Abstract
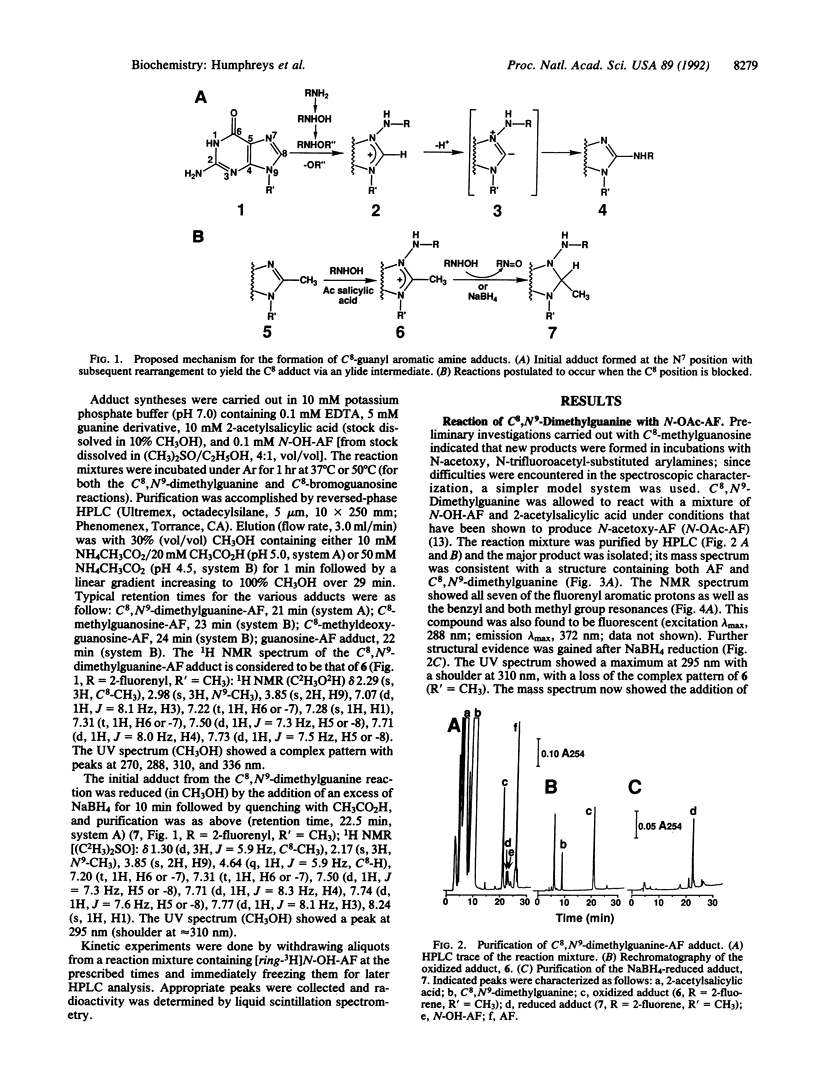
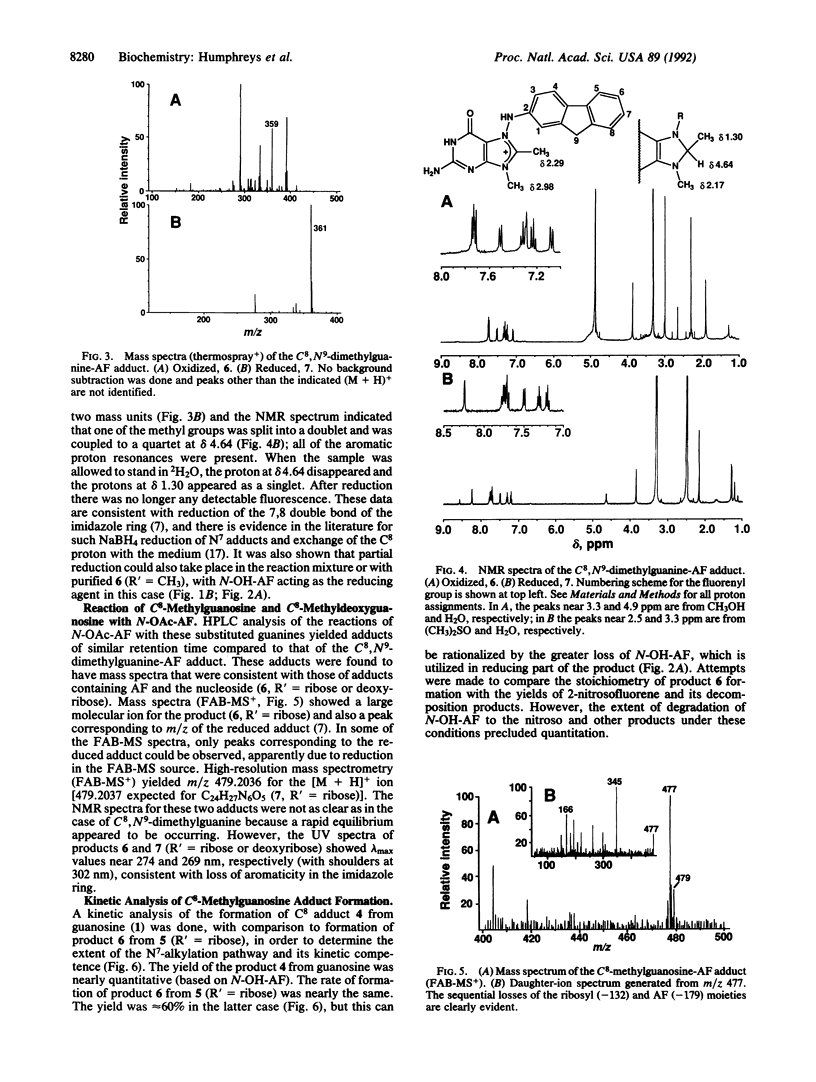
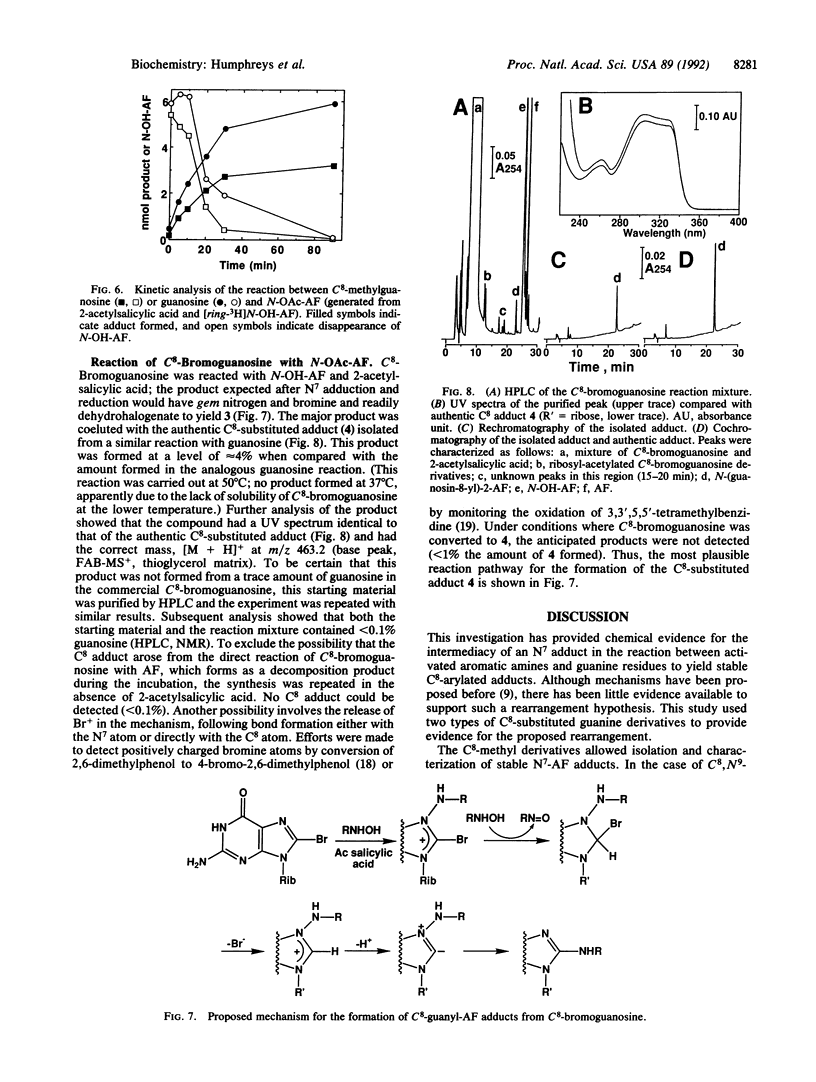
Aromatic amines are bioactivated to electrophilic compounds that react with DNA, predominantly at the C8 position of guanine bases. This site is weakly nucleophilic and it has been proposed that the C8 adduct is the final product after initial N7-adduct formation. To consider this possibility, we reacted several C8-substituted guanine derivatives with N-acetoxy-2-aminofluorene, prepared in situ from 2-acetylsalicylic acid and N-hydroxy-2-aminofluorene. With C8,N9-dimethylguanine, an adduct was isolated in good yield that was consistent, by NMR and mass spectral characterization, with a structure involving carcinogen substitution at the N7 position of guanine and linked through the 2-aminofluorenyl nitrogen--N-(C8,N9- dimethylguanin-N7-yl)-2-aminofluorene. This adduct could be easily reduced with NaBH4, consistent with the proposed N7-adduct structure. The same reaction was also carried out with C8-methylguanosine and C8-methyldeoxyguanosine and similar adducts were isolated. In contrast, C8-bromoguanosine reacted with N-acetoxy-2-aminofluorene to yield the C8-substituted arylamine adduct N-(guanosin-C8-yl)-2-aminofluorene directly. These products are uniquely consistent with a scheme in which C8-adduct formation is preceded by an initial electrophilic substitution on the N7 atom, which is postulated to be a general reaction for activated arylamines and heterocyclic amines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapeau M. C., Marnett L. J. Enzymatic synthesis of purine deoxynucleoside adducts. Chem Res Toxicol. 1991 Nov-Dec;4(6):636–638. doi: 10.1021/tx00024a006. [DOI] [PubMed] [Google Scholar]
