Abstract
Background: Although right parasternal approach (RPA) decreases the incidence of mediastinal infection, this approach is associated with lung hernia and flail chest. Our RPA employs thoracotomy with bending rib cartilages and wound closure performed by repositioning the ribs with underlying sheet reinforcement.
Methods: We evaluated 16 patients who underwent aortic valve replacement via the RPA from January 2010 to August 2013. We compared outcomes of 15 male patients had the RPA with 30 male patients had full median sternotomy.
Results: One patient with a history of radical breast cancer treatment underwent RPA with concomitant right coronary artery bypass grafting. No hospital deaths occurred. Four patients developed hospital-associated morbidity (re-exploration for bleeding, prolonged ventilation, cardiac tamponade, and perioperative myocardial infarction). There were no conversions to full median sternotomy, mediastinal infections, and lung hernias. Preoperative computed tomography showed that the distance from the right sternal border to the aortic root was significantly associated with operation times. With RPA, there was no significant difference in outcomes, despite significantly longer operation times compared with full median sternotomy.
Conclusion: Our RPA provides satisfactory outcomes without lung hernia, especially in patients unsuitable for sternotomy. Preoperative computed tomography is useful for identifying appropriate candidates for the RPA.
Keywords: minimally invasive cardiac surgery, multidetector computed tomography, right parasternal approach
Introduction
Minimally invasive aortic valve surgery is performed by various approaches, including the right parasternal approach (RPA), inverse-T partial sternotomy, transverse sternotomy, L-shaped partial sternotomy, and minithoracotomy though the second or third intercostal space.1–11) Minimally invasive aortic valve surgery has been changing to more limited and cosmetic approaches to improve patients’ activities of daily living. These changes have been accompanied by the development of particular instruments and maneuvers. In 1996, Cosgrove, et al.1) first reported the performance of minimally invasive aortic valve surgery via the RPA. The RPA without median sternotomy prevents deep sternal wound infection and greatly facilitates later reoperation.1–4) However, the RPA is associated with the risk of lung hernia and flail chest, which may require surgical treatment and contribute to poor wound healing.2,12) We have developed a new RPA that includes bending rib cartilages into thoracic cavity (without rib removal) accompanied with muscle flap and facilitates wound closure by sheet reinforcement, which may prevent lung hernia and flail chest.
Minimally invasive aortic surgery via right infra-axillary thoracotomy, right anterior minithoracotomy, and anterolateral minithoracotomy using a port access approach with or without the aid of thoracoscopy has been recently reported.9–11,13,14) Therefore, novel and more advanced techniques are needed to facilitate the smooth performance of such operations without surgical errors. However, these approaches may be difficult to implement for beginning surgeons. The RPA can serve as the first step in minimally invasive aortic valve surgery with peripheral cardiopulmonary bypass (CPB); such an approach may help the surgeon to develop the skills necessary for other approaches.
In this study, we evaluated aortic valve replacement via the RPA using our thoracotomy method. We assessed the availability of the RPA compared with conventional full median sternotomy.
Materials and Methods
Patients
We retrospectively reviewed all patients who underwent aortic valve surgery in our institution from January 2010 to August 2013. Sixteen patients underwent aortic valve replacement via the RPA without rib removal, and 80 patients underwent single aortic valve replacement via full median sternotomy (conventional approach). Patients with peripheral artery disease, a severely calcified ascending aorta, or mobile plaques in the aortic arch and descending aorta were excluded in aortic valve replacement via the RPA. Additionally, we preferably used the RPA in patients who had aortic valve regurgitation and relatively younger male patients because these patients have a high muscle mass and activity that may lead to cutting of sternal wire and dehiscence of the medial sternum by exercise.
