Abstract
Purpose
Brn3b is a class IV POU domain transcription factor that plays an important role in the development of retinal ganglion cells (RGCs), RGC survival, and particularly axon growth and pathfinding. Our previous study demonstrated that recombinant adenoassociated virus serotype 2 (rAAV-2)–mediated overexpression of Brn3b in RGCs promoted neuroprotection in a rodent model of glaucoma. However, the mechanisms underlying neuroprotection of RGCs in rats overexpressing Brn3b in animal models of glaucoma remain largely unknown. The goal of this study was to understand some of the mechanisms underlying the neuroprotection of RGCs overexpressing Brn3b during intraocular pressure (IOP) elevation in Brown Norway rats.
Methods
One eye of Brown Norway rats (Rattus norvegicus) was injected with an AAV construct encoding either green fluorescent protein (GFP; recombinant adenoassociated virus–green fluorescent protein, rAAV-hSyn-GFP) or Brn3b (rAAV-hSyn-Brn3b). Expression of antiapoptotic proteins, including B cell lymphoma/leukemia-2 (Bcl-2) family proteins (Bcl-2 and Bcl-xL), and p-AKT, was observed following immunostaining of rat retinas that overexpress Brn3b. In a different set of experiments, intraocular pressure was elevated in one eye of Brown Norway rats, which was followed by intravitreal injection with AAV constructs encoding either GFP (rAAV-CMV-GFP) or Brn3b (rAAV-CMV-Brn3b). Retinal sections were stained for prosurvival factors, including Bcl-2, Bcl-XL, and p-AKT.
Results
AAV-mediated expression of transcription factor Brn3b promoted statistically significant upregulation of the Bcl-2 protein and increased expression of p-AKT in RGCs of Brown Norway rats. In addition, following IOP elevation, AAV-mediated Brn3b expression also statistically significantly increased levels of Bcl-2 in the RGC layer in Brown Norway rats.
Conclusions
Adenoassociated virus–mediated Brn3b protein overexpression may promote neuroprotection by upregulating key antiapoptotic proteins, including Bcl-2, Bcl-xL, and p-AKT, in animal models of glaucoma.
Introduction
Glaucoma, a leading cause of irreversible blindness, is projected to affect 79.6 million people by 2020 [1]. It is a heterogeneous group of optic neuropathies characterized by axon degeneration, cupping of the optic disc, and loss of retinal ganglion cells (RGCs) that contribute to visual field defects and vision loss [1,2]. Increased intraocular (IOP) remains a major risk factor in glaucoma, and most current treatments are aimed at reduction of IOP in patients. Multiple theories has been proposed to explain the pathophysiology of glaucoma, including mechanical stress due to elevated IOP, disruption of retrograde transport of neurotrophins [2], ocular ischemia [3-5], glutamate-mediated excitotoxicity [6], and oxidative stress [7-9]. IOP management in patients with glaucoma is aimed at limiting the initial insult that produces optic nerve degeneration and RGC apoptosis. Despite current strategies to lower IOP in patients with glaucoma, some neurodegenerative effects at the optic nerve and retina continue to occur in many patients. Thus, understanding the molecular mechanisms that contribute to RGC apoptosis can lead to the development of more effective treatments for patients with glaucoma [10].
The brain-specific homeobox/POU domain protein (Brn) family of class-4 POU domain transcription factors consists of three closely related genes: Brn3a, Brn3b, and Brn3c [11,12]. Brn3 genes are expressed in either a discrete or overlapping pattern in the developing and mature mammalian nervous system [13-17]. In the retina, Brn3b is specifically expressed in retinal ganglion precursor neurons, as well as mature RGCs [15,16,18]. Previous studies have shown that Brn3b plays an important role in the regulation of RGC survival, axon growth, and pathfinding [13,19,20]. A prominent phenotype in Brn3b-deficient mice, but not Brn3a- or Brn3c-deficient mice, was the loss of nearly 70% of RGCs between E15.5 and birth [18,21,22]. Consistent with a reduction in the number of RGCs, a decrease in optic nerve fibers and thinning of the optic nerve were also observed in Brn3b-deficient mice [18]. Our previous study demonstrated the neuroprotective effects of AAV-mediated Brn3b overexpression in an ocular hypertension rat model of glaucoma [23]. However, the mechanisms that contribute to neuroprotective effects of Brn3b overexpression during ocular hypertension are unknown. One obvious candidate gene responsible for the neuroprotective effects of Brn3b is the antiapoptotic protein, B cell leukemia/lymphoma 2 (Bcl-2), since there is a putative binding site for Brn3b in the upstream promoter region of the bcl-2 gene.
The Bcl-2 gene family encodes proteins with similar structural domains (Bcl-2 homology domains designated BH1 to BH4) that play a key role in the regulation of cell survival. Typically, proteins that contain all four domains (e.g., Bcl-2, Bcl-xL, and Mcl-1) are antiapoptotic, while those that contain fewer domains are proapoptotic (e.g., Bax, Bad, Bim, Bid, and Puma). Pro- and antiapoptotic members of the Bcl-2 family are major regulators of cell death and survival. Previous studies have shown the role of the Bcl-2 gene family in RGC survival, in acute and chronic models of optic nerve lesion [10,24]. Bcl-2 was found to prevent cell death when overexpressed in a variety of cell types, particularly in neurons. For instance, mice that overexpress Bcl-2 in neurons, under the regulation of the neuron-specific enolase (Nse) promoter displayed an increased number of RGCs, after developmental pruning and after optic nerve axotomy [25-29]. More recently, gene transfer of BAG1, a Bcl-2 associated protein, rescued RGCs following optic nerve crush as well as axotomy [30]. Among antiapoptotic Bcl-2 family members, Bcl-xL is predominantly expressed in the rat retina, and levels decrease following optic nerve crush [31]. Bcl-2 and Bcl-xL retinal mRNA levels were found to decrease after optic nerve axotomy [32]. Moreover, adenoassociated virus (AAV)–mediated expression of Bcl-xL promoted the survival of axotomized RGCs [33-35]. Targeted deletion of Bcl-xL has been shown to contribute to apoptotic cell death of post-mitotic immature neurons of the central nervous system (CNS) [36]. These findings suggest that Bcl-2 and Bcl-xL are key regulators of RGC survival following a variety of insults that contribute to optic nerve injury.
AKT, a PI 3-kinase activated protein kinase, acts as the principal mediator of cell survival in diverse cell types [37-41]. Several studies suggest that activation of the AKT pathway leads to retinal ganglion cell survival, not only during development but also in different animal models of glaucoma, including those involving ischemia-perfusion injury and optic nerve injury [42-50].
The purpose of the current study was to determine whether the transcription factor Brn3b by itself promotes an increase in the levels of the prosurvival Bcl-2 family of proteins. In addition, the involvement of members of the Bcl-2 family and p-AKT during AAV-mediated Brn3b overexpression following ocular hypertension was also investigated.
Methods
Plasmid construction and recombinant AAV-2 production
Plasmid construction and recombinant AAV-2 production were performed according to the method described by Stankowska et al. [23]. Using the AAV Helper-free system (Agilent Technologies, Santa Clara, CA), recombinant AAV vectors were prepared with plasmids pAAV-IRES-hrGFP (hrGFP is a humanized recombinant green fluorescent protein, GFP), pAAV-RC, and pHelper. pAAV-Brn3b vector encoding transcription factor Brn3b was constructed by insertion of mouse Brn3b cDNA (Origene, Rockville, MD) into pAAV-IRES-hrGFP (Agilent Technologies). After the DNA sequence was validated, plasmids were used to produce rAAV-CMV-Brn3b and adenoassociated virus–cytomegalovirus–green fluorescent protein (rAAV-CMV-GFP) viruses. Gene expression in both vectors was driven by the CMV promoter.