- Chung F. L., Wang M. Y., Hecht S. S. Detection of exocyclic guanine adducts in hydrolysates of hepatic DNA of rats treated with N-nitrosopyrrolidine and in calf thymus DNA reacted with alpha-acetoxy-N-nitrosopyrrolidine. Cancer Res. 1989 Apr 15;49(8):2034–2041. [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Hecht S. M., Adams B. L., Kozarich J. W. Chemical transformations of 7,9-disubstituted purines and related heterocycles. Selective reduction of imines and immonium salts. J Org Chem. 1976 Jun 25;41(13):2303–2311. doi: 10.1021/jo00875a019. [DOI] [PubMed] [Google Scholar]
- Kohda K., Kawazoe Y., Minoura Y., Tada M. Separation and identification of N4-(guanosin-7-yl)-4-aminoquinoline 1-oxide, a novel nucleic acid adduct of carcinogen 4-nitroquinoline 1-oxide. Carcinogenesis. 1991 Aug;12(8):1523–1525. doi: 10.1093/carcin/12.8.1523. [DOI] [PubMed] [Google Scholar]
- Kohda K., Tada M., Hakura A., Kasai H., Kawazoe Y. Formation of 8-hydroxyguanine residues in DNA treated with 4-hydroxyaminoquinoline 1-oxide and its related compounds in the presence of seryl-AMP. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1141–1148. doi: 10.1016/0006-291x(87)90527-4. [DOI] [PubMed] [Google Scholar]
- Lee M. S., King C. M. New syntheses of N-(guanosin-8-yl)-4-aminobiphenyl and its 5'-monophosphate. Chem Biol Interact. 1981 Mar 1;34(2):239–248. doi: 10.1016/0009-2797(81)90135-6. [DOI] [PubMed] [Google Scholar]