We evaluated the outcomes among the patients who had undergone aortic valve replacement via the RPA. From the indication for the RPA group above mentioned, in male patients excluding female and octogenarian patients, we also compared the perioperative outcomes of 15 patients who underwent simple aortic valve replacement via the RPA with those of 30 patients who underwent the conventional approach. Morbidity was defined as the development of severe postoperative complications, including re-exploration for bleeding, low output syndrome, prolonged ventilation (>72 h), cardiac tamponade, perioperative myocardial infarction, acute renal failure (creatinine level of >2.0 mg/dl or a >2-fold increase in the creatinine level), hemodialysis, ventricular fibrillation, complete atrioventricular block, pneumonia, mediastinal infection, and prosthetic valve infection.
This retrospective study was approved by the Institutional Review Board of Osaka City General Hospital and complied with the current ethical guidelines of the Helsinki Declaration. All of the patients were informed about the need for individual consent for later retrospective studies, which included collection and analysis of their clinical and follow-up data, at the same time as the necessity for undergoing cardiovascular surgery was explained.
Surgical Procedures
RPA
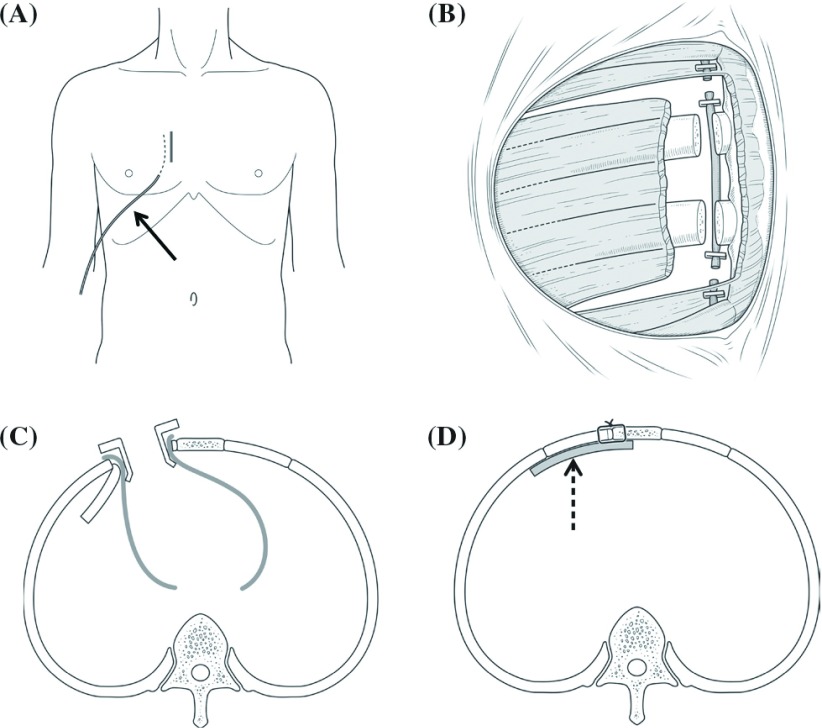
After anesthetic induction and left double-lumen endotracheal tube ventilation, a Swan-Ganz catheter with a pacing lead was inserted, and defibrillation pads were placed on the right and left chest before sterile draping. A right parasternal longitudinal skin incision was made (Fig. 1A). We performed a longitudinal skin incision to ensure that there was a wide operative field for cutting the other ribs if there was a technical difficulty. The right pectoralis major muscle was split at the level of the third and fourth intercostal spaces, and a muscle flap was created (Fig. 1B). The right internal thoracic artery was then doubly ligated, and the third and fourth costal cartilages were cut and manually bent into the left thoracic cavity without removal of the ribs (Fig. 1C). The bended ribs were covered by surgical covering cloth. After the pericardium was cut, we exposed the operative field using pericardial traction with a rib retractor (Fig. 1C). After systemic heparinization, CPB was established with a cannula to the right femoral artery and a venous cannula to the right atrium through the right femoral vein. Vacuum-assisted venous drainage was applied. To ensure sufficient arterial flow or venous drainage, we also inserted an arterial or venous cannula into the right axillary artery or the superior vena cava, respectively, in some patients. A left ventricular vent was inserted via the right upper pulmonary vein. After performing aortic cross-clamping and partial transverse aortotomy, identical cold-blood cardioplegia was administered selectively into the orifices of the coronary arteries, followed by intermittent selective administration into the orifices of the coronary arteries or retrograde administration. The operative field was flooded with CO2. Aortic valve replacement was performed using a mechanical or bioprosthetic valve of the appropriate size with consideration of the patient’s preference following our preoperative explanation. Pledgeted 2-0 polyester sutures were placed in a para- or supra- annular position around the annulus. The aortotomy was closed in a double-suture fashion, and the aortic clamp was released. Function of the implanted prosthetic valve and general cardiac function was evaluated by transesophageal echocardiography while weaning from CPB. Heparin was neutralized with protamine. For wound closure, an expanded polytetrafluoroethylene sheet (GORE® PRECLUDE® Pericardial Membrane; W.L. Gore & Associates, Inc., Flagstaff, Arizona, USA) was placed under the ribs to prevent lung hernia (Fig. 1D). After repositioning and fixing the ribs by wire or stitches, a local anesthesia tube providing continuous administration of 0.2% ropivacaine was placed under the pectoralis major muscle to control the perioperative pain. The local anesthesia tube was removed on postoperative day 3.