The control virus AAV2.hSyn.eGFP.WPRE.bGH was purchased from the Penn Vector Core facility (Philadelphia, PA) and abbreviated as rAAV-hSyn-GFP. The pAAV-hSyn.Brn3b-DDK.WPRE.bGH plasmid was prepared by insertion of a mouse Brn3b cDNA sequence containing the DDK tag (Origene, Rockville, MD) into pAAV.hSyn.eGFP.WPRE.bGH (in place of the eGFP cDNA sequence), as described by Stankowska et al. [23]. The custom-made plasmid sequence was confirmed with DNA sequencing and sent to Penn Vector Core for AAV-2 virus production. The custom-made virus AAV2.hSyn.Brn3b-DDK.WPRE.bGH was abbreviated in the current study as rAAV-hSyn-Brn3b. To improve the specificity and reduce off-target effects of AAV-2 virus, we used the viral constructs driven by the neuron-specific human synapsin promoter [23].
Animals
All animal-related procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the UNT Health Science Center and were in compliance with the ARVO statement for the use of Animals in Ophthalmic and Vision Research. Male retired breeder Brown Norway rats (Rattus norvegicus; Charles River Laboratories, Wilmington, MA) in the age group of 8 to 12 months were used in this study.
Morrison’s ocular hypertension model of glaucoma in rats
Male retired male breeder Brown Norway rats were used to study the effect of overexpression of transcription factor Brn3b in the RGCs of retinas with elevated IOP. The procedure described by Morrison et al. [51] was used to elevate the IOP in one eye of the rats. To elevate the IOP, the animals were maintained in a reduced constant light environment (90 lux) for a minimum of 3 days before surgery. On the day of the surgery, the animals were anesthetized using a cocktail comprising of 50 mg/ml ketamine, 5 mg/ml xylazine and 1 mg/ml acepromazine (100 μl/100 g body weight administered intraperitoneally) and injected with 1.8 M NaCl via an episcleral vein, while the contralateral eye served as the control. A microglass needle was inserted into the episcleral vein, and approximately 50 µl of hypertonic saline was injected with a force sufficient to blanch the aqueous plexus. This procedure produced scarring of the trabecular meshwork that resulted in a rise in IOP (7 to 10 days following surgery) and subsequent damage to the optic nerve and RGCs.
IOP measurements
IOP measurements using a Tonolab tonometer (Icare Finland Oy, Espoo, Finland) were performed on conscious animals following slight sedation with intramuscular (i.m.) administration of acepromazine (2 mg/kg), and IOP was measured 2 to 5 min after the injection. During each IOP measurement session, ten average readings were obtained from the contralateral control eyes and the eyes with elevated IOP. A plot of mean IOP versus time was performed and to assess total IOP exposure (mmHg-day), which was computed by determining the difference of the area under the curve (AUC) between the IOP-elevated eye and the contralateral control eye (mmHg-days = AUC of the eye with elevated IOP – AUC of the control eye).
Intravitreal injections of AAV-2 constructs
Intravitreal injections were performed using an ultrafine 30.5 G disposable needle connected to a 50 µl Hamilton syringe (Hamilton Company, Reno, NV) as described by Zhou et al. [52] in the anesthetized rats. Five microliters (ranging from 1×109 to 3.5×109 genomic copies) of the AAV virus were injected (while the position of the needle was continuously monitored) in the center of the vitreous cavity to avoid lens injury. Transduction of AAV viruses did not cause any inflammatory or damaging effect to the optic nerve or the retina. Experiments were performed three times using three Brown Norway rats for the control AAV vector and three rats for the AAV-Brn3b groups (total n = 18).
Cryosections
Animals were euthanized by intraperitoneal administration of pentobarbital (120 mg/kg body weight administered intraperitoneally followed by intracardial administration), and the eyes were enucleated and fixed in 4% paraformaldehyde for 3 h at room temperature and then submerged in 20% sucrose in PBS (1×: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) overnight at 4 °C. Fixed eyes were embedded in optimal cutting temperature compound (OCT; Miles Diagnostics, Elkhart, IN) and frozen at −80 °C. Transverse 10 µm thick retinal sections were cut using a cryostat (Leica Biosystems, Buffalo Grove, IL) and used for immunohistochemical analysis. Sections were viewed with a Zeiss (Thornwood, NY) LSM 510 META confocal scanning microscope.
Immunohistochemistry analysis
To validate upregulation of Brn3b and changes in the expression of Bcl-2, Bcl-xL, and p-AKT in RGCs, colocalization of these proteins with the RGC marker βIII-tubulin (1:500; Sigma-Aldrich, MO) was performed. Retinal cryosections were hydrated in PBS for 15 min and then blocked in PBS containing 5% normal donkey serum and 5% bovine serum albumin (BSA) for 1 h at room temperature. The sections were double-immunostained with mouse anti-βIII-tubulin antibody in combination with either rabbit anti-Brn3b antibody (1:250 dilution, Antibody Research Corporation, St. Charles, MO), rabbit anti-Bcl-2 antibody (1:100 dilution, catalog no. sc-492; Santa Cruz Technology, Dallas, TX), rabbit anti-Bcl-xL antibody (1:300 dilution, catalog no. 2764; Cell Signaling Technology, Beverly, MA), rabbit anti-p-AKT antibody (1:25 dilution, catalog no. 9271; Cell Signaling Technology), or rabbit anti-GFP antibody (1:100 dilution, catalog no. G10362; ThermoFisher, Waltham, MA) and incubated overnight at 4 °C.
Sections were then washed 3 times for 5 min each with PBS and incubated for 1 h with the appropriate donkey anti-IgG secondary antibody conjugated with Alexa 488, Alexa 647, or Alexa 547 (1:1,000 dilution; Molecular Probes, Invitrogen, Eugene, OR). Sections in which the primary antibody incubation was excluded served as negative controls (blanks) and were used to assess nonspecific staining with the secondary antibody.
Results
Overexpression of Brn3b in rat retinal ganglion cells after intravitreal injection of rAAV-hSyn-Brn3b
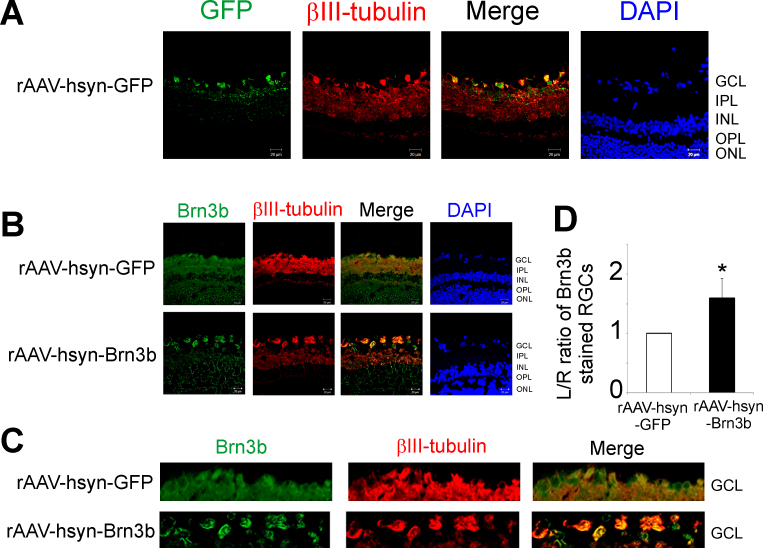
To study the effect of overexpression of Brn3b in RGCs, the viral vector rAAV-hSyn-GFP or rAAV-hSyn-Brn3b was injected intravitreally into the left eye (L), while the right eye (R) served as the corresponding control. The rats were maintained for 3 weeks following virus injection and then euthanized. Frozen rat retinal sections were obtained and subjected to immunohistochemistry to detect the GFP and Brn3b levels. To confirm virus transduction of the RGCs of the rats injected with rAAV-hSyn-GFP, the rat retinal sections were immunostained with a rabbit anti-GFP antibody. Staining for GFP was seen mainly in the ganglion cell layer (GCL), and mild staining was also seen in the inner plexiform layer (IPL) layer (Figure 1A). Increased Brn3b immunostaining (pseudogreen) was detected in the retinas of the rats administered rAAV-hSyn-Brn3b, mainly in the GCL, and diffuse staining was also detected in the inner nuclear layer (INL) and the outer nuclear layer (ONL; Figure 1B). βIII-tubulin was used as an RGC marker, and the antibody was found to stain mainly the RGC layer and the IPL. As expected, ratios of Brn3b staining of RGCs counts between the left and right eyes were statistically significantly higher (1.5 fold) in the rats injected with rAAV-hSyn-Brn3b, compared to those injected with rAAV-hSyn-GFP (n = 6; Figure 1D).
