Abstract
Oligometastases from solid tumors are currently recognized as a distinct clinical entity, corresponding to an intermediate state between local and widespread disease. It has been suggested that local ablative therapies (including surgery, radiofrequency ablation and radiation therapy) play an important role in this setting, in combination or not with systemic therapies, particularly in delaying disease progression and hopefully in increasing the median survival time. Stereotactic body radiation therapy (SBRT) rapidly emerged in recent years as one of the most effective and less toxic local treatment modalities for lung, liver, adrenal, brain and bone metastases. The aim of this review was to focus on its clinical role for oligometastatic disease in four major cancer subtypes: lung, breast, colorectal and prostate. On the basis of the available evidence, SBRT is able to provide high rates of local tumor control without significant toxicity. Its global impact on survival is uncertain; however, in specific subpopulations of oligometastatic patients there is a trend towards a significant improvement in progression-free and overall survival rates; these important data might be used as a platform for clinical decision-making and establish the basis for the current and future prospective trials investigating its role with or without systemic treatments.
Keywords: stereotactic body radiotherapy, stereotactic ablative radiotherapy, oligometastases, radiotherapy, radiosurgery
INTRODUCTION
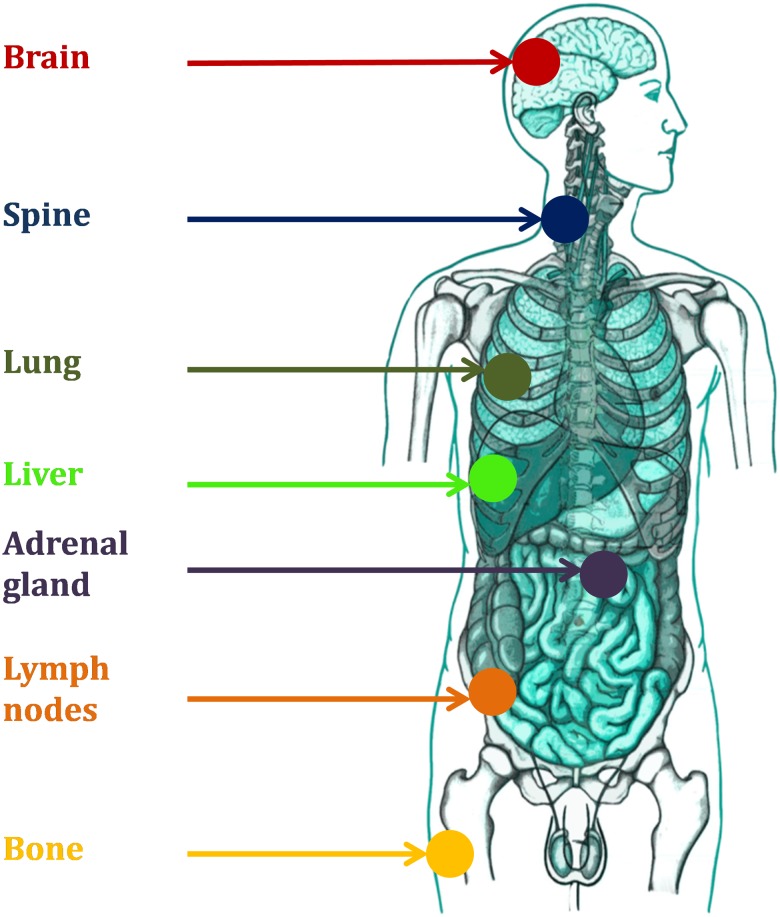
Though metastases are generally widely disseminated, a minimal metastatic state has been recently recognized, with a distinct natural history and an intermediate prognosis between that of localized and metastatic disease [1]. In 1995, S. Hellman and R. Weichselbaum first coined the term ‘oligometastases’: the definition of such a state came after years of research on the natural history of human solid tumors, especially breast cancer [2]. Within this framework, they also developed the idea that local treatments such as radiation therapy would probably have an increasing role, especially in those patients with slowly progressing cancer. Several years later, methods of morphological and metabolic imaging have improved dramatically, new systemic therapies are able to prolong survival and the oligometastatic state is increasingly encountered in most common tumors, including lung, breast, colorectal and prostate cancer. A combination of local therapies with systemic treatments at different time-points in the natural history of the disease is currently being offered to patients in a number of institutions worldwide, both in clinical studies and outside experimental trials [3–6]. Recent data suggest that oligometastases may be relatively common, at least among certain tumour types, for example breast cancer [7]. It is accepted that the oligometastatic state can occure in three categories of patients: patients who present with oligometastatic disease at diagnosis, those with oligoprogressive disease after cytoreductive therapy, and those with oligorecurrent disease after curative locoregional therapy [8]. The number of metastases generally accepted as truly ‘oligo’ are less than or equal to five in no more than three different organs, even if the definition can be discussed and adapted to specific clinical presentations. Radiotherapy may play a unique role in this scenario, as the technological advances in the field of radiation oncology allow for rapid non-invasive delivery of very high radiation doses to specific sites. Stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy (SABR) has been extensively investigated in recent years, showing high local control rates (LCRs) and promising progression-free survival estimates in selected metastatic patients, at the price of a very limited toxicity [5]. By the term SBRT, we currently refer to a ‘philosophy’ of cancer treatment with highly focused radiation doses in one or few sessions (less than or equal to eight), administered with ablative intent [9]. Figure 1 depicts the most common treated sites.
Fig. 1.
Metastatic sites treatable with stereotactic radiotherapy.
