection. It is assumed that foreign body stimulation by a prosthesis may increase the exudation from surrounding tissue, resulting in increased drainage time or more frequent wound seroma or even local infection. However, in this study, the incidence of wound seroma and local infection did not differ significantly between the 3 groups. We therefore think that the prosthetic materials examined in our study are feasible for chest wall reconstruction. The postoperative chest drainage time in the Gore-Tex group was significantly shorter than in the other prosthesis group, suggesting the advantage of the Gore-Tex soft tissue patch.
Many patients with chest wall malignancy undergo chemotherapy or radiotherapy after surgery. Therefore, whether or not reduced immunity due to the chemotherapy or radiotherapy will affect the prosthesis is another concern. In this study, only one patient with Marlex mesh reconstruction was complicated by delayed infection which presented 33 days postoperatively.
A further concern for prosthetic reconstruction is the risk of degradation or shrinkage, which can result in chest pain and chest wall deformity. Several experimental studies have investigated the shrinkage of ePTFE biomaterials in animal models of abdominal wall reconstruction,8–10) with a reported range of shrinkage of 7.6%8) to 50.8%.9) Such a degree of shrinkage would have been worrisome in the present patients. To date, no experimental or clinical studies on the shrinkage of prostheses following chest wall reconstruction have been reported. In this study, the results indicated that the shrinkage of Gore-Tex following chest wall reconstruction was either absent or acceptable in all patients at long-term follow up: shrinkage was absent in four patients, minimal in five, and moderate in two, with the largest degree being 10.2%. This result is remarkably lower than reported by studies on abdominal wall reconstruction. Furthermore, no significant differences in shrinkage were observed between the different materials used. Figure 2 shows the CT images of a patient with <5% shrinkage, with a CT interval of 69.3 months.
It is assumed that a prosthesis will not shrink by itself, but only as a consequence of adjacent scar tissue formation caused by infectious or non-infectious inflammatory changes.11) Granulation formation is a normal process of these inflammatory changes. Under the condition of prosthetic chest wall reconstruction, the foreign body reaction caused by a prosthesis will lead to the non-infectious inflammation and prompt granulation formation. Therefore, if a prosthesis has good biocompatibility, granulation formation will not increase. In this study, no granulation formation around the Gore-Tex patches was evident on CT follow up. This was coincident with our results of clinical shrinkage measurement, and also indicated the good biocompatibility of Gore-Tex.
Whether or not the shrinkage of Gore-Tex will develop over time is another issue. If shrinkage does develop over time, dehiscence would be a potential risk for long-term survival despite the low initial shrinkage. In this study, we found that Gore-Tex shrinkage was not related to follow-up time, which indirectly indicates that Gore-Tex shrinkage does not develop over time. In addition, no dehiscence, chest wall deformity, or chest pain was evident at long-term follow up.
Two patients underwent chest wall reconstruction twice. One case was due to tumor recurrence. In the first operation, chest wall reconstruction was accomplished using a local muscle flap, and the second time, Gore-Tex was used (Fig. 1). The other patient underwent chest wall reconstruction a second time because of an infection due to Gore-Tex patch. After prosthesis removal, a local muscle flap was used.
Conclusion
Chest wall reconstruction with an autologous material or a prosthesis is feasible and can provide satisfactory outcomes. Sometimes, both approaches are needed and are in fact complementary. In this first study to investigate the shrinkage of Gore-Tex following clinical chest wall reconstruction, Gore-Tex had a shorter drainage time than reconstruction with other prostheses. In addition, with its characteristic of being radiolucent on CT, Gore-Tex is suitable for follow-up evaluations. No granulation formation around the Gore-Tex was evident, and shrinkage was absent or acceptable. It also led to good long-term outcomes with no evident side effects.
Disclosure statement
The authors declare no conflict of interest.
References
- 1).Pairolero PC, Arnold PG. Thoracic wall defects: surgical management of 205 consecutive patients. Mayo Clin Proc 1986; 61: 557-63. [DOI] [PubMed] [Google Scholar]
- 2).Murakawa T, Nakajima J, Maeda K, et al. Reappraisal of fascia lata grafts for reconstruction of chest wall defects. Asian Cardiovasc Thorac Ann 2002; 10: 285-6. [DOI] [PubMed] [Google Scholar]
- 3).Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999; 117: 588-91; discussion 591-2. [DOI] [PubMed] [Google Scholar]
- 4).Soysal O, Walsh GL, Nesbitt JC, et al. Resection of sternal tumors: extent, reconstruction, and survival. Ann Thorac Surg 1995; 60: 1353-8; discussion 1358-9. [DOI] [PubMed] [Google Scholar]
- 5).Watson WL, James G. Fascia lata grafts for chest wall defects. J Thorac Surg 1947; 14: 399-406. [PubMed] [Google Scholar]
- 6).Southwick HW, Economou SG, Otten JW. Prosthetic replacement of chest-wall defects; an experimental and clinical study. AMA Arch Surg 1956; 72: 901-7. [DOI] [PubMed] [Google Scholar]
- 7).le Roux BT, Shama DM. Resection of tumors of the chest wall. Curr Probl Surg 1983; 20: 345-86. [DOI] [PubMed] [Google Scholar]
- 8).Bellón JM, Rodríguez M, García-Honduvilla N, et al. Partially absorbable meshes for hernia repair offer advantages over nonabsorbable meshes. Am J Surg 2007; 194: 68-74. [DOI] [PubMed] [Google Scholar]
- 9).Johnson EK, Hoyt CH, Dinsmore RC. Abdominal wall hernia repair: a long-term comparison of Sepramesh and Dualmesh in a rabbit hernia model. Am Surg 2004; 70: 657-61. [PubMed] [Google Scholar]
- 10).García-Ureña MA, Vega Ruiz V, Díaz Godoy A, et al. Differences in polypropylene shrinkage depending on mesh position in an experimental study. Am J Surg 2007; 193: 538-42. [DOI] [PubMed] [Google Scholar]
- 11).Gonzalez R, Fugate K, McClusky D, et al. Relationship between tissue ingrowth and mesh contraction. World J Surg 2005; 29: 1038-43. [DOI] [PubMed] [Google Scholar]