Figure 1.
Transduction of rAAV-hSyn-GFP and rAAV-hSyn-Brn3b and overexpression of Brn3b in RGCs of Brown Norway rats. A: Immunohistochemical analyses for green fluorescent protein (GFP; green) in frozen retinal sections from rats injected with the recombinant adenoassociated virus–hSyn–green fluorescent protein (rAAV-hSyn-GFP) virus. Binding of the GFP antibody was detected using an Alexa 488-conjugated donkey anti-rabbit immunoglobulin G (IgG) antibody. Cells were counterstained with 4',6-diamidino-2-phenylindole (DAPI) to detect nuclei. B: Brn3b (pseudogreen) and βIII-tubulin (pseudored) immunostaining in retinal frozen sections from rats intravitreally injected with either rAAV-hSyn-GFP or rAAV-hSyn-Brn3b. The immunostaining was detected by using corresponding Alexa 546 (pseudogreen) and Alexa 647 (pseudored)-conjugated donkey anti-immunoglobulin G (IgG) secondary antibodies. Cells were counterstained with DAPI (blue) to detect cell nuclei. NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; OS, outer segment. Scale bar indicates 20 µm. C: Representative images of the GCL showing Brn3b staining in rats injected with rAAV-hSyn-GFP or rAAV-hSyn-Brn3b. D: Plot of the ratio of fluorescence intensity of Brn3b immunostaining in RGCs between left (L: intravitreally injected) and right (R: contralateral) eyes in 24 regions. The L/R ratio was compared between rats injected with rAAV-hSyn-GFP and rAAV-hSyn-Brn3b. A significant increase in Brn3b staining in RGCs was observed in rats that overexpressed Brn3b compared to the control vector. Fluorescent intensity values are shown as mean ± standard error of the mean (SEM), n = 6. The Mann–Whitney rank-sum test was used for statistical analysis (*p<0.002).
To further demonstrate the immunostaining for Brn3b in the RGC layer, additional immunohistochemical staining was performed using the NeuN antibody. As seen in Appendix 1, costaining for Brn3b and NeuN occurred mainly in the RGC layer of the retina.
Upregulation of Bcl-2 levels in RGCs of rats injected intravitreally with rAAV-hSyn-Brn3b
Additional experiments were performed to determine whether the neuroprotective protein Bcl-2 was upregulated in the retinas of the rats injected with rAAV-hSyn-Brn3b. In addition to Bcl-2, the status of Bcl-xL was also assessed in rats administered rAAV-hSyn-Brn3b intravitreally. Bcl-xL has been shown to be neuroprotective for RGCs in optic nerve injury models in several studies [32-36].
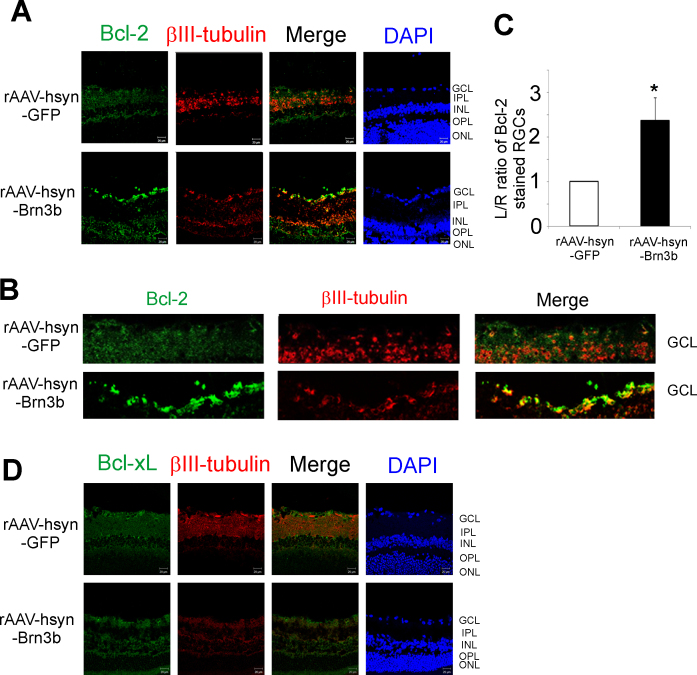
Briefly, 3 weeks following intravitreal injection of rAAV-hSyn-GFP or rAAV-hSyn-Brn3b, the rats were euthanized, and frozen sections of the retina were collected and analyzed with immunohistochemistry for either Bcl-2 expression (pseudogreen, Figure 2A) or Bcl-xL expression (pseudogreen, Figure 2D) in the RGCs of the rats. An increase in Bcl-2 was found mainly in the GCL, and a mild increase in immunostaining was also observed in the INL in the rat eyes injected with rAAV-hSyn-Brn3b compared to the rat eyes injected with rAAV-hSyn-GFP, where minimal staining for Bcl-2 was found (Figure 2A). Immunostaining with the βIII-tubulin antibody was used as an RGC marker. The fluorescence intensity ratios (left to right eyes) for Bcl-2 staining in the RGCs were statistically significantly higher (greater than twofold) in the rat retinas that overexpressed Brn3b compared to the retinas transduced with the control vector (Figure 2C). To determine whether Brn3b-mediated Bcl-2 upregulation has detrimental effects on visual function, we performed an optomotor test. The results indicated that intravitreal administration of the rAAV-2-hSyn-Brn3b does not affect the visual acuity in Brown Norway rats (Appendix 3; n = 2).
Figure 2.
Transcription factor Brn3b-mediated changes in Bcl-2 and Bcl-xL expression in RGCs of Brown Norway rats. A: Bcl-2 (pseudogreen), and βIII-tubulin (pseudored) expression in retinal sections from Brown Norway rat eyes injected with either the recombinant adenoassociated virus–hSyn–green fluorescent protein (rAAV-hSyn-GFP) virus (control) or the rAAV-hSyn-Brn3b virus, detected with secondary antibodies conjugated with Alexa 546 (pseudogreen) or Alexa 647 (pseudored) dye. Cells were counterstained with 4',6-diamidino-2-phenylindole (DAPI; blue) to detect cell nuclei. B: Magnified image of the retinal ganglion cell (RGC) layers showing Bcl-2 immunostaining of RGCs of retinas transduced with either rAAV-hSyn-GFP or rAAV-hSyn-Brn3b. Scale bar indicates 20 µm. C: Ratio of fluorescence intensity for Bcl-2 staining was measured at 24 different regions in the ganglion cell layers using ImageJ. The fluorescence intensity ratios are shown as mean ± standard error of the mean (SEM), n = 6. A statistically significant increase in the intensity of Bcl-2 staining was found in the RGCs of the rats injected with rAAV-hSyn-Brn3b, compared to those injected with rAAV-hsyn-GFP. Statistical analysis was performed with the Mann–Whitney rank-sum test (*p<0.002). D: Bcl-xL (pseudogreen) and βIII-tubulin (pseudored) immunostaining in frozen retinal sections from rats intravitreally injected with either rAAV-hSyn-GFP or rAAV-hSyn-Brn3b.