However, determining the clinical advantages or superior survival of this strategy when compared with systemic therapy or observation alone is challenging because of the predominantly retrospective nature of existing data; this has raised substantial concerns about positive selection biases in reported series (better performance status, longer disease-free interval, smaller metastatic burden, less aggressive course). In many cases it is unclear whether better than expected outcomes are seen due to the efficacy of the ablative treatments or to patient selection (more indolent tumor biology) [10]. Prognostic data are lacking for adequate patient selection; the ability to predict rapidly versus slowly progressive disease would be of major importance for the design of customized therapeutic strategies as well as for prospective clinical trials. In general, evidence supporting the concept of prolongation of progression-free survival (and sometimes overall survival [OS]) by the combined use of systemic and local therapies comes from observational cohort studies using surgery and/or radiotherapy taken together. Perhaps the best evidence for a local ablative approach for oligometastatic disease comes from surgery for lung or liver oligometastases from colorectal cancer (CRC) performed sequentially following systemic treatment [11]. However, in recent years only limited prospective data from pioneer researchers has become available.
The purpose of the present review is to focus on the role of SBRT as local ablative therapy for most common oligometastatic cancer subtypes, with the aim of discussing its results and possible applications as a disease-specific local therapy option either with or without systemic therapies.
SBRT FOR OLIGOMETASTATIC NON–SMALL CELL LUNG CANCER
Oligometastatic non–small cell lung cancer (NSCLC), presenting with one to five synchronous or metachronous metastatic lesions, has recently been considered a distinct disease state [12]. Locally ablative therapies are often used for such clinical presentations; however, the subset of patients who may benefit from these interventions at metastatic sites or at the primary lesion has not been conclusively identified. These issues are reflected by the heterogeneous survival outcomes reported in several retrospective and a limited number of prospective studies on oligometastatic lung cancer [4]. A single-arm Phase II study enrolled 39 patients presenting with Stage IV disease with less than four synchronous sites of distant metastasis [13]. The primary lesion was treated with radiotherapy alone or in combination with chemotherapy, and all sites of distant metastases were treated with surgery or SBRT. The median OS was 13.5 months, though six patients were progression-free after 2 years. The median progression-free survival (PFS) was 12.1 months. An individual patients' data meta-analysis of outcome of SBRT in oligometastatic NSCLC with one to five synchronous or metachronous metastases treated with surgical metastectomy, SBRT/radiosurgery, or radical external-beam radiotherapy (and curative treatment of the primary lung cancer) showed a median survival time of 19 months among patients with controlled primary tumors. Aggressive treatment to the primary tumor and metastases improved OS, supporting the hypothesis that oligometastatic NSCLC represents a clinically distinct and potentially curable disease. Collen et al. also reported on a Phase II prospective study of SBRT to primary tumor and metastatic locations in oligometastatic NSCLC [14]. SBRT could be delivered after induction chemotherapy, but after radiation no further treatment was given until progression. With 26 patients enrolled and 16.4 months of median follow-up time, a total of 87 sites were irradiated, including 48 metastases. With 30% showing complete metabolic response and 30% showing partial response rate, the median OS was 23 months, with 67% of patients alive at 1 year. The median progression-free survival (PFS) was 11.2 months.
Recently, an individual patient data meta-analysis on oligometastatic lung cancer patients showed that factors predictive of OS were: synchronous versus metachronous metastases (P < 0.001), N-stage (P = 0.002), and adenocarcinoma histology (P = 0.036); the model remained predictive in the validation set (c-statistic = 0.682). After recursive partitioning analysis (RPA), three risk groups were identified: low-risk, metachronous metastases (5-year OS, 47.8%); intermediate-risk, synchronous metastases and N0 disease (5-year OS, 36.2%); and high-risk, synchronous metastases and N1/N2 disease (5-year OS, 13.8%) [15].
The majority of published studies on oligometastatic NSCLC actually describe the outcomes for patients with resected solitary brain metastases. As an alternative to surgery, stereotactic brain radiosurgery (SRS) has the advantage of being able to treat unresectable metastases, easily treating multiple tumors in different regions of the CNS in a single course, and imparting acceptable rates of local control for small- to medium-sized tumors using a single modality [16]. Flannery et al. described the outcomes for a cohort of 42 patients treated for a solitary, synchronous brain metastasis from NSCLC [17]. The patients had Stage I–III thoracic disease, and slightly more than half (62%) were treated definitively in the chest area with chemoradiotherapy with or without surgery. For 26 patients whose thoracic disease was treated with radical intent, the median OS was ∼26 months, twice that of patients whose thoracic disease was treated with chemotherapy alone. All patients received brain SRS. In all but five cases, the patients died due to progression of the disease outside the CNS, suggesting that an aggressive local approach to treating brain metastases in patients who can tolerate definitive treatment for their primary disease may be appropriate. The median OS for the whole population was 18 months. It should also be noted that, in the previously cited prospective trial by De Ruysscher et al. [13], most of the enrolled patients were oligometastatic for a single brain lesion, with encouraging results in terms of median OS of the whole cohort and few long-term survivors.
SBRT was mostly used as an effective means of treating pulmonary oligometastases [4]. In the case of NSCLC, it is often not possible to determine whether one or both lung lesions represent primary lesions. If one or more lesions are presumed to be metastatic, according to the AJCC 2009, lesions in the ipsilateral or contralateral lung signify T4 or M1a classifications, respectively, in both cases indicating a better prognosis than extrapulmonary metastatic disease [18]. De Rose et al. recently reported on a series of 60 patients affected with lung oligometastases from NSCLC with controlled primary tumors: 90 lung lesions were treated, and with a median follow-up time from diagnosis of 28 months the local control at 2 years was 88.9%, with OS rates at 1 and 2 years of 94.5% and 74.6%, respectively. The median PFS was 32.2 months, and the median OS was 32.1 months [19].
