Abstract
Multiple synchronous primary lung cancers presenting with different histologic types are uncommon. Among reported cases with different histologic findings, only a few had small cell lung cancer (SCLC) and adenocarcinoma. This unusual combination of lung cancers has not been well reported. In this report, we describe two cases of synchronous primary lung cancer presenting with lymph node metastasis of SCLC and early-stage adenocarcinoma. Epidermal growth factor receptor (EGFR) mutation was not detected in either SCLC or adenocarcinoma in the two cases.
Keywords: synchronous primary lung cancer, small cell carcinoma, adenocarcinoma
Introduction
Multiple primary lung cancers arising synchronously or metachronously are uncommon. Among possible combinations, squamous cell carcinoma is by far the most common. Recently, frequency of diagnosing adenocarcinomas is increasing with the widespread use of computed tomography (CT). However, combinations with small cell lung cancer (SCLC) and adenocarcinoma are very rare. Through our literature search, we identified five cases of synchronous primary lung cancer (SPLC) presenting with SCLC and adenocarcinoma. Here, we describe two cases of SPLC presenting with SCLC and adenocarcinoma. We also report the clinical characteristics, thus adding these two cases to the five previously reported cases.
Case Report
Case 1
A 70-year-old man was referred to our hospital for an abnormal shadow on chest CT. His past medical history was unremarkable but he had a smoking history (Brinkman index: 2040). Physical examination revealed no abnormalities. Serum levels of CYFRA and Pro-GRP were elevated to 3.5 ng/mL (normal range: <2.0) and 85.9 pg/mL (normal range: <81.0), respectively. Chest CT showed an 18-mm-diameter part-solid nodule in the right Segment 10 (Fig. 1A) and enlarged right hilar lymph node (Fig. 1B). Positron emission tomography/CT (PET/CT) revealed low-intensity fluorodeoxyglucose (FDG) uptake of the lesion, with the max standardized uptake value (SUVmax) of 1.70 (Fig. 1C). There was also weak FDG uptake in the right #12 lower lobar node (SUVmax: 2.9) (Fig. 1D). Lung cancer (cT1aN1M0 stage IIA) was suspected, and right lower lobectomy with lymph node dissection was performed in June 2013.
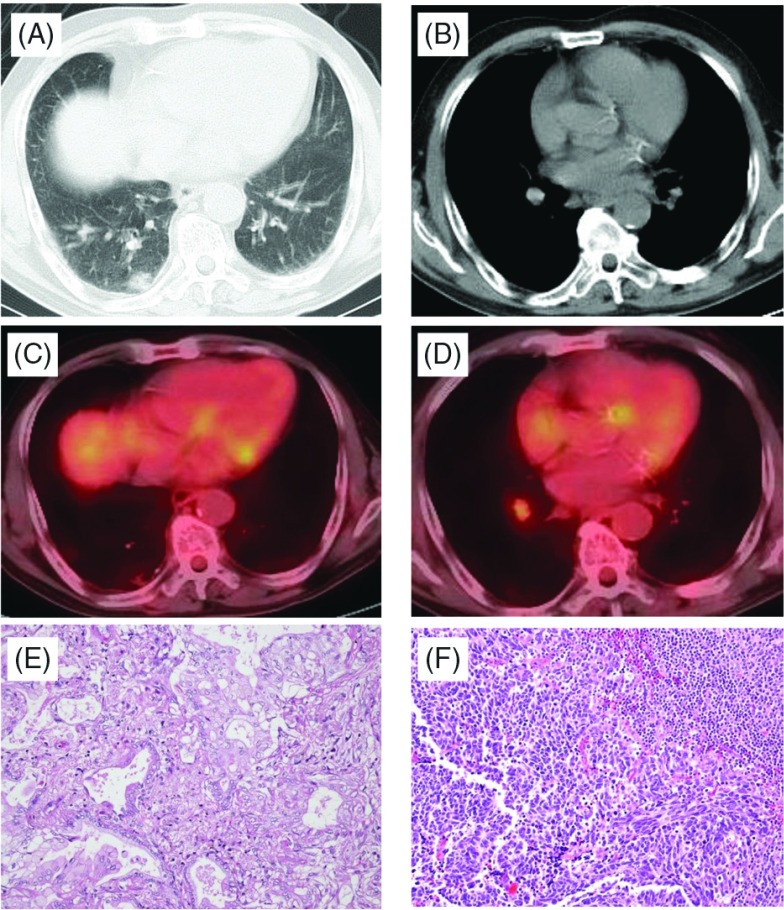
Fig. 1.