However, no change in the Bcl-xL levels was seen in the RGCs of the rats injected with rAAV-hSyn-Brn3b compared to those injected with rAAV-hSyn-GFP (n = 6; Figure 2D). The data suggest that Bcl-2, but not Bcl-xL, expression was markedly increased in the RGCs in rat eyes injected with rAAV-hSyn-Brn3b compared to those injected with rAAV-hSyn-GFP. As the immunostaining for Bcl-xL was minimally detected in the frozen retinal sections, we performed an immunoblot analysis using the Bcl-xL antibody in optic nerve extracts from naïve Brown Norway rats. It was found that the antibody recognized a doublet band at 30 kDa (of the expected size) in the extracts from the left and right optic nerves, indicating the immunoreactivity and specificity of the antibody (Appendix 2). This demonstrates that the lack of immunostaining in the immunohistochemical analysis (Figure 2D) was not due to weak immunoreactivity of the anti-Bcl-xL antibody.
Increased levels of p-AKT in rat RGCs that overexpress rAAV-hSyn-Brn3b
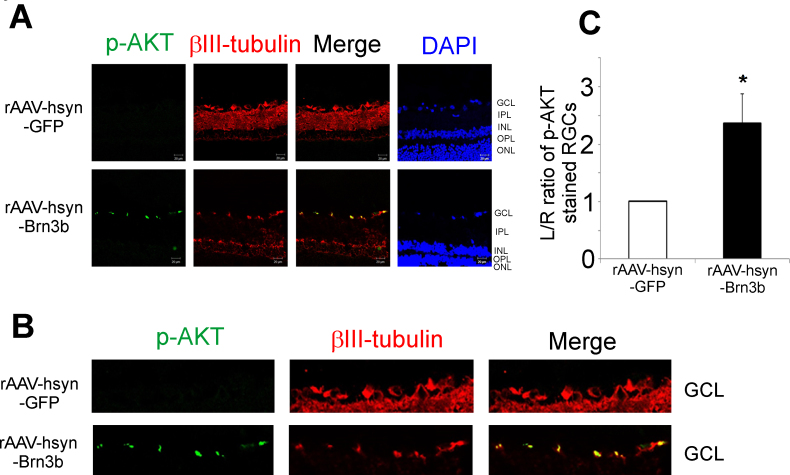
Several lines of evidence have demonstrated AKT is a key mediator of RGC survival in multiple models of glaucoma [42-50]. Therefore, we assessed immunostaining of p-AKT in retinal frozen sections from rats administered either rAAV-hSyn-Brn3b or rAAV-hSyn-GFP intravitreally. As seen in Figure 3A, intravitreal administration of rAAV-hSyn-Brn3b in rats produced robust upregulation of p-AKT in the RGCs compared to the rats administered rAAV-hSyn-GFP. Staining for βIII-tubulin was used as an RGC marker (n = 6). Densitometric analysis revealed a statistically significant increase in the expression of p-AKT in the retinas of rats intravitreally injected with rAAV-hSyn-Brn3b compared to those injected with rAAV-hSyn-GFP (Figure 3C). Based upon the quantitation of the fluorescence intensities, overexpression of Brn3b in the RGCs of rats produced a nearly 2.5-fold increase in p-AKT, compared to the rats intravitreally injected with rAAV-hSyn-GFP.
Figure 3.
Transcription factor Brn3b promoted an increase in the levels of p-AKT in retinas of rats injected with rAAV-hSyn-Brn3b. A: Immunostaining for p-AKT (pseudogreen), βIII-tubulin (pseudored) expression in retinal sections from Brown Norway rats intravitreally injected with either the recombinant adenoassociated virus–hSyn–green fluorescent protein (rAAV-hSyn-GFP; vector control) or rAAV-hSyn-Brn3b virus. The immunostaining was detected using corresponding Alexa 546 (pseudogreen) or Alexa 647 (pseudored) conjugated secondary antibody. Scale bar indicates 20 µm. B: A magnified view of the retinal ganglion cell (RGC) layers of retinas transduced with either rAAV-hSyn-GFP or rAAV-hSyn-Brn3b. C: A significant 2.4-fold increase in p-AKT expression was observed in the RGCs of rats injected with rAAV-hSyn-Brn3b. Ratios of fluorescence intensity values are shown in mean ± standard error of the mean (SEM), n = 6. The Mann–Whitney rank-sum test was used for statistical analysis (*p<0.002).
Overexpression of Brn3b using AAV-2 vectors in the RGCs of Brown Norway rats with elevated IOP
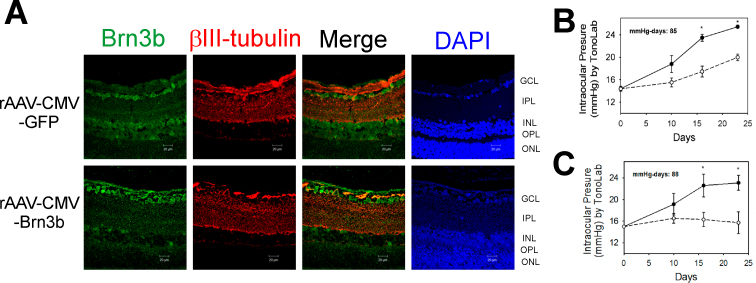
Injection of hypertonic saline into episcleral veins using the method of Morrison et al. [51] was used to elevate IOP in one eye of Brown Norway rats. One week after IOP elevation, viral vectors (either rAAV-CMV-GFP or rAAV-CMV-Brn3b) were injected intravitreally into the IOP-elevated eye. Three weeks following intravitreal injection, the rats were euthanized, and the retinas were collected, embedded in paraffin, and 5 µm thick sections were obtained. Retinal sections were subjected to immunohistochemistry to detect Brn3b levels. As seen in Figure 4A, increased immunostaining (pseudogreen) for Brn3b was detected in the rat retinas administered rAAV-CMV-Brn3b, mainly in the GCL, and diffuse staining was also detected in the INL. Ectopic Brn3b localization in the outer retina was also observed in our previous study [23]. As seen in Figure 4B,C, IOP was elevated after 7 to 10 days after surgery and remained elevated for 3 weeks until the rats were euthanized. Representative mean values of IOP exposure during the course of the experiment were 85 and 88 mmHg-days for the rats administered AAV-CMV-GFP and AAV-CMV-Brn3b, respectively. βIII-tubulin immunostaining was used as an RGC marker to identify RGCs.
Figure 4.
AAV-mediated overexpression of Brn3b in RGCs of Brown Norway rats with elevated IOP following intravitreal injection of rAAV-CMV-Brn3b. A: Representative images show Brn3b (pseudogreen) and βIII-tubulin (pseudored) immunostaining in retinas of rats intravitreally injected with either recombinant adenoassociated virus–cytomegalovirus–green fluorescent protein (rAAV-CMV-GFP) or rAAV-CMV-Brn3b. Brn3b staining was detected mainly in the GCL. B: Intraocular pressure (IOP) elevation profile in Brown Norway rats administered rAAV-CMV-GFP. C: IOP elevation profile in Brown Norway rats administered rAAV-CMV-Brn3b. IOP was elevated in one eye (closed circles), while the other eye served as the contralateral control eye (open circles). IOP elevation was performed in three Brown Norway rats followed by intravitreal injection with either rAAV-CMV-GFP or rAAV-CMV-Brn3b. IOP values were plotted as mean ± standard error of the mean (SEM; solid line, IOP-elevated and virus-injected eye; dashed line, untreated contralateral eye). Asterisk indicates p<0.001 statistically significant elevation of IOP in the IOP-elevated eye compared with contralateral eye using the Student t test. Scale bar indicates 20 µm.