Moreover, small retrospective series have described the outcomes following resection of solitary adrenal metastases in oligometastatic NSCLC. Interestingly, in a surgical series, a comparison of median survival favored metachronous over synchronous metastases (12 months versus 31 months); however, 5-year survival rates were comparable, at ∼25% [20]. A recent report described the use of SBRT for treating 13 patients with a solitary adrenal metastasis from NSCLC using doses ranging from 20 to 40 Gy delivered in five fractions. The median PFS was 12 months, the median OS was 23 months, and the crude rate of local control was 77% [21]. Two recent reports, unrestricted to NSCLC adrenal metastases but with the majority of patients included being affected by oligometastatic lung cancer, LCRss were promising, but PFS rates were disappointing [22, 23].
Patients with metastatic lung cancer driven by epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) gene rearrangements, treated with the tyrosine-kinase inhibitors (TKIs), represent a distinct group. These patients have superior survival rates compared with patients not carrying these mutations and treated with standard cytotoxic therapies [24–27]. Weickhardt et al. reported a single institution retrospective study of 25 patients who developed oligoprogressive disease in the CNS alone or with four or fewer extracranial metastases when on treatment with either erlotinib or crizotinib monotherapy [28]. Patients were treated with SBRT, SRS, whole brain radiotherapy, or surgery and maintained on the same drug. This strategy was associated with a median of 6 additional months before a second progression. In particular, with a median follow-up of 20 months, the median PFS (from local treatment to second progression) was 6.2 months. In a similar study, 18 patients with EGFR-mutant, non-CNS, oligoprogressive disease received ablative treatment while continuing TKI monotherapy. The median time to progression was 10 months and the median OS following first local treatment was 41 months [29]. Iyengar et al. recently reported on a Phase II trial on SBRT and erlotinib in patients previously treated with chemotherapy [30]. The patients included had less than six extracranial metastatic sites, and those progressing after SBRT were allowed to remain in the trial if progression occurred outside the treatment field. A total of 24 patients were accrued, and 52 lesions treated, most commonly in the lung or mediastinum. The trial met its primary endpoint, with a 69% rate of PFS at 6 months, a median PFS of 14.7 months and a median OS of 20.4 months. The pattern of progression was mainly at new sites (allowing for other possible local ablative treatments). At the same time, Grade 3 toxicity was not negligible (in particular, pulmonary toxicity), a possible consequence of the combination. Given the paucity of data, despite these encouraging results, SBRT and continuation of the same drug in oligoprogressive patients under treatment with TKIs is not presently considered as a standard of care. Variations to this approach are expected when new results from prospective trials will be available.
Table 1 summarizes the characteristics and results of the studies using SBRT as metastasis-directed local therapy for oligometastatic NSCLC.
Table 1.
Studies investigating the use of SBRT in oligometastatic non–small cell lung cancer
| Study | Patients | Eligibility criteria | Study design | Site of metastases | Therapy | Median follow-up (months) | Median PFS (months) | Median OS (months) | Other therapy (percentage) |
|---|---|---|---|---|---|---|---|---|---|
| De Ruysscher et al. [13] | 39 | Stage IV with <5 metastases | single-arm Phase II | brain, bone, adrenal gland | SRS/SBRT/surgery | 27.7 | 12.1 | 13.5 | chemoradiotherapy for primary tumor (92%) |
| Collen et al. [15] | 26 | Stage IV with <5 metastases | single-arm Phase II | lung, bone, adrenal gland, brain, liver, lymph nodes | SBRT | 16.4 | 11.2 | 23 | induction chemotherapy (65%) |
| Flannery et al. [17] | 42 | synchronous solitary brain metastases | retrospective observational | solitary brain | SRS | 64.5 | NR | 18 | local thoracic therapy (62%) |
| De Rose et al. [19] | 60 | synchronous or metachronous lung metastases with controlled primary tumor | retrospective observational | lung | SBRT | 28 | 32.2 | 32.1 | chemotherapy (49%) |
| Holy et al. [21] | 13 | synchronous or metachronous adrenal metastases | retrospective observational | adrenal glands | SBRT | 12 | 12 | 23 | NR |
| Weickhardt et al. [28]a | 25 | oligoprogression under TKIs | subgroup analysis of prospective trial | brain, bone, lung, adrenal gland, liver | SBRT and surgery | 20 | 6.2 (from treatment to second progression) | NR | NA |
| Yu et al. [29]a | 18 | extracranial oligoprogression under TKIs | subgroup analysis of prospective trial | lung, lymph nodes, adrenal glands | SBRT, surgery, RFA and TKIs | NR | 10 | 41 | NA |
| Iyengar et al. [30]a | 24 | oligoprogression after first line chemotherapy (<6 metastases) | single-arm Phase II | lung, lymph nodes, adrenal glands, bone, liver | SBRT + erlotinib | 11.6 | 14.7 | 20.4 | previous chemotherapy (100%) |
aCombination of local therapies and TKIs. RFA = radiofrequency ablation, SRS = stereotactic radiosurgery, SBRT = stereotactic body radiation therapy, TKIs = tyrosine-kinase inhibitors, PFS = progression-free survival, OS = overall survival, NA = not applicable, NR = not reported.
Although the reported outcomes of these single-arm studies appear promising, some caution is warranted. Many questions remain, such as the influence of tumor biology, the use of biomarkers for patient selection, and the effect of novel systemic therapies, including immunotherapy [10, 31]. As for other cancer subtypes, emerging therapies could lead to a scenario where the better control of occult disease will possibly amplify the effect of local ablative therapies on visible deposits.