Fig. 1.
Operative schema of the right parasternal approach. The solid arrow shows a local anesthesia tube. The broken arrow shows an expanded polytetrafluoroethylene sheet.
Conventional approach
A full median sternotomy was performed under general anesthesia. After systemic heparinization, CPB was established with an arterial cannula to the ascending aorta and a two-stage cannula through the right atrial appendage. We used the same CPB and aortic valve replacement procedures as in the above-described RPA with the exception of the vacuum-assisted venous drainage. Identical cold-blood cardioplegia was administered through the aortic root, followed by intermittent administration in an antegrade (selectively into the orifices of the coronary arteries) or retrograde fashion. After aortic valve replacement, the median sternotomy was closed with interrupted or mattress wire sutures.
Preoperative computed tomography
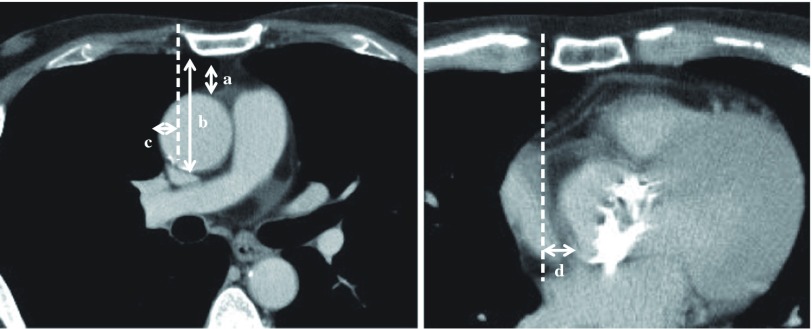
Every patient who underwent aortic valve surgery via the RPA first underwent preoperative multidetector computed tomography (CT) angiography to identify those who were not suitable for the RPA. We evaluated the preoperative CT results to identify factors that may affect the performance of an operation via the RPA. We measured the distances from the lower border of the sternum to the upper and lower borders of the ascending aorta, from the right sternal border to the right border of the ascending aorta in the fourth intercostal space, and from the right sternal border to the right border of the aortic root at the aortic valve level (Fig. 2).
Fig. 2.
Preoperative computed tomography. (a, b) Distance from the lower border of the sternum to the upper and lower borders of the ascending aorta. (c) Distance from the right sternal border to the right border of the ascending aorta in the fourth intercostal space. (d) Distance from the right sternal border to the right border of the aortic root at the aortic valve level.
Statistical analysis
The data were analyzed using Dr. SPSS II for Windows software (SPSS, Troy, New York, USA). Numerical variables are expressed as the mean ± standard deviation and were analyzed using the nonparametric Mann–Whitney U test for comparisons between two groups, as appropriate. Categorical variables are expressed as numbers or percentages of patients and were compared using the χ2 test and Fisher’s exact test, as appropriate. Correlations among the operation time, CPB time, and aortic cross-clamp time were also analyzed. Correlation coefficients were evaluated by Spearman’s rank method. Values of p <0.05 were considered statistically significant.