Intravitreal administration of rAAV-CMV-Brn3b promotes an increase in the expression of Bcl-2 in RGCs of rats with elevated IOP
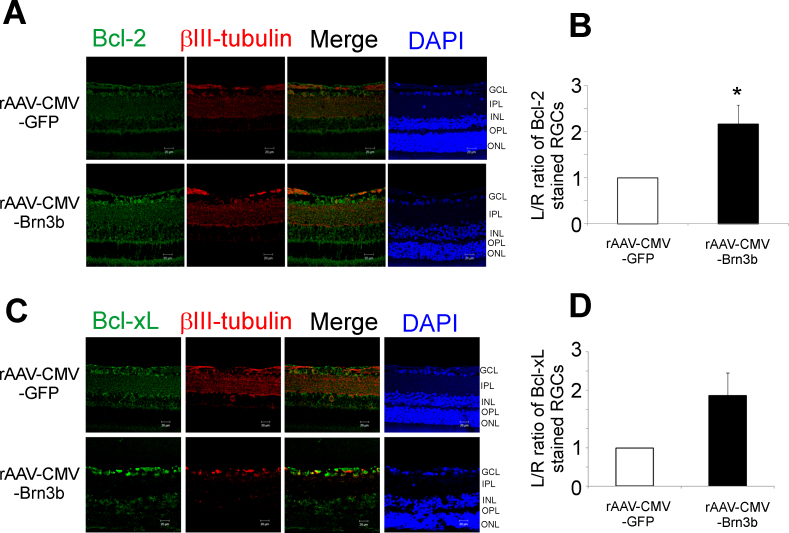
To determine whether transcription factor Brn3b has the ability to upregulate Bcl-2 and Bcl-xL under glaucomatous conditions, rats were administered rAAV-CMV-Brn3b or rAAV-CMV-GFP following elevation of IOP. After the rats were euthanized, the retinal sections were immunostained for either Bcl-2 or Bcl-xL. Increase in Bcl-2 (pseudogreen) levels was mainly seen in the GCL, and mild staining was also observed in the IPL and the outer plexiform layer (OPL) in the retinas of the rats injected intravitreally with rAAV-CMV-Brn3b, compared to those injected with rAAV-CMV-GFP following IOP elevation (Figure 5A). Quantification of the L/R ratio of fluorescence intensities in RGCs showed a statistically significant increase in immunostaining for Bcl-2 (Figure 5B). Rats injected with rAAV-CMV-Brn3b showed an increase in Bcl-xL expression mainly in the GCL and mild staining in the INL, compared to the rats intravitreally injected with rAAV-CMV-GFP (n = 3; Figure 5C). However, there was only an increasing trend (not statistically significant) in Bcl-xL expression in rats injected with rAAV-CMV-Brn3b, compared to those injected with rAAV-CMV-GFP (Figure 5D). These data suggest that following IOP-mediated damage, overexpression of transcription factor Brn3b could upregulate Bcl-2 as a mechanism to promote RGC survival as described by Stankowska et al. [23].
Figure 5.
rAAV-2-mediated overexpression of Brn3b produced an increase in Bcl-2 expression in the RGCs of Brown Norway rats with elevated IOP. A: Brown Norway rats with elevated intraocular pressure (IOP) were intravitreally injected with either recombinant adenoassociated virus–cytomegalovirus promoter–green fluorescent protein (rAAV-CMV-GFP) or rAAV-CMV-Brn3b. Retinal sections obtained were immunostained for Bcl-2 (pseudogreen) and βIII-tubulin (pseudored). B: The L/R ratio of fluorescence intensity of Bcl-2 immunostaining in retinal ganglion cells (RGCs) from rats intravitreally injected with either rAAV-CMV-GFP or rAAV-CMV-Brn3b is plotted. Densitometry analysis shows a significant increase in Bcl-2 expression in RGCs transduced with rAAV-CMV-Brn3b, compared to RGCs transduced with rAAV-CMV-GFP in the rats with elevated IOP. Student t test was used for statistical analysis (*p<0.05). Values are represented as mean ± standard error of the mean (SEM), n = 3. Scale bar indicates 20 µm. C: Brown Norway rats had elevated IOP and subsequently were intravitreally injected with either rAAV-CMV-GFP or rAAV-CMV-Brn3b. Retinal sections obtained were immunostained for Bcl-xL (pseudogreen) and βIII-tubulin (pseudored). D: A plot of the L/R ratio of fluorescence intensities of Bcl-xL immunostaining in the RGCs of rats intravitreally injected with either rAAV-CMV-GFP or rAAV-CMV-Brn3b. An increasing trend in Bcl-xL expression (not statistically significant) was observed in rats intravitreally injected with rAAV-CMV-Brn3b, compared to those administered the control vector.
Immunostaining for p-AKT in the retina during AAV-mediated upregulation of transcription factor Brn3b following IOP elevation in rats
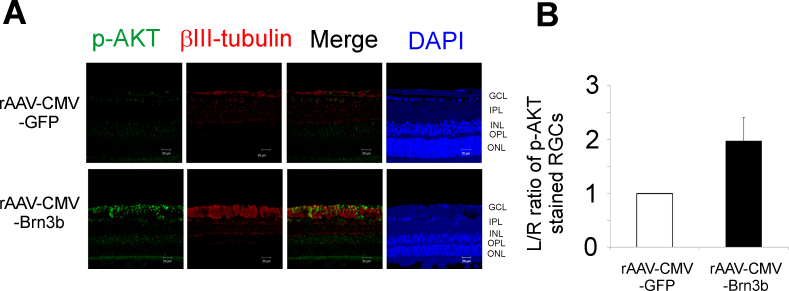
Our previous experiments demonstrated that intravitreal administration of rAAV-hSyn-Brn3b (without IOP elevation) produced increased expression of p-AKT in RGCs (Figure 3). To determine the effect of Brn3b overexpression during IOP elevation on p-AKT levels, rats with elevated IOP were injected intravitreally with either rAAV-CMV-Brn3b or rAAV-CMV-GFP, and immunostaining for p-AKT was performed. As shown in Figure 6A, immunohistochemical analysis shows upregulation of p-AKT in the NFL as well as in the GCL with rat eyes injected with rAAV-CMV-Brn3b, compared to those injected with rAAV-CMV-GFP (n = 3). There was an increasing trend (not statistically significant) in the levels of p-AKT in the RGCs of the rats intravitreally injected with rAAV-CMV-Brn3b following IOP elevation, compared to those intravitreally injected with rAAV-CMV-GFP (Figure 6B).
Figure 6.
Levels of p-AKT in the retinas of rats with elevated IOP overexpressing Brn3b. A: Immunostaining for p-AKT in retinal ganglion cells (RGCs) of Brown Norway rats intravitreally injected with either the recombinant adenoassociated virus–cytomegalovirus–green fluorescent protein (rAAV-CMV-GFP) or rAAV-CMV-Brn3b following intraocular pressure (IOP) elevation. Retinal sections obtained were immunostained for p-AKT (pseudogreen) and βIII-tubulin (pseudored). B: An increase in immunostaining (not statistically significant) for p-AKT was observed in RGCs overexpressing Brn3b (rAAV-CMV-Brn3b) compared to RGCs overexpressing the control vector (rAAV-CMV-GFP; determined with the L/R ratios of fluorescence intensities of p-AKT staining in RGCs). Values are represented as mean ± standard error of the mean (SEM), n = 3. Scale bar indicates 20 µm.