SBRT FOR OLIGOMETASTATIC BREAST CANCER
Breast cancer is probably the first model used to illustrate the natural history of solid tumors, and the mechanisms underlying the metastatic spread [2]. Therefore, it is also one of the cancer subtypes where the hypothesis of oligometastases was first formulated, and it served as a model to illustrate the rationale for the use of local therapies to a few metastatic sites, in combination with systemic agents [7]. Over the past years, while systemic therapies have improved the control of subclinical breast cancer metastases and prolonged progression-free intervals [32], most long-term survivors have received aggressive local therapies following systemic therapy [33]. This is likely due to the fact that patients with oligometastatic disease usually progress in sites of known metastases, and not in new metastatic locations [7]. Very few studies have been published on the use of SBRT for oligometastatic breast cancer patients. The University of Rochester researchers published seminal and most relevant data regarding the use of SBRT in oligometastatic breast cancer [34–36]. In particular, a pooled analysis of women treated with two subsequent protocols for oligometastatic cancer [35] showed promising results: the 4-year OS rate was 59% and the 4-year PFS rate was 38%, with almost 90% of metastases locally controlled by radiation therapy. The median PFS was 23 months, while median OS was not reached (Table 2). A plateau was evident in the Kaplan–Meier projection after 40 months. Paradoxically, the increased use of SBRT for treating oligometastatic cancers (and particularly breast cancer) made it difficult to design and conduct prospective clinical trials, because in most Centers SBRT is offered as a ‘routine’ option; this was evident from a recent international survey, in which it was found that 83% of radiation oncologists were offering SBRT to oligometastatic cancer patients after 2005 and more than half of the remaining were planning to start in the next 3 years [37]. At the same time, especially for breast cancer, where the apparent benefit of this strategy is greater, a lack of evidence is perceived and trials are ongoing: NRG Oncology is conducting a Phase I dose de-escalation study (NRG BR001) in which breast, lung and prostate cancer patients with two metastases in close proximity or those with three to four metastases will receive radiation to all known sites of disease, with dose selected on the basis of tumor location and the potential for normal tissue toxicity. An alternative approach, comparing SBRT at fixed dose levels plus standard systemic therapy versus standard systemic therapy plus/minus palliative radiotherapy is used in the COMET trial, which includes different cancer subtypes (SABR COMET, NCT01446744). NRG Oncology is also conducting a randomized Phase II trial (NRG BR002) on oligometastatic breast cancer patients to determine whether the addiction of ablative therapy to all known metastases is superior to standard therapy alone in terms of PFS (with the aim of planning a Phase III study with OS as the primary endpoint if the results are positive). As for other oligometastatic tumors, the identification of favorable/unfavorable prognostic groups remains challenging. From surgical series, known favorable prognostic factors are estrogen-receptor positivity, response to systemic therapies, fewer and smaller metastases and longer disease-free interval [38]. Patients receiving ablative radiotherapy for bone-only metastases, single metastases and stable or responding metastases have shown improved outcomes [35].
Table 2.
Studies investigating the use of SBRT in oligometastatic breast and colorectal cancer
| Study | Patients | Eligibility criteria | Study design | Site of metastases | Therapy | Median follow-up (months) | Median PFS (months) | Median OS (months) | Other therapy (percentage) |
|---|---|---|---|---|---|---|---|---|---|
| Breast cancer | |||||||||
| Milano et al. [35] | 40 | <5 extracranial metastases | subgroup analysis of a prospective Phase II trial | liver, lung, lymph nodes, bone | SBRT | NR | 23 | not reached | adjuvant chemotherapy/hormonal therapy (80%) |
| Colorectal cancer | |||||||||
| Kim et al. [55] | 13 | isolated lung metastases | retrospective observational | lung | SBRT | 28 | NR (PFS at 3 years: 11.5%) | NR (OS at 3 years: 64.7%) | chemotherapy (100%) |
| Takeda et al. [56] | 15 | lung oligometastases | retrospective observational with LC as primary endpoint | lung | SBRT | 29 | NA (LC at 2 years: 72%)a | NA | NA |
| Filippi et al. [57] | 40 | metachronous, at first lung progression | retrospective observational | lung | SBRT | 20 | 8 | 46 | pre-post SBRT chemotherapy (20%) |
| Qiu et al. [58] | 65 | synchronous/metachronous lung progression | retrospective observational | lung | SBRT | 6.4 | 5.7 | 20.3 | previous chemotherapy for metastatic disease (69%) |
| Comito et al. [59] | 82 | 1–3 lung or liver metastases | retrospective observational | lung, liver | SBRT | 24 | 14 | 32 | pre-post SBRT chemotherapy (95%) |
aLocal control rates were compared with metastases from other origins and primary lung cancer, with statistically significant inferiority (P < 0.05). SRS = stereotactic radiosurgery, SBRT = stereotactic body radiation therapy, PFS = progression-free survival, OS = overall survival, LC = local control, NA = not applicable, NR = not reported.
SBRT FOR OLIGOMETASTATIC COLORECTAL CANCER
CRC is one of the tumors that most often present oligorecurrence, most commonly in the liver and lung. Surgical removal of liver and lung lesions is apparently associated with better survival, despite the absence of controlled randomized data. Liver resection may achieve 5-year OS rates in the range of 37–58% [39–42]; also, pulmonary resection is able to achieve 5-year survival rates in the range of 38–50% [43, 44]. Approximately 70–90% of CRC metastatic patients, however, are unresectable [45]. Radiofrequency ablation (RFA) directed to liver metastases has been used as an alternative to surgical resection, but the use of RFA has limitations related to the size and location of the target lesions [46–48]. Schlijper et al. reviewed the clinical reports published as at 2011 on the application of surgery, RFA or SBRT in patients with pulmonary oligometastases from CRC, selected for a minimum follow-up of 24 months and a minimum inclusion of 50 patients. Twenty-three surgical series fulfilled the selection criteria, four of which were prospective. Survival rates for surgery were 64–88% at 2 years and 29–71.2% at 5 years. Limited data were available for RFA, with survival rates ranging from 64 to 73% at 2 years and 34.9 to 45% at 5 years [49]. No studies on SBRT fulfilled the selection criteria.