Results
RPA
The mean age of the 16 patients (15 males, one female) who underwent the RPA was 61.3 ± 16.3 years (27–78 years). Of the 13 patients who underwent operations for aortic valve regurgitation, seven had degenerative disorders, five had bicuspid valves, and one had sustained trauma. Of the three patients who underwent operations for aortic valve stenosis, two had bicuspid valves and one had a degenerative disorder. One patient with traumatic aortic valve regurgitation had comorbidities comprising sternal and rib fractures, inducing a left lung contusion. Additionally, one patient underwent aortic valve replacement accompanied by right coronary artery bypass grafting. In this patient, previous cancer treatment of the left breast involving radical mastectomy and radiation therapy precluded the performance of conventional median sternotomy and left thoracotomy because of insufficient blood flow and a skin defect in the mid to left thorax. Eleven patients had a mechanical valve and five had a bioprosthetic valve.
The mean skin incision length was 8.8 ± 1.8 cm. No hospital deaths occurred. Four patients (25.0%) developed hospital morbidities, including re-exploration for bleeding (n = 1), prolonged ventilation (>72 h) (n = 1), cardiac tamponade (n = 1), and perioperative myocardial infarction (n = 1). The patient who developed perioperative myocardial infarction had an anomaly of the left coronary artery orifice that resulted in insufficient cardioplegia administration. We could not perform adequate cardioversion for ventricular fibrillation because of the limited surgical field; therefore, this patient required transverse sternotomy. One patient had mild paravalvular leakage, but an additional operative procedure was not needed. After the operation, there were no lung hernias or flail chest. No wound or mediastinal infections occurred, with good wound healing for 7 to 10 days after the operation. The local anesthesia adequately controlled the perioperative pain without many analgesic medications. The 3-year mortality rate of the RPA was 0%. Only one patient underwent pacemaker implantation for complete atrioventricular block 1 year after the operation.
Factors affecting the operation via the RPA
The distance from the right sternal border to the right border of the aortic root was significantly associated with the operation time, CPB time, and aortic cross-clamp time with correlation coefficients of 0.721, 0.701, and 0.775, respectively (p <0.01). Patients with the aortic root located on the right side of the sternum tended to have shorter operation times, CPB times, and aortic cross-clamp times. There were no significant correlations between postoperative complications and operation times.
Comparison between RPA and the conventional approach
The preoperative characteristics are shown in Table 1. The preoperative data of the RPA group were comparable with those of the conventional approach group. Aortic valve regurgitation occurred significantly more frequently in the RPA group than in the conventional approach group (p <0.01). This was because patients with less calcification and mobile plaques are young and commonly develop aortic valve regurgitation. The perioperative data are shown in Table 2. The operation time and CPB time were significantly longer in the RPA group than in the conventional approach group (p <0.01). No hospital deaths occurred in either group. No significant difference was observed in hospital morbidity between the two groups.
Table 1.
Preoperative characteristics
| Variable | Right parasternal approach (n = 15) | Conventional approach (n = 30) | p value |
|---|---|---|---|
| Age, years | 60.5 ± 16.5 | 64.5 ± 10.0 | 0.664 |
| Aortic valve disease | |||
| Stenosis | 2 | 22 | <0.001 |
| Regurgitation | 13 | 8 | <0.001 |
| Bicuspid valve | 7 | 8 | 0.086 |
| Hypertension | 10 | 22 | 0.732 |
| Diabetes mellitus | 4 | 12 | 0.514 |
| Hyperlipidemia | 4 | 16 | 0.118 |
| Smoking | 8 | 15 | 1.000 |
| Chronic renal failure (Hemodialysis) | 3 (0) | 6 (0) | 1.000 |
| Old cerebral infarction | 2 | 2 | 0.591 |
| euroScore II | 1.23 ± 0.79 | 1.48 ± 1.21 | 0.219 |
| Ejection fraction, % | 60.6 ± 7.0 | 60.6 ± 13.0 | 0.283 |
Data are expressed as mean ± SD or number of patients
Table 2.