Case 1. Chest computed tomography (CT) showing a part-solid nodule (A) and #12 lymph node swelling (B). Positron emission tomography (PET)-CT showing a part-solid nodule (C) and the #12 lymph node (D). Histologic findings (Hematoxylin-eosin staining) show adenocarcinoma (E) and small cell lung cancer (SCLC) (F).
Pathological examination of the S10 tumor revealed a minimally invasive mucinous adenocarcinoma (Fig. 1E), but the #12 lymph node revealed small cell carcinoma with a Ki-67 index of approximately 90% (Fig. 1F). Immunohistochemical staining was positive for cytokeratin AE1/AE3, thyroid transcription factor 1 (TTF-1), and synaptophysin, and the final histological diagnosis was lymph node metastasis of small cell carcinoma. However, the primary site was not identified inside or outside the lung. Accordingly, the patient was diagnosed with stage IIA disease (TXN1M0) of SCLC and stage IA (T1aN0M0) adenocarcinoma. Epidermal growth factor receptor (EGFR) gene mutation in both adenocarcinoma and lymph node metastasis of SCLC were examined. However, no mutation was detected in either lesion.
Because of prolonged alveolar air leak, he was discharged on 38th post-operative day, and underwent post-operative combination chemotherapy with the regimen for SCLC (carboplatin and etoposide). After the completion of four courses of chemotherapy, no recurrence was observed during the 12-month follow-up period.
Case 2
A 72-year-old man was referred to our hospital for an abnormal shadow on chest CT during his medical check-up. His past medical history was unremarkable but he had a smoking history (Brinkman index: 2000). Physical examination was also unremarkable. Of serum tumor markers, only Pro-GRP was slightly elevated, at 155 pg/mL (normal range, <81.0). Chest CT showed a 23-mm-diameter ground-glass opacity (GGO) with a cavity in the right Segment 6 (Fig. 2A) and right hilar lymph node enlargement (Fig. 2B). Bronchoscopy was unremarkable. PET/CT revealed low-intensity FDG uptake in the GGO lesion with the SUVmax of 13.0 (Fig. 2C). There was also intermediate FDG uptake (SUVmax: 3.58) in the right #12 lymph node (Fig. 2D). Lung cancer (cT1bN1M0 stage IIA) was suspected, and surgery was planned. Right lower lobectomy with lymph node dissection was performed in August 2013.