However, SBRT was investigated in the treatment of liver and lung metastases with promising results, using either a single dose or a small number of fractions, in studies unrestricted to CRC patients [50–52]. In particular, for liver metastases, results of prospective trials showed very favorable results in terms of local control and PFS [53, 54].
Few studies are focused on the use of SBRT for oligometastatic CRC patients. Kim et al. first reported on 13 cases of isolated pulmonary metastasis from CRC treated using SBRT. All patients underwent chemotherapy for salvage treatment. The median follow-up was 28 months; the 3-year OS and PFS rates were 64.7% and 11.5%, respectively [55].
Takeda et al. analyzed 15 patients with lung oligometastases from CRC, 19 from other origins, and 183 primary lung cancers. The primary endpoint of that study was to compare local control. The median follow-up for the CRC cohort was 29 months, for lung metastases from other origins 15 months and for primary NSCLC 24 months. The 1-year and 2-year LCRs for CRC lung metastases and from other origins were 80% and 72%, and 94% and 94%, respectively. The LCR for CRC metastases was significantly worse than from the other origins and primary lung cancers with pathological and clinical diagnosis (P < 0.05, P < 0.0001 and P < 0.005, respectively) [56].
In a recent retrospective cohort study including 40 patients treated with SBRT at the time of first lung progression, with a median follow-up of 20 months, the median PFS and OS were 8 and 46 months, respectively [57]. These results are quite promising in view of the negative selection for factors such as age or comorbidities typical of the non-surgical population, because the reported 2-year OS after surgery is in the range of 64–88%. Qiu et al. also reported on a mono-institutional cohort of 65 patients treated for CRC lung metastases: the median OS was 20.3 months and the median PFS was 5.7 months. Nearly all (98%) patients developed distant progression. Extra lung and liver involvement at the time of initial metastases (hazard ratio 2.10) and extra lung involvement at the time of SBRT (hazard ratio 2.67) were the only independent predictors of OS [58]. A prospective observational series by Comito et al. included 82 patients with one to three inoperable metastases confined to one organ (liver or lung), treated with SBRT for a total of 112 lesions. The median follow-up was 24 months, and the median OS was 32 months; the OS rate at 1, 2 and 3 years was 85%, 65% and 43%, respectively. Univariate analysis showed a correlation only between OS and cumulative tumor volume >3 cm (P < 0.02). The median PFS was 14 months, with a PFS rate of 56% and 40% at 1 and 2 years, respectively [59]. Table 2 summarizes the results of the studies investigating the use of SBRT for CRC oligometastases.
As for other oligometastatic scenarios, there is a lack of prospective controlled data comparing surgery or stereotactic radiotherapy versus observation or systemic therapy alone. The ongoing PulMICC trial (UK, clinicaltrials.gov identifier NCT01106261) is an example of a feasibility study with the aim to determine whether it will be possible to recruit sufficient patients for a larger Phase III randomized trial powered to detect statistical differences in OS between metastasectomy and active monitoring. This trial is completing patient recruitment, and will hopefully give us important information not only on clinical endpoints (OS is the secondary endpoint), but also on which patients are routinely offered surgery. The ORCHESTRA trial (A Randomized Multicenter Clinical Trial for Patients with Multi-Organ Colorectal Cancer Metastases Comparing the Combination of Chemotherapy and Maximal Tumor Debulking versus Chemotherapy Alone, NCT 01792934) will also hopefully provide useful clinical evidence.
SBRT FOR OLIGOMETASTATIC PROSTATE CANCER
Available data on metastasis-directed therapies for oligometastatic prostate cancer consists of small and heterogeneous studies, but a recent systematic review on the role of local therapies in patients with regional and/or distant recurrences after curative treatment helped in clarifying the possible role of local therapies (including SBRT) in this patient subset [60]. As pointed out by the authors, interest in ablative metastasis-directed therapies for recurrent/metastatic prostate cancer emerged after the introduction of novel imaging modalities (for example choline Positron Emission Tomography, PET) that increased the detection of oligometastic patients, potentially justifying a local approach either with or without systemic treatments. Fifteen single-arm case series were identified with a total of 450 patients. In most of the patients (98%), oligometastatic recurrence was diagnosed through choline-PET co-registered with computed tomography scans. Nodal, bone and visceral metastases were treated in 78%, 21% and 1% of the patients, respectively. Patients were treated with either RT (66%) or lymph node dissection (34%). Adjuvant androgen deprivation was used for 61% of patients. In the case of nodal metastases, prophylactic nodal irradiation was administered in 49% of patients. Overall, 51% of patients were progression free at 1–3 years after local therapies, with most of them receiving adjuvant treatment. For SBRT, Grade 2 toxicity was observed in 8.5% of patients, and there was one case of Grade 3 toxicity. For lymph node dissections, 11% and 12% of Grade 2 and Grade 3 complications, respectively, were reported.