Intra- and postoperative clinical data
| Variable | Right parasternal approach (n = 15) | Conventional approach (n = 30) | p value |
|---|---|---|---|
| Duration of procedure, min | |||
| Operation | 320 ± 107 | 209 ± 40 | <0.001 |
| CPB | 160 ± 68 | 108 ± 26 | 0.007 |
| Aortic cross-clamp | 113 ± 48 | 85 ± 21 | 0.055 |
| Intraoperative transfusion, U per patient | |||
| Packed red blood cells | 1.1 ± 2.3 | 3.1 ± 4.0 | 0.105 |
| Fresh frozen plasma | 3.5 ± 3.8 | 2.2 ± 2.6 | 0.327 |
| Postoperative transfusion, U per patient | |||
| Packed red blood cells | 1.7 ± 4.7 | 1.2 ± 2.3 | 0.619 |
| Fresh frozen plasma | 2.3 ± 3.2 | 1.9 ± 3.1 | 0.651 |
| Intensive care unit stay, day | 1.7 ± 1.2 | 1.3 ± 0.8 | 0.262 |
| Extubation time, h | 17.0 ± 17.6 | 11.1 ± 8.3 | 0.352 |
| Re-intubation, n | 0 | 1 | 1.000 |
| Hospital stay, days | 16.7 ± 9.5 | 17.7 ± 14.9 | 0.952 |
| Morbidity, n | 4 | 5 | 0.454 |
| Re-exploration for bleeding | 1 | 1 | 1.000 |
| Low output syndrome | 0 | 1 | 1.000 |
| Prolonged ventilation | 1 | 1 | 1.000 |
| Perioperative myocardial infarction | 1 | 0 | 0.333 |
| Cardiac tamponade | 1 | 1 | 1.000 |
| Acute renal failure (Hemodialysis) | 0 (0) | 1 (0) | 1.000 |
| Ventricular fibrillation | 0 | 1 | 1.000 |
| Complete atrioventricular block | 0 | 0 | 1.000 |
| Mediastinal infection | 0 | 1 | 1.000 |
| New atrial fibrillation, n | 7 | 12 | 0.754 |
| Perivalvular leakage, n | 1 | 0 | 0.333 |
Data are expressed as mean ± SD or number of patients. CPB: cardiopulmonary bypass
Discussion
Upper or lower partial sternotomy is commonly performed in minimally invasive aortic valve surgery.5–9,15) Although the RPA has been used in the past, partial sternotomy with no specialized equipment has recently become the predominant approach. A few studies have reported transition from the RPA to partial sternotomy in minimally invasive aortic valve surgery.5,6) The reasons for this transition may be that partial sternotomy can easily convert to full median sternotomy and that the RPA is associated with the potential to sacrifice the right internal thoracic artery. Moreover, the RPA may result in lung hernia or flail chest, which may require surgical treatment and contribute to poor wound healing.2,12) However, the RPA without median sternotomy decreases the possibility of mediastinitis and has a cosmetic advantage in that the skin incision is covered by clothing.1–4) In patients who are not suitable for median sternotomy, as in our patients who underwent radical breast cancer treatments and sustained sternal fractures, the RPA may be more acceptable. Additionally, we preserved the anatomical integrity of the chest wall without lung hernia or fail chest by avoiding rib detachment with placement of an expanded polytetrafluoroethylene sheet under the ribs. Minale, et al.4) also reported that by avoiding rib resection, the RPA prevents flail chest. Considering the reduction in the incidence of mediastinitis and preservation of the thoracic shape with the RPA, this approach may be an alternative and preferable technique for appropriate patients.
Many studies have reported conversion to full median sternotomy because of difficulty with operative maneuvers and bleeding control.2–4,6–11) One of our patients was also converted to transverse sternotomy to achieve appropriate cardioversion for ventricular fibrillation. This is because the limited operative field obscured the whole heart, leading to myocardial damage secondary to inappropriate cardioplegia. A recent report described the performance of aortic valve surgery via the RPA with a transverse skin incision.15) This transverse incision may permit easy conversion to extended surgery by transverse sternotomy when unexpected intraoperative complications occur.