- Mico B. A., Branchflower R. V., Pohl L. R., Pudzianowski A. T., Loew G. H. Oxidation of carbon tetrachloride, bromotrichloromethane, and carbon tetrabromide by rat liver microsomes to electrophilic halogens. Life Sci. 1982 Jan 11;30(2):131–137. doi: 10.1016/0024-3205(82)90644-0. [DOI] [PubMed] [Google Scholar]
- Minchin R. F., Ilett K. F., Teitel C. H., Reeves P. T., Kadlubar F. F. Direct O-acetylation of N-hydroxy arylamines by acetylsalicylic acid to form carcinogen-DNA adducts. Carcinogenesis. 1992 Apr;13(4):663–667. doi: 10.1093/carcin/13.4.663. [DOI] [PubMed] [Google Scholar]
- Scribner J. D. Determinants of nucleic acid adduct formation. Natl Cancer Inst Monogr. 1981 Dec;(58):173–181. [PubMed] [Google Scholar]
- Scribner J. D., Miller J. A., Miller E. C. Nucleophilic substitution on carcinogenic N-acetoxy-N-arylacetamides. Cancer Res. 1970 Jun;30(6):1570–1579. [PubMed] [Google Scholar]
- Tarpley W. G., Miller J. A., Miller E. C. Rapid release of carcinogen-guanine adducts from DNA after reaction with N-acetoxy-2-acetylaminofluorene or N-benzoyloxy-N-methyl-4-aminoazobenzene. Carcinogenesis. 1982;3(1):81–88. doi: 10.1093/carcin/3.1.81. [DOI] [PubMed] [Google Scholar]
- Tomasz M. Extreme lability of the C-8 proton: a consequence of 7-methylation of guanine residues in model compounds and in DNA and its analytical application. Biochim Biophys Acta. 1970 Jan 21;199(1):18–28. doi: 10.1016/0005-2787(70)90690-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Nagao M., Esumi H., Sugimura T. Food-derived mutagens and carcinogens. Cancer Res. 1992 Apr 1;52(7 Suppl):2092s–2098s. [PubMed] [Google Scholar]