Of the 15 studies, six used SBRT as a metastasis-directed therapy [61–66]. Casamassima et al. [61] reported on a series of 25 patients with nodal metastases, with a median follow-up time of 24 months that PFS at 3 years was 17%. Seven patients also received prophylactic nodal irradiation. Muacevic et al. [62] described the results of SBRT to bone metastases only in 40 patients, with a median follow-up of 14 months. The median PFS was not reached, and 75% of the patients were alive at 17.5 months. In the series by Ahmed et al., 15 patients received SBRT for bone metastases, 1 for nodal metastasis and 1 for liver metastasic, with a median PFS of 12 months. Most of the patients also received adjuvant androgen deprivation therapy [63]. Jereczek-Fossa et al. reported on a series of 34 oligorecurrent patients (including recurrent primary), with a median follow-up time of 16.9 months; the median PFS was not reported (42.6% at 30 months) [64]. The two series by Schick et al. [65] and Decaestecker et al. [66] included patients with nodal, bone and visceral metastases. The first study, retrospective on 50 patients, reported a median time to distant recurrence of 15.6 months; the latter prospective, again including 50 patients, reported this time as 57.6 months. Median PFS was not reached (58.6% at 3 years) in the first study, and it was 19 months in the second. The median follow-up times were very similar (31 and 25 months, respectively). A recent multi-institutional analysis on oligometastatic treatment-naïve patients was conducted with the aim of estimating the potential benefit of SBRT in terms of distant PFS, reducing the heterogeneity by pooling individual patient data from various studies. In total, 163 metastases were treated in 119 patients. The median distant PFS was 21 months, and no Grade 3 or more toxicity occurred. Given this result, the authors concluded that SBRT is safe and associated with a prolonged distant PFS [67].
Table 3 summarizes the results of the reported studies on the use of SBRT for oligometastatic/oligorecurrent prostate cancer.
Table 3.
Studies investigating the use of SBRT in oligometastatic prostate cancer
| Study | Patients | Eligibility criteria | Study design | Site of metastases | Therapy | Median follow-up (months) | Median PFS (months) | Median OS (months) | Other therapy (percentage) |
|---|---|---|---|---|---|---|---|---|---|
| Casamassima et al. [61] | 25 | nodal recurrence only | retrospective observational | lymph nodes | SBRT | 29 | PFS rate at 3 years 17% | OS rate at 3 years 92% | NR |
| Muacevic et al. [62] | 40 | bone metastases | prospective | bone | SBRT | 14 | NR | not reached (75% alive at 17.5 months) | chemotherapy (20%), previous RT (20%), ADT (47.5%) |
| Ahmed et al. [63] | 17 | <5 metastases | retrospective observational | bone, lymph nodes, liver | SBRT | 6 | 12 | NR (cancer-specific survival 100% at 12 months) | |
| Jereczek-Fossa et al. [64] | 34 | recurrent primary, node or metastatic | retrospective observational | nodal | SBRT | 16.9 | NR (30 months PFS 42.6%) | NR | ADT (55%) |
| Schick et al. [65] | 50 | synchronous or metachronous oligometastases | retrospective observational | lymph nodes, bone, visceral | SBRT | 31 | 3-year PFS (clinical) 58.6% | NR (OS at 3 years 92%) | ADT (100%) |
| Decaestecker et al. [66] | 50 | oligorecurrent metastatic, <3 lesions | prospective observational | lymph nodes, bone, visceral | SBRT | 24 | 19 | NR (androgen deprivation–free survival 25 months) | ADT (70%) |
| Ost et al. [67] | 119 | <3 metastases, treatment-naive, recurrent | pooled analysis of individual patient data | lymph nodes, bone, visceral | SBRT | 36 | 21 | NR (OS at 5 years 88%) | ADT (50%) |
SRS = stereotactic radiosurgery, SBRT = stereotactic body radiation therapy, PFS = progression-free survival, OS = overall survival, ADT = androgen deprivation therapy, NA = not applicable, NR = not reported.
The interpretation of these results is complex due to the extreme heterogeneity of the reported series, and no conclusions can be drawn on the role of SBRT. What emerged is that SBRT is a safe, low-toxic treatment, in comparison with surgery, and its potential in delaying progression is high. Again, as for other cancer subtypes, prospective studies are needed to validate this hypothesis.
FUTURE PERSPECTIVES
Identifying patients who are most likely to benefit from metastasis-directed radiation therapy is probably the most significant challenge, as well as obtaining high quality prospective data on treatment efficacy [7]. As the LCR achievable with either surgery or stereotactic radiotherapy has increased over time, with acceptable toxicity, it is important to select those patients who will have the maximal benefit. Currently, oligometastatic patients are defined eligible for local therapies through the use of clinical variables extracted from retrospective series, such as the number of metastases, the disease-free interval, the tumor type, the control of the primary tumor, the possibility of using efficient systemic therapies (e.g. for oncogene-driven lung cancer) and the presence of specific biological features (for example estrogen-receptor positivity for breast cancer). Most of these criteria are the same as those used to select patients for lung or liver surgery. Intracranial involvement, traditionally considered a worse prognostic factor, may not be interpreted the same in the era of radiosurgery to multiple intracranial sites [68]. However, these relatively simple clinical criteria appear insufficient for proper patient selection, being surrogates for the tumor's biology. Research efforts have been made to correlate biological markers with clinical outcomes. A study on resected pulmonary metastases and of the combination of primary and metastatic tumors has identified a microRNA signature that may select patients whose disease is unlikely to progress rapidly [69]. The same group showed that, when restricted to lung metastases, microRNA signatures are able not only to classify patients as oligo versus polymetastatic but also to differentiate those patients with a low recurrence probability following surgical resection [70]. These studies need a prospective validation, but represent a significant step forward in the identification of biomarkers in this setting. Other researchers focused their attention on metabolic response after ablative therapy: one study showed that only 10% of patients with a partial response on first post-radiotherapy PET, and 29% of those in complete response, progressed during follow-up [71]. In metastatic breast cancer, circulating tumor cells (CTCs) showed the potential to be a powerful predictive toll for response to systemic therapy [72]. Theoretically, CTCs might also be used as a powerful biomarker for both evaluating the presence of a true oligometastatic state and predicting outcome after ablative therapies. The eradication of previously detectable CTCs could indicate that the primary source of CTCs was the treated lesions and not occult sites reseeding [7].