Minimally invasive aortic surgeries via right infra-axillary thoracotomy, right anterior minithoracotomy, and anterolateral minithoracotomy using a port access approach were recently reported.9–11,13,14) These approaches provide more acceptable cosmesis and preservation of the right internal thoracic artery than does the RPA. However, in these approaches, the deeper and narrower operative field necessitates more difficult operative maneuvers and particular instruments, such as a thoracic videoscope, a knotting pusher, and CPB with cardioplegia. The RPA requires knowledge of establishing peripheral CPB, as do these new approaches, with a relatively wider operative field than with the intercostal approaches. Additionally, the RPA permits concomitant coronary artery bypass grafting in patients with coronary artery disease.3,4) Therefore, the RPA may be an optimal first step in minimally invasive aortic valve surgery with peripheral CPB. Learning the techniques of the above-mentioned new approaches is not necessary, and the RPA meets the patient’s needs if concomitant coronary bypass grafting is required.
Exposure of the operative field is important to allow for smooth performance of minimally invasive cardiac surgery. Pericardial traction may be required to obtain a good operative field, although many studies did not adequately report the importance of pericardial traction because of a lack of detail of the operative techniques used. A recent study reported the achievement of operative field exposure using pericardial traction and fixation to the skin edge during minimally invasive aortic valve surgery.16) We exposed the operative field using pericardial traction with a rib retractor, which provided a good operative field by pulling the right heart. One of our patients had severe adhesions caused by bone fractures, and pericardial traction could not provide a good operative field and may have led to paravalvular leakage. Recent reports have shown that a knotting pusher is useful for achieving effective ligation in a narrow operative field and may prevent paravalvular leakage.13,15,17) In a poor operative field, a knotting pusher may be a useful instrument, preventing paravalvular leakage by facilitating effective ligation.
The relationship between the ascending aorta or the heart and sternum may affect the facility of surgery, which in turn is associated with operation times and perioperative complications. Multidetector CT is a useful instrument that clearly shows the anatomy of the thoracic cavity, heart, and arteries, thus facilitating preoperative planning that will potentially prevent surgical errors in minimally invasive cardiac surgery.10,11) Glauber, et al. reported that patients with (1) the ascending aorta located on the right with respect to a vertical line drawn from the right sternal border to the ascending aorta and (2) a distance of ≤10 cm from the ascending aorta to the sternum are candidates for minimally invasive aortic valve replacement via right anterior thoracotomy.10) We observed that placement of the aortic root on the right side of the sternum was correlated with shorter operation times and easy handling of operative maneuvers, although the ascending aorta was not significantly associated with operation times. The learning curve may affect the relationship between CT and the operation time, but we found that only the first two or three patients had longer operation times by un-established maneuvers and the other patients had established maneuvers performed. Therefore, preoperative multidetector CT appears to be an indispensable examination that affects the selection of patients and operative maneuvers.
The RPA has a lower possibility of mediastinal infection, lower blood transfusion volume, cosmetic advantage, and shorter hospital stay and cost than does the conventional approach.2–4) Many studies have shown that minimally invasive aortic surgery may have further advantages, including blood conservation, a short hospital stay, and a short ventilation time.3,5,7–9) However, we did not observe any significant differences in perioperative outcomes. The reasons for this lack of finding may be because this study included a small number of patients and insufficient surgical techniques affected the perioperative outcomes. We showed that the RPA had a significantly longer operation time compared with full median sternotomy. Many studies have also reported that minimally invasive aortic valve surgery is associated with a longer operation time compared with the conventional approach.3,8,9) However, recent studies involving a large number of patients showed that a shorter operation time was associated with minimally invasive aortic valve surgery.5,7) Other studies have reported that minimally invasive aortic valve surgery is associated with a shorter operation time in recent years compared with previous years, which indicates the presence of a technical learning curve.6,8) Therefore, further investigations are needed to identify the availability of the RPA.