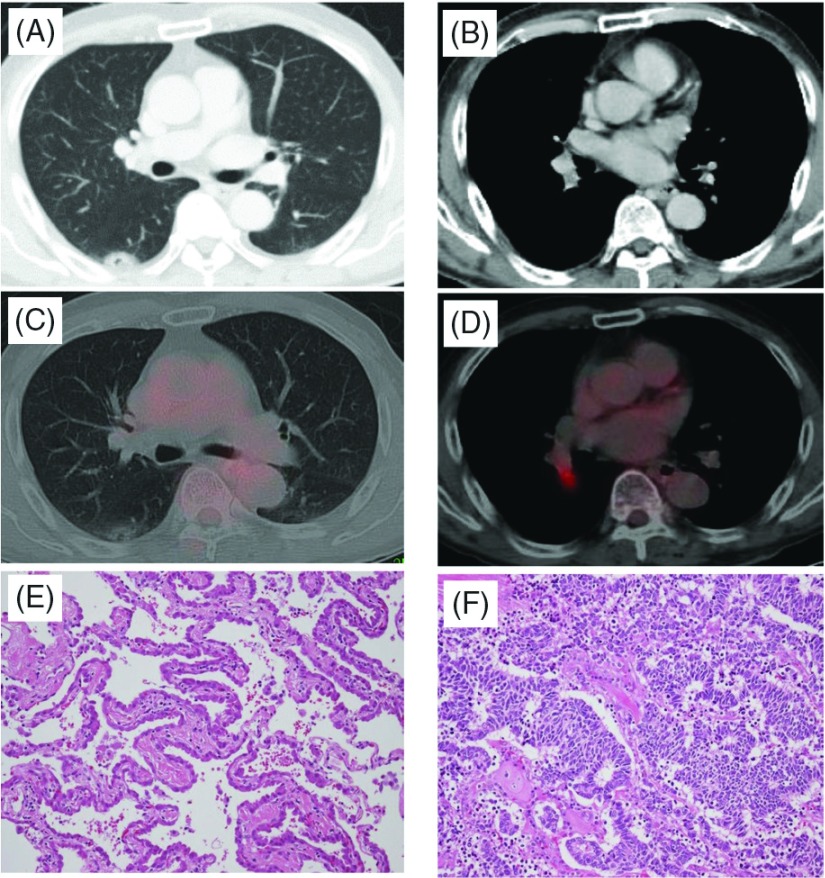
Fig. 2.
Case 2. Chest computed tomography (CT) showing a ground-glass opacity (A) and #12 lymph node swelling (B). Positron emission tomography (PET)-CT showing a part-solid nodule (C) and the #12 lymph node (D). Histologic findings (Hematoxylin-eosin staining) showing adenocarcinoma (E) and small cell lung cancer (SCLC) (F).
Pathological examination of the S6 tumor revealed adenocarcinoma in situ (Fig. 2E), but the #12 lymph node revealed metastasis of small cell carcinoma (Fig. 2F). The Ki-67 index was more than 80% with positive reactivity for cytokeratin AE1/AE3, TTF-1, and synaptophysin immunohistologically. These findings were consistent with small cell carcinoma metastasis to the hilar lymph node. However, the primary site of small cell carcinoma was not identified inside or outside the lung. Accordingly, the patient was diagnosed with stage IIA disease (TXN1M0) of SCLC and stage 0 (TisN0M0) adenocarcinoma. EGFR gene mutation in both adenocarcinoma and lymph node metastasis of SCLC were examined. However, no mutation was detected in either lesion.
His post-operative course was uneventful, and he was discharged on the 6th post-operative day. He underwent post-operative combination chemotherapy with the same regimen as that of Case 1. After four courses of chemotherapy, no recurrence was observed during the 10-month follow-up period.
Discussion
To date, the incidence and histology of SPLC have been described in a number of reports. According to several studies, SPLC occurs in 1%–16% of all lung cancer patients and is most commonly of the same histologic type.1) Hiraki, et al. reported that, among possible combinations, squamous cell carcinoma and squamous cell carcinoma were the most common; however, the synchronous presentation of small cell lung cancer and adenocarcinoma was present in only five of 16434 patients (0.03%) with lung cancers.2)
With the widespread use of low-dose helical CT for lung cancer screening, a noted increase in the detection of small peripheral adenocarcinoma, such as in Figs. 1A and 2A, has been recognized. Lobectomy with systematic lymph node dissection has been accepted as a standard treatment for primary non-small cell lung cancer (NSCLC). In our two cases, histologic diagnosis was made at the time of surgery. Although comprehensive histologic investigation of the resected specimens was performed, we were not able to identify the primary lesion of small cell carcinoma in either case. Therefore, these two patients showed a very rare combination of primary adenocarcinoma and SCLC with an unknown primary site. When the lymphatic pathway toward the hilar lymph nodes is taken into consideration, the metastasis might have developed from a primary site existing in the lower lobe. There was no abnormal uptake of FDG leading to the suspected existence of SCLC in not only other pulmonary lobes but also other organs on PET performed at the time of pre-operative staging.