Discussion
The Bcl-2 family proteins that include anti- and proapoptotic proteins are key regulators of apoptosis. Changes in the ratio of these proteins may result either in cell survival or cell death [53]. The Bcl-2 family comprises antiapoptotic members (Bcl-2, Bcl-xL, Bcl-W, Mcl-1) that contain four Bcl-2 homology (BH) domains (BH1 to BH4), and proapoptotic members, Bax, Bak, and Bok, which have three BH domains (BH1 to BH3) and Bid, Bad, and Bim (with only a BH3 domain) [54]. Two distinct forms of Bcl-x have been identified: Bcl-xL (233 amino acids) and Bcl-xS (170 amino acids: in which BH1 and BH2 are removed by alternate spicing). Bcl-xL inhibits cell death whereas Bcl-xS facilitates it, independent of heterodimerization with other Bcl-2 family members [55]. Bcl-2 is an integral membrane protein whereas Bcl-xL becomes tightly associated with the membrane after injury signals. This prevents mitochondrial outer membrane permeabilization (MOMP) by neutralizing the activity of proapoptotic members [53,56]. Dimerization is important for the function of Bcl-2; Bcl-2 can form homodimers to protect cells from death or can form heterodimers with BAX, preventing cell death and activating cell survival [57]. In mammals, widespread expression of Bcl-2 occurs in CNS and peripheral nervous system (PNS) neurons during embryonic development, and the selective retention of Bcl-2 in the adult PNS is consistent with the role of Bcl-2 in regulating neuronal survival [58,59]. Mouse RGCs lose Bcl-2 expression after E18 [27]; thus, they have limited capacity to survive following axotomy.
Bcl-xL is expressed in the developing brain, but unlike Bcl-2 expression, Bcl-xL expression continues to increase into adult life [60]. Bcl-xL-null mice die around E13 and exhibit massive cell death of immature hematopoietic cells and neurons [36]. Cell death occurs primarily in immature neurons that have not established synaptic connections. Therefore, Bcl-xL might be important for the survival of immature neurons before the establishment of synaptic connections with their targets [56]. Bcl-2 and Bcl-xL act by inhibiting proapoptotic members of the Bcl-2 family through heterodimerization [58].
AKT, also referred as PKB (RAC-PK), is homologous to the PKA and PKC families of protein kinases [61]. The activity of AKT/PKB is regulated by serum and growth factors that activate phosphatidylinositol (PI) 3-kinase (PI3K) in vivo [62]. PI3K enzymes catalyze the formation of the lipid 3′-phosphorylated phosphoinositides, which regulate the localization and activity of a key component of cell survival, the Ser/Thr kinase, AKT [63]. AKT phosphorylation at Ser473 site is required for cell survival [64]. In different models of glaucoma, it is been shown that activated AKT has neuroprotective effects on RGCs [65-67].
One of the important goals in research on neurodegenerative diseases is to develop therapies that effectively block the apoptotic cell death of susceptible neuronal populations. RGCs, which undergo cell death via apoptosis in optic neuropathies such as glaucoma, are important targets of such neuroprotective strategies [68]. Clearly, the development of new therapies relies on a complete understanding of the changes in gene expression, which contribute to neuroprotective effects against damaging stimuli.
In a previous study conducted at our laboratory, we found significant protection of RGCs and optic nerve axons from IOP-mediated injury following AAV-mediated overexpression of Brn3b in the RGCs of Brown Norway rats [23]. The present study explored some mechanisms by which Brn3b promotes neuroprotection of RGCs in rats with elevated IOP. Two approaches were taken to study Brn3b-mediated changes in gene expression: either direct administration of the AAV-hSyn-Brn3b vector by itself (in the absence of IOP elevation) or administration of AAV-CMV-Brn3b following IOP elevation. In both models, overexpression of Brn3b produced significant upregulation of the prosurvival gene Bcl-2 in the RGCs. A statistically significant increase in the expression of p-AKT was found in the RGCs of the rat retinas that overexpressed Brn3b only via direct administration of AAV-hSyn-Brn3b vectors (in the absence of IOP elevation), compared to those administered the control vector. No statistically significant change in Bcl-xL expression was found in the rat RGCs that overexpressed Brn3b in both models. Our current findings support a key role of the antiapoptotic molecule Bcl-2 in mediating the neuroprotective effects of transcription factor Brn3b in an ocular hypertension model of glaucoma.
Brn3b is endogenously expressed in adult retinas, as well as in different parts of the brain, which includes the superior colliculus, interpeduncular nucleus, and trigeminal ganglion [69]. The role of Brn3b in mature RGC physiology is not completely understood. Brn3b plays a crucial role in the development and differentiation of RGCs, evidenced by the loss of approximately 70% of the RGCs in Brn3b-knockout mice compared to the wild-type mice [15,18,20,22]. In different animal models of glaucoma, a decrease in Brn3b expression precedes RGC loss and neurodegeneration [70,71].
Following development, Bcl-2 is not robustly expressed in retinal neurons, including RGCs. This compromises the intrinsic regenerative capacity of RGCs following injury. However, the homolog Bcl-xL appears to be a dominant antiapoptotic gene expressed in the retina of adult rats [31]. Bcl-2 is evolutionarily conserved and plays a key inhibitory role against cell death in a range of organisms from Caenorhabditis elegans to humans [72,73]. In an experimental model of optic nerve lesion, RGCs were robustly protected by Bcl-2 overexpression and demonstrated good preservation of pattern ERG responses [72]. Bcl-2 overexpression reduced neuronal loss during development that resulted in hypertrophy of the nervous system. Mice that overexpress Bcl-2 have enlarged optic nerves suggestive of enhanced RGC survival during development [74]. Bcl-2 overexpression has been shown to enhance RGC survival to nearly 65%, 3.5 months after axotomy in adult mice [26]. Numerous studies have demonstrated a neuroprotective role for Bcl-2, and constitutive expression of the Bcl-2 protein has been shown to promote the survival of various cells when exposed to adverse stimuli [25,58,75]. Our data suggest the possibility that Bcl-2 upregulation may be associated with the enhancement of RGC survival in rats intravitreally injected with rAAV-CMV-Brn3b after IOP elevation that may account for the neuroprotective effects of Brn3b found in our previous study [23].
Using reverse transcription (RT)–PCR and in situ hybridization techniques, Bcl-2 and Bcl-x mRNA expression in RGCs was found to be decreased following optic nerve axotomy [32]. Intraocular delivery of Bcl-xL fusion proteins reduced RGC death after optic nerve axotomy [33,35]. Adenoviral overexpression of Bcl-xL in mature axotomized rats enhanced RGC survival in vivo [34]. However, in the current study, no appreciable changes were found in the immunoreactive levels of Bcl-xL following intravitreal administration of AAV-hSyn-Brn3b. Mere overexpression of Brn3b was not sufficient to induce Bcl-xL, but overexpression in conjunction with elevated pressure was able to bring about an appreciable increase (albeit not significant) in Bcl-xL levels, suggesting the possibility that mechanotransduction might be a contributor to these effects.