Conclusion
Our aortic valve replacement procedure via the RPA without rib removal provides satisfactory outcomes without lung hernia and mediastinal infection. The RPA may also be cosmetically acceptable, especially in patients who are not suitable for sternotomy and require coronary artery bypass grafting. Multidetector CT should be performed to identify patients who may be appropriate for the RPA because of the reduction in surgical errors and intraoperative complications.
Disclosure Statement
The authors have no conflict of interest to declare.
References
- 1).Cosgrove DM, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg 1996; 62: 596-7. [PubMed] [Google Scholar]
- 2).Cosgrove DM, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg 1998; 65: 1535-8; discussion 1538-9. [DOI] [PubMed] [Google Scholar]
- 3).Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997; 226: 421-6; discussion 427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Minale C, Reifschneider HJ, Schmitz E, et al. Minimally invasive aortic valve replacement without sternotomy. Experience with the first 50 cases. Eur J Cardiothorac Surg 1998; 14 Suppl 1: S126-9. [DOI] [PubMed] [Google Scholar]
- 5).Mihaljevic T, Cohn LH, Unic D, et al. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004; 240: 529-34; discussion 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Tabata M, Umakanthan R, Cohn LH, et al. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur J Cardiothorac Surg 2008; 33: 537-41. [DOI] [PubMed] [Google Scholar]
- 7).Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006; 81: 1599-604. [DOI] [PubMed] [Google Scholar]
- 8).Gilmanov D, Bevilacqua S, Murzi M, et al. Minimally invasive and conventional aortic valve replacement: a propensity score analysis. Ann Thorac Surg 2013; 96: 837-43. [DOI] [PubMed] [Google Scholar]
- 9).Brinkman WT, Hoffman W, Dewey TM, et al. Aortic valve replacement surgery: comparison of outcomes in matched sternotomy and PORT ACCESS groups. Ann Thorac Surg 2010; 90: 131-5. [DOI] [PubMed] [Google Scholar]
- 10).Glauber M, Miceli A, Bevilacqua S, et al. Minimally invasive aortic valve replacement via right anterior minithoracotomy: early outcomes and midterm follow-up. J Thorac Cardiovasc Surg 2011; 142: 1577-9. [DOI] [PubMed] [Google Scholar]
- 11).Plass A, Scheffel H, Alkadhi H, et al. Aortic valve replacement through a minimally invasive approach: preoperative planning, surgical technique, and outcome. Ann Thorac Surg 2009; 88: 1851-6. [DOI] [PubMed] [Google Scholar]
- 12).Sundara Pandiyan M, Mathew Kavunkal A, Titus VT, et al. Successful repair of chronic instability of anterior chest wall following right parasternal approach for closure of atrial septal defect in a young female. Interact Cardiovasc Thorac Surg 2006; 5: 740-1. [DOI] [PubMed] [Google Scholar]
- 13).Ito T, Maekawa A, Hoshino S, et al. Right infraaxillary thoracotomy for minimally invasive aortic valve replacement. Ann Thorac Surg 2013; 96: 715-7. [DOI] [PubMed] [Google Scholar]
- 14).Totsugawa T, Kuinose M, Hiraoka A, et al. Anterolateral approach for minimally invasive aortic valve replacement. Gen Thorac Cardiovasc Surg 2014; 62: 290-5. [DOI] [PubMed] [Google Scholar]
- 15).Fuji G, Ito T, Maekawa A, et al. Minimally invasive approach (para-sternum small incision) for aortic valve replacement. Jpn J Cardiovasc Surg 2013; 42: 11-5. [Google Scholar]
- 16).Malaisrie SC, Barnhart GR, Farivar RS, et al. Current era minimally invasive aortic valve replacement: techniques and practice. J Thorac Cardiovasc Surg 2014; 147: 6-14. [DOI] [PubMed] [Google Scholar]
- 17).Shibata T, Hattori K, Kato Y, et al. Thimble type knot pusher: bioprosthesis stents no longer interfere with tying. Interact Cardiovasc Thorac Surg 2010; 11: 131-2. [DOI] [PubMed] [Google Scholar]