According to the analysis of 26 patients with combined SCLC, with squamous cell carcinoma (n = 21), adenocarcinoma (n = 4), or adenosquamous carcinoma (n = 1) by Hage, et al.,3) the cumulative five-year survival rate after surgical resection was 31% for p-Stage I and 0% for p-Stage >II. They concluded that, in patients with Stage I combined or pure SCLC, surgery can facilitate a long-term disease free interval or may even be curative.
We performed a literature search, and extracted five case reports describing SPLC of adenocarcinoma and SCLC.2,4–7) The clinical characteristics of the seven patients, including our two patients, are presented in Table 1. Those who received chemotherapy prior to diagnosis or surgery are excluded. The ages ranged from 55 to 73 (median: 65) years. Six males and one female had a smoking habit. In our cases, the mutation status of the EGFR gene in both adenocarcinoma and lymph node metastasis of SCLC was investigated. However, no mutation was detected in either lesion (Table 1). In the case reported (Case 5) by Norkowski, et al., one of two adenocarcinomas had EGFR mutation; however, the other one and SCLC had wild-type EGFR. In addition, we list five reports which described the EGFR mutation status in resected specimens with combinations of SCLC and adenocarcinoma.7–11) Fifteen patients were extracted. They also had received no chemotherapy, target therapy, or radiation therapy prior to surgery. Their ages ranged between 47 and 76 years. Of the 15 patients, four were women in whom three were never-smokers. Of the 15, seven patients had EGFR mutation in their adenocarcinoma component. In three patients (19%), the same type of EGFR mutation was found in both adenocarcinoma and SCLC components. One of six Japanese cases had an L858R mutation in exon 21,8) and one of five Chinese cases had a deletion in exon 19.11) Toyokawa, et al.10) reported a male patient with EML4-ALK fusion in his SCLC component. According to the report of Norkowski, et al.,7) four (44%) of nine SCLC components developing in association with adenocarcinoma, either synchronously or metachronously, were EGFR-mutated. They suggest that such associated cases should be tested for EGFR mutations. The mutation rate is higher than that (4%) detected in non-combined SCLC.10)
Table 1.
Characteristic of SPLC patients presenting with SCLC and adenocarcinoma
| Sex Age | BI | Diagnosis | Tumor location for SCLC/Ad | Specimen | Treatment for SCLC/Ad | Survival/cause of death | EGFR mutation | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Kodama, et al. 1985 | M 55 | 1400 | SCLC Papillary Ad | Right upper/ Right lower | Bronchoscopy/ Percutaneous | Right pnemonectomy | 5 months, dead Dermatomyositis Interstitial pneumonia | ND |
| 2 | Hida, et al. 1993 | M 63 | 1200 | SCLC Moderately differentiated Ad | Right lower/ Right upper | TBLB | Cisplatin + doxorubicin + cyclophosphamide + etposide/Thoracic RT (50 Gy) | 9 months, dead Heart attack | ND |
| 3 | Hiraki, et al. 1999 | M 57 | 1800 | SCLC Moderately differentiated Ad | Subcarinal LN/Right upper | LN biopsy TBLB | Cisplatin + etoposide + thoracic RT (50 Gy)/ Thoracioscopic partial resection | 13 months, alive SCLC recurrence | ND |
| 4 | Kobashi, et al. 2006 | M 73 | 500 | SCLC Well differentiated Ad | Left upper (S1+2)/Left upper (S3) | TBLB | Cisplatin + Irinotecan/ Left upper lobectomy | 3 months, alive No recurrence | ND |