The need for prospective controlled studies has been already discussed for the various examined histologies; currently, a significant number of clinical trials in different diseases are exploring the effect of SBRT in terms of better PFS, prolongation of the time interval ‘free’ from systemic treatments, or even OS. All these efforts will be crucial for elucidating the exact role of SBRT in oligometastatic disease, and their result are awaited with great anticipation.
CONCLUSIONS
SBRT appears a safe and efficient way to treat oligometastases from different primary tumors, with very high LCRs and low toxicity. Due to the predominant nature of existing data, mainly retrospective, and the intrinsic heterogeneity and complexity of this population, there are still doubts about the wide application of SBRT in clinical practice. However, as for surgery, the encouraging results obtained so far have increased the use of SBRT worldwide. In specific subpopulations, we showed that there is a trend towards a significant improvement in PFS and OS rates. Ongoing and future trials are warranted to better establish its role, and important translational studies are ongoing and we hope will provide us with very helpful information about patient selection in the future.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the Japan Radiation Research Society and the Japanese Society for Radiation Oncology.
REFERENCES
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 2.Hellman S. Natural history of small breast cancers. J Clin Oncol 1994;12:2229–34. [DOI] [PubMed] [Google Scholar]
- 3.Lo SS, Fakiris AJ, Teh BS et al. Stereotactic body radiation therapy for oligometastase. Expert Rev Anticancer Ther 2009;9:621–35. [DOI] [PubMed] [Google Scholar]
- 4.Schultz DB, Filippi AR, Thariat J et al. Stereotactic ablative radiotherapy for pulmonary oligometastases and oligometastatic lung cancer. J Thorac Oncol 2014;9:1426–33. [DOI] [PubMed] [Google Scholar]
- 5.Tree AC, Khoo VS, Eeles RA et al. Stereotactic body radiation therapy for oligometastases. Lancet Oncol 2013;14:e28–37. [DOI] [PubMed] [Google Scholar]
- 6.Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol 2013;31:1–8. [DOI] [PubMed] [Google Scholar]
- 7.Salama JK, Chmura AJ. The role of surgery and ablative radiotherapy in oligometastatic breast cancer. Semin Oncol 2014;41:790–7. [DOI] [PubMed] [Google Scholar]
- 8.Niibe I, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol 2010;40:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senan S, Guckenberger M, Ricardi U. Stage I NSCLC and oligometastatic disease. In Ball D, Scagliotti GV (eds). The IASLC Multidisciplinary Approach to Thoracic Oncology. Aurora, CO: International Association for the Study of Lung Cancer, 2014. [Google Scholar]
- 10.Palma DA, Salama JK, Lo SS et al. The oligometastatic state: separate truth from wishful thinking. Nat Rev Clin Oncol 2014;11:549–57. [DOI] [PubMed] [Google Scholar]
- 11.Ksienski D, Woods R, Speers C et al. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (MCRC). Ann Surg Oncol 2010;17:3085–93. [DOI] [PubMed] [Google Scholar]
- 12.Villaruz LC, Kubicek GJ, Socinski MA. Management of non-small cell lung cancer with oligometastasis. Curr Oncol Rep 2012;14:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Ruysscher D, Wanders R, van Baardwijk A et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547–55. [DOI] [PubMed] [Google Scholar]
- 14.Collen C, Christian N, Schallier D et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic non-small cell lung cancer patients. Ann Oncol 2014;25:1954–9. [DOI] [PubMed] [Google Scholar]
- 15.Ashworth AB, Senan S, Palma DA et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346–55. [DOI] [PubMed] [Google Scholar]
- 16.Chang EL, Wefel JS, Hess KR et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation. Lancet Oncol 2009;10:1037–44. [DOI] [PubMed] [Google Scholar]
- 17.Flannery TW, Suntharalingam M, Regine WF et al. Long-term survival in patients with synchronous, solitary brain metastases from non-small cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys 2008;72:19–23. [DOI] [PubMed] [Google Scholar]
- 18.American Joint Committee on Cancer. AJCC Cancer Staging Manual, 7th edn Chicago: Springer, 2009. [Google Scholar]
- 19.De Rose F, Cozzi L, Navarria P et al. Clinical outcome of stereotactic ablative body radiotherapy for lung metastatic lesions in non-small cell lung cancer oligometastatic patients. Clin Oncol 2016;28:13–20. [DOI] [PubMed] [Google Scholar]
- 20.Tanvetyanon T, Robinson LA, Schell MJ et al. Ouctomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small cell lung cancer: a systematic review and pooled analysis. J Clin Oncol 2008;26:1142–7. [DOI] [PubMed] [Google Scholar]
- 21.Holy R., Piroth M, Pinkawa M et al. Stereotactic body radiation therapy for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011;187:245–51. [DOI] [PubMed] [Google Scholar]
- 22.Casamassima F, Livi L, Masciullo S et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys 2012;82:919–23. [DOI] [PubMed] [Google Scholar]
- 23.Scorsetti M, Alongi F, Filippi AR et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: a retrospective analysis of 34 patients. Acta Oncol 2012;51:618–23. [DOI] [PubMed] [Google Scholar]
- 24.Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin-paclitaxelin pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- 25.Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- 26.Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 27.Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. [DOI] [PubMed] [Google Scholar]
- 28.Weickhardt AJ, Scheier B, Burke JM et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu HA, Sima CS, Huang J et al. Local therapy with continued EGFR-tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyengar P, Kavanagh BD, Wardak Z et al. Phase II trial of stereotactic radiotherapy combined with erlotinib for patients with limited but progressive disease metastatic non-small cell lung cancer. J Clin Oncol 2014;32:3824–30. [DOI] [PubMed] [Google Scholar]
- 31.Palma DA, Videtic GM. Oligometastatic non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2015;93:223–6. [DOI] [PubMed] [Google Scholar]
- 32.Blackwell KI, Burnstein HJ, Storniolo AM et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 study. J Clin Oncol 2012;30:2585–92. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg PA, Hortobagyi GN, Simth TL et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol 1996;14:2197–205. [DOI] [PubMed] [Google Scholar]
- 34.Milano MT, Katz AW, Muhs AG et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2007;112:650–8. [DOI] [PubMed] [Google Scholar]
- 35.Milano MT, Zhang H, Metcalfe SK et al. Oligometastatic breast cancer treated with curative intent stereotactic body radiation therapy. Breast Cancer Res Treat 2009;115:601–8. [DOI] [PubMed] [Google Scholar]
- 36.Milano MT, Katz AW, Zhang H et al. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2011;83:878–86. [DOI] [PubMed] [Google Scholar]
- 37.Lewis S, Porceddu S, Nakamura N et al. (2 February 2015) Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: an international survey of >1000 radiation oncologists. Am J Clin Oncol (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 38.Abbott DE, Brouquet A, Mittendorf EA et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery 2012;151:710–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon SS, Tanabe KK. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist 1999;4:197–208. [PubMed] [Google Scholar]
- 40.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241–6. [DOI] [PubMed] [Google Scholar]
- 41.Fong Y, Fortner J, Sun RL et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elias D, Liberale G, Vernerey D et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 2005;12:900–9. [DOI] [PubMed] [Google Scholar]
- 43.Inoue M, Ohta M, Iuchi K et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238–44. [DOI] [PubMed] [Google Scholar]
- 44.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007;84:324–38. [DOI] [PubMed] [Google Scholar]
- 45.Kanemitsu Y, Kato T, Hirai T et al. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg 2004;91:112–20. [DOI] [PubMed] [Google Scholar]
- 46.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206–13. [DOI] [PubMed] [Google Scholar]
- 47.Siperstein A, Berber E, Ballem N et al. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559–67. [DOI] [PubMed] [Google Scholar]
- 48.Chua TC, Thornbury K, Saxena A et al. Radiofrequency ablation as an adjunct to systemic chemotherapy for colorectal pulmonary metastases. Cancer 2010;116:2106–14. [DOI] [PubMed] [Google Scholar]
- 49.Schlijper R, Grutters J, Houben R et al. What to choose as radical local treatment for lung metastases from colo-rectal cancer: surgery or radiofrequency ablation? Canc Treat Rev 2014;40:60–7. [DOI] [PubMed] [Google Scholar]
- 50.Alongi F, Arcangeli S, Filippi AR et al. Review and uses of stereotactic body radiation therapy for oligometastases. Oncologist 2012;17:1100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rusthoven KE, Kavanagh BD, Cardenes H et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572–8. [DOI] [PubMed] [Google Scholar]
- 52.Filippi AR, Badellino S, Guarneri A et al. Outcomes of single fraction stereotactic ablative radiotherapy in lung metastases. Tech Canc Res Treat 2014;13:37–45. [DOI] [PubMed] [Google Scholar]
- 53.Lee M, Kim J, Dinniwell R et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585–91. [DOI] [PubMed] [Google Scholar]
- 54.Scorsetti M, Arcangeli S, Tozzi A et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastasis? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013;86:336–42. [DOI] [PubMed] [Google Scholar]
- 55.Kim MS, Yoo SY, Cho CK et al. Stereotactic body radiation therapy using three fractions for isolated lung recurrence from colorectal cancer. Oncology 2009;76:212–9. [DOI] [PubMed] [Google Scholar]
- 56.Takeda A, Kunieda E, Ohashi T et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101:255–9. [DOI] [PubMed] [Google Scholar]
- 57.Filippi AR, Badellino S, Ceccarelli M et al. Stereotactic Ablative Radiation Therapy as first local therapy for lung oligometastases from colorectal cancer: a single-institution cohort study. Int J Radiat Oncol Biol Phys 2015;91:524–9. [DOI] [PubMed] [Google Scholar]
- 58.Qiu H, Katz AW, Chowdhry H et al. (11 August 2015) Stereotactic body radiotherapy for lung metastases from colorectal cancer: prognostic factors for disease control and survival. Am J Clin Oncol (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 59.Comito T, Cozzi L, Clerici E et al. Stereotactic Ablative Radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer 2014;14:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ost P, Bossi A, Decaestecker K et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 2015;67:852–63. [DOI] [PubMed] [Google Scholar]
- 61.Casamassima F, Masi L, Menichelli C et al. Efficacy of eradicative radiotherapy for limited nodal metastases detected with choline PET scan in prostate cancer patients. Tumori 2011;97:49–55. [DOI] [PubMed] [Google Scholar]
- 62.Muacevic A, Kufeld M, Rist C et al. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases from prostate cancer. Urol Oncol 2011;31:455–61. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed KA, Barney BM, Davis BJ et al. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol 2012;2:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jereczek-Fossa BA, Beltramo G, Fariselli L et al. Robotic image-guided stereotactic radiotherapy for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:889–97. [DOI] [PubMed] [Google Scholar]
- 65.Schick U, Jorcano S, Nouet P et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol 2013;52:1622–8. [DOI] [PubMed] [Google Scholar]
- 66.Decaestecker K, De Meerleer G, Lambert B et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014;9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ost P, Jereczeck-Fossa BA, Van As N et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naïve recurrence: a