Some cytokines such as STAT3 promote a survival response in neurons through the PI3K/AKT pathway [40,41]. PI3K/AKT signaling promotes RGC survival during retinal development, optic nerve injury, and ischemia-reperfusion injury and in acute ocular hypertension [44-46,48-50]. p-AKT levels have a correlation to increased RGC survival, particularly survival of melanopsin-expressing RGCs (mRGCs) after optic nerve injury [42,47]. However, in the current study, we found only an increasing trend for p-AKT levels during AAV-mediated overexpression of Brn3b. During ocular hypertension, AKT has direct effects on the effectors of the apoptotic pathway, including Bcl-2 [37-41]. AKT signaling through enhanced cAMP response element-binding protein (CREB) activity could increase Bcl-2 promoter activity leading to increased cell survival [46,56,76]. p-AKT also regulates Bcl-2 activity in various types of cells [37-41,46,56,76]. In addition to Brn3b-mediated elevation in Bcl-2 expression, it is possible that a transient elevation in p-AKT could promote increased Bcl-2 expression, contributing to increased RGC survival. Taken together, findings from the current study provide insight into molecular and biochemical mechanisms that contribute to the neuroprotective effects of Brn3b during ocular hypertension, which have implications for the development of strategies for neuroprotection in glaucoma.
Acknowledgments
The work was supported by a grant from the Department of Defense (W81XWH-10–2-0003) to Thomas Yorio (P.I.), and Raghu Krishnamoorthy (Co-I.) and partial support from a National Eye Institute grant (R01EY019952) to Raghu Krishnamoorthy.
Appendix 1.
To access the data, click or select the words “Appendix 1.” Brn3b expression in retinas of rats intravitreally injected with rAAV-hsyn-Brn3b. Brown Norway rats were intravitreally injected with either rAAV-hsyn-GFP or rAAV-hsyn-Brn3b and maintained for 2 weeks. Following sacrifice, frozen retinal sections were obtained and stained with an antibody against Brn3b (pseudo-green) and Neu N (pseudo-red). An increase in immunoreactive levels of Brn3b was observed in retinal sections obtained from AAV-hsyn-Brn3b injected rats, compared to those injected with AAV-hsyn-GFP (control vector). Co-staining of Brn3b with NeuN was observed in all retinal sections.
Appendix 2.
To access the data, click or select the words “Appendix 2.” Immunoreactivity and specificity of Bcl-xL antibody. The specificity and immunoreactivity of the Bcl-xL antibody was tested by a immunoblot analysis of optic nerve extracts from Brown Norway rats. Briefly, optic nerve extracts obtained from the left (L) and right (R) eyes of a Brown Norway rat were run on a SDS–PAGE gel, and transferred to a nitrocellulose membrane. Primary antibody incubation was performed with a rabbit anti-Bcl-xL antibody and secondary antibody incubation was done with a donkey anti-rabbit IgG Horse radish peroxidase (HRP) conjugate. The blots were developed with an enhanced chemiluminescence kit. A single doublet band (30 kDa) was observed indicating the specificity of the Bcl-xL antibody used in this study.
Appendix 3.
To access the data, click or select the words “Appendix 3.” rAAV-hsyn-Brn3b virus injection was performed in one eye (black bar, n=2) and compared with the contralateral eye (white bar, n=2). Visual acuity was measured using the Optomotor test as described by Stankowska et al., (2015). There was no change in visual acuity 4 weeks following administration of rAAV-hsyn-Brn3b virus, compared to the untreated companion eye.
References
- 1.Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007;18:110–4. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust N Z J Ophthalmol. 1995;23:85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. %U. [DOI] [PubMed] [Google Scholar]
- 3.Begg IS, Drance SM. Progress of the glaucomatous process related to recurrent ischaemic changes at the optic disc. Exp Eye Res. 1971;11:141. doi: 10.1016/s0014-4835(71)80081-7. [DOI] [PubMed] [Google Scholar]
- 4.Hayreh SS. Posterior ciliary arterial occlusive disorders. Trans Ophthalmol Soc U K. 1971;91:291–303. [PubMed] [Google Scholar]
- 5.Cioffi GA. Care guidelines and optic nerve assessment. J Glaucoma. 1996;5:A12. [PubMed] [Google Scholar]
- 6.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996;37:1618–24. [PubMed] [Google Scholar]
- 7.Levin LA. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43(Suppl 1):S98–101. doi: 10.1016/s0039-6257(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 8.Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008;173:339–52. doi: 10.1016/S0079-6123(08)01124-2. [DOI] [PubMed] [Google Scholar]
- 9.Kong GYX, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- 10.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–81. doi: 10.1016/j.preteyeres.2011.11.002. %U. [DOI] [PubMed] [Google Scholar]
- 11.Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–85. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang M, Zhou L, Nathans J. Similarities and differences among inner retinal neurons revealed by the expression of reporter transgenes controlled by Brn-3a, Brn-3b, and Brn-3c promotor sequences. Vis Neurosci. 1996;13:955–62. doi: 10.1017/s0952523800009184. [DOI] [PubMed] [Google Scholar]
- 13.Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210:469–80. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- 14.Wang SW, Mu X, Bowers WJ, Kim D-S, Plas DJ, Crair MC, Federoff HJ, Gan L, Klein WH. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development. 2002;129:467–77. doi: 10.1242/dev.129.2.467. [DOI] [PubMed] [Google Scholar]
- 15.Xiang M, Gan L, Li D, Zhou L, Chen ZY, Wagner D, O'Malley BW, Jr, Klein W, Nathans J. Role of the Brn-3 family of POU-domain genes in the development of the auditory/vestibular, somatosensory, and visual systems. Cold Spring Harb Symp Quant Biol. 1997;62:325–36. [PubMed] [Google Scholar]
- 16.Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125:3935–46. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- 17.Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron. 1993;11:689–701. doi: 10.1016/0896-6273(93)90079-7. [DOI] [PubMed] [Google Scholar]
- 18.Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci USA. 1996;93:3920–5. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erkman L, Yates PA, McLaughlin T, McEvilly RJ, Whisenhunt T, O'Connell SM, Krones AI, Kirby MA, Rapaport DH, Bermingham JR, O'Leary DD, Rosenfeld MG. A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron. 2000;28:779–92. doi: 10.1016/s0896-6273(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang SW, Gan L, Martin SE, Klein WH. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol Cell Neurosci. 2000;16:141–56. doi: 10.1006/mcne.2000.0860. [DOI] [PubMed] [Google Scholar]
- 21.Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O'Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–6. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- 22.Camp AS, Ruggeri M, Munguba GC, Tapia ML, John SWM, Bhattacharya SK, Lee RK. Structural correlation between the nerve fiber layer and retinal ganglion cell loss in mice with targeted disruption of the Brn3b gene. Invest Ophthalmol Vis Sci. 2011;52:5226–32. doi: 10.1167/iovs.10-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stankowska DL, Minton AZ, Rutledge MA, Mueller BH, Phatak NR, He S, Ma HY, Forster MJ, Yorio T, Krishnamoorthy RR. Neuroprotective Effects of Transcription Factor Brn3b in an Ocular Hypertension Rat Model of Glaucoma. Invest Ophthalmol Vis Sci. 2015;56:893–907. doi: 10.1167/iovs.14-15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickells RW, Semaan SJ, Schlamp CL. Involvement of the Bcl2 gene family in the signaling and control of retinal ganglion cell death. Prog Brain Res. 2008;173:423–35. doi: 10.1016/S0079-6123(08)01129-1. [DOI] [PubMed] [Google Scholar]