| 5 | Norkowski, et al. 2013 | F 65 | 900 | SCLC Well differentiated Acinar and papillary Ad | Left upper (S1+2)/Left upper (S3) | Resection | ND | ND | SCLC none Upper lobe Ad: none Lower lobe Ad: exson 21 L858R |
| 6 | Present Case 1 | M 70 | 2040 | SCLC Minimally invasive Ad | Right hilar LN/Right lower (S10) | Resection | Carboplatin + etoposide/Right lower lobectomy | 12 months, alive No recurrence | SCLC: none Ad: none |
| 7 | Present Case 2 | M 72 | 2000 | SCLC Ad in situ | Right hilar LN/Right lower (S6) | Resection | Carboplatin + etoposide/Right lower lobectomy | 10 months, alive No recurrence | SCLC: none Ad: none |
SPLC: synchronous primary lung cancer; BI: Brinkman index; SCLC: small cell lung cancer; Ad: adenocarcinoma; EGFR: epidermal growth factor receptor; TBLB: transbronchial lung biopsy; ND: not described; RT: radiation therapy; LN: lymph node
We encountered two cases with SPLC of SCLC and early-stage adenocarcinoma. On the basis of the stage of SCLC at the time of surgery, post-operative chemotherapy was administered. We were not able to define whether the primary site of SCLC was inside or outside of the adenocarcinoma in either case. Gene analysis of both histologic components may yield interesting findings.
Consent
Written informed consent was obtained from the patients for the publication of this report and any accompanying images.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1).Ferguson MK, DeMeester TR, DesLauriers J, et al. Diagnosis and management of synchronous lung cancers. J Thorac Cardiovasc Surg 1985; 89: 378-85. [PubMed] [Google Scholar]
- 2).Hiraki A, Ueoka H, Yoshino T, et al. Synchronous primary lung cancer presenting with small cell carcinoma and non-small cell carcinoma: diagnosis and treatment. Oncol Rep 1999; 6: 75-80. [PubMed] [Google Scholar]
- 3).Hage R, Elbers JR, Brutel de la Rivière A, et al. Surgery for combined type small cell lung carcinoma. Thorax 1998; 53: 450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Kodama K, Doi O, Akita N, et al. A resected case of unilateral double primary lung cancer (small cell carcinoma and adenocarcinoma) with dermatomyositis and intersititial pneumonia. Jpn J Lung Cancer 1985; 25: 85-92. (in Japanese) [Google Scholar]
- 5).Hida T, Ariyoshi Y, Sugiura T, et al. Synchronous lung cancer presenting with small cell carcinoma and adenocarcinoma. Chest 1993; 104: 1602-4. [DOI] [PubMed] [Google Scholar]
- 6).Kobashi Y, Fukuda M, Yoshida K, et al. Synchronus presentation of early-stage small cell carcinoma and adenocarcinoma in the same lung lobe. Intern Med 2006; 45: 287-91. [DOI] [PubMed] [Google Scholar]
- 7).Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013; 8: 1265-71. [DOI] [PubMed] [Google Scholar]
- 8).Fukui T, Tsuta K, Furuta K, et al. Epidermal growth factor receptor mutation status and clinicopathological features of combined small cell carcinoma with adenocarcinoma of the lung. Cancer Sci 2007; 98: 1714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008; 14: 6092-6. [DOI] [PubMed] [Google Scholar]
- 10).Toyokawa G, Taguchi K, Ohba T, et al. First case of combined small-cell lung cancer with adenocarcinoma harboring EML4-ALK fusion and an exon 19 EGFR mutation in each histological component. J Thorac Oncol 2012; 7: e39-41. [DOI] [PubMed] [Google Scholar]
- 11).Lu HY, Mao WM, Cheng QY, et al. Mutation status of epidermal growth factor receptor and clinical features of patients with combined small cell lung cancer who received surgical treatment. Oncol Lett 2012; 3: 1288-92. [DOI] [PMC free article] [PubMed] [Google Scholar]