- 25.Bonfanti L, Strettoi E, Chierzi S, Cenni MC, Liu XH, Martinou JC, Maffei L, Rabacchi SA. Protection of retinal ganglion cells from natural and axotomy-induced cell death in neonatal transgenic mice overexpressing bcl-2. J Neurosci. 1996;16:4186–94. doi: 10.1523/JNEUROSCI.16-13-04186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cenni MC, Bonfanti L, Martinou JC, Ratto GM, Strettoi E, Maffei L. Long-term survival of retinal ganglion cells following optic nerve section in adult bcl-2 transgenic mice. Eur J Neurosci. 1996;8:1735–45. doi: 10.1111/j.1460-9568.1996.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–9. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 28.Chierzi S, Strettoi E, Cenni MC, Maffei L. Optic nerve crush: axonal responses in wild-type and bcl-2 transgenic mice. J Neurosci. 1999;19:8367–76. doi: 10.1523/JNEUROSCI.19-19-08367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J Neurosci. 1996;16:2064–73. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planchamp V, Bermel C, Tönges L, Ostendorf T, Kügler S, Reed JC, Kermer P, Bähr M, Lingor P. BAG1 promotes axonal outgrowth and regeneration in vivo via Raf-1 and reduction of ROCK activity. Brain. 2008;131:2606–19. doi: 10.1093/brain/awn196. [DOI] [PubMed] [Google Scholar]
- 31.Levin LA, Schlamp CL, Spieldoch RL, Geszvain KM, Nickells RW. Identification of the bcl-2 family of genes in the rat retina. Invest Ophthalmol Vis Sci. 1997;38:2545–53. [PubMed] [Google Scholar]
- 32.Chaudhary P, Ahmed F, Quebada P, Sharma SC. Caspase inhibitors block the retinal ganglion cell death following optic nerve transection. Brain Res Mol Brain Res. 1999;67:36–45. doi: 10.1016/s0169-328x(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 33.Malik JM, Shevtsova Z, Bahr M, Kugler S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol Ther. 2005;11:373–81. doi: 10.1016/j.ymthe.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Kretz A, Kugler S, Happold C, Bahr M, Isenmann S. Excess Bcl-XL increases the intrinsic growth potential of adult CNS neurons in vitro. Mol Cell Neurosci. 2004;26:63–74. doi: 10.1016/j.mcn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Liu XH, Collier RJ, Youle RJ. Inhibition of axotomy-induced neuronal apoptosis by extracellular delivery of a Bcl-XL fusion protein. J Biol Chem. 2001;276:46326–32. doi: 10.1074/jbc.M108930200. [DOI] [PubMed] [Google Scholar]
- 36.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh DY. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–10. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 37.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–82. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Shacka JJ, Roth KA. Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr Drug Targets CNS Neurol Disord. 2005;4:25–39. doi: 10.2174/1568007053005127. [DOI] [PubMed] [Google Scholar]
- 39.Leaver SG, Cui Q, Bernard O, Harvey AR. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur J Neurosci. 2006;24:3323–32. doi: 10.1111/j.1460-9568.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- 40.Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci. 2001;18:270–82. doi: 10.1006/mcne.2001.1018. [DOI] [PubMed] [Google Scholar]
- 41.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 42.Nakazawa T, Shimura M, Tomita H, Akiyama H, Yoshioka Y, Kudou H, Tamai M. Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr Eye Res. 2003;26:55–63. doi: 10.1076/ceyr.26.1.55.14254. [DOI] [PubMed] [Google Scholar]
- 43.Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci. 2002;43:3319–26. [PubMed] [Google Scholar]
- 44.Chavarria T, Valenciano AI, Mayordomo R, Egea J, Comella JX, Hallbook F, de Pablo F, de la Rosa EJ. Differential, age-dependent MEK-ERK and PI3K-Akt activation by insulin acting as a survival factor during embryonic retinal development. Dev Neurobiol. 2007;67:1777–88. doi: 10.1002/dneu.20554. [DOI] [PubMed] [Google Scholar]
- 45.Luo JM, Cen LP, Zhang XM, Chiang SW, Huang Y, Lin D, Fan YM, van Rooijen N, Lam DS, Pang CP, Cui Q. PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal ganglion cells via different mechanisms after optic nerve injury. Eur J Neurosci. 2007;26:828–42. doi: 10.1111/j.1460-9568.2007.05718.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, Cen LP, Luo JM, Wang N, Zhang MZ, van Rooijen N, Pang CP, Cui Q. Differential roles of phosphatidylinositol 3-kinase/akt pathway in retinal ganglion cell survival in rats with or without acute ocular hypertension. Neuroscience. 2008;153:214–25. doi: 10.1016/j.neuroscience.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Li SY, Yau SY, Chen BY, Tay DK, Lee VW, Pu ML, Chan HH, So KF. Enhanced survival of melanopsin-expressing retinal ganglion cells after injury is associated with the PI3 K/Akt pathway. Cell Mol Neurobiol. 2008;28:1095–107. doi: 10.1007/s10571-008-9286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai RK, Chang CH, Sheu MM, Huang ZL. Anti-apoptotic effects of human granulocyte colony-stimulating factor (G-CSF) on retinal ganglion cells after optic nerve crush are PI3K/AKT-dependent. Exp Eye Res. 2010;90:537–45. doi: 10.1016/j.exer.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Qi Y, Chen L, Zhang L, Liu WB, Chen XY, Yang XG. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Socodato R, Brito R, Portugal CC, de Oliveira NA, Calaza KC, Paes-de-Carvalho R. The nitric oxide-cGKII system relays death and survival signals during embryonic retinal development via AKT-induced CREB1 activation. Cell Death Differ. 2014;21:915–28. doi: 10.1038/cdd.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y, Pernet V, Hauswirth WW, Di Polo A. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Mol Ther. 2005;12:402–12. doi: 10.1016/j.ymthe.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 54.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 55.Minn AJ, Boise LH, Thompson CB. Bcl-x(S) anatagonizes the protective effects of Bcl-x(L). J Biol Chem. 1996;271:6306–12. doi: 10.1074/jbc.271.11.6306. [DOI] [PubMed] [Google Scholar]
- 56.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 57.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 58.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–67. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 59.Merry DE, Veis DJ, Hickey WF, Korsmeyer SJ. bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994;120:301–11. doi: 10.1242/dev.120.2.301. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Garcia M, Garcia I, Ding L, O'Shea S, Boise LH, Thompson CB, Nunez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci USA. 1995;92:4304–8. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–7. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 62.Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 63.Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139:809–15. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 65.Quigley HA, Addicks EM. Regional differences in the structure of the lamina cribrosa and their relation to glaucomatous optic nerve damage. Arch Ophthalmol. 1981;99:137–43. doi: 10.1001/archopht.1981.03930010139020. [DOI] [PubMed] [Google Scholar]
- 66.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–49. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 67.Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–26. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 68.Weinreb RN, Levin LA. Is neuroprotection a viable therapy for glaucoma? Arch Ophthalmol. 1999;117:1540–4. doi: 10.1001/archopht.117.11.1540. [DOI] [PubMed] [Google Scholar]
- 69.Turner EE, Jenne KJ, Rosenfeld MG. Brn-3.2: a Brn-3-related transcription factor with distinctive central nervous system expression and regulation by retinoic acid. Neuron. 1994;12:205–18. doi: 10.1016/0896-6273(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 70.Soto I, Oglesby E, Buckingham BP, Son JL, Roberson ED, Steele MR, Inman DM, Vetter ML, Horner PJ, Marsh-Armstrong N. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28:548–61. doi: 10.1523/JNEUROSCI.3714-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weishaupt J, Klöcker N, Bähr M. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. J Mol Neurosci. 2005;26:17–26. doi: 10.1385/JMN:26:1:017. [DOI] [PubMed] [Google Scholar]
- 72.Chierzi S, Cenni MC, Maffei L, Pizzorusso T, Porciatti V, Ratto GM, Strettoi E. Protection of retinal ganglion cells and preservation of function after optic nerve lesion in bcl-2 transgenic mice. Vision Res. 1998;38:1537–43. doi: 10.1016/s0042-6989(97)00332-5. [DOI] [PubMed] [Google Scholar]
- 73.Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007;6:2236–40. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- 74.Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huartes J. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–30. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 75.Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–6. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- 76.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–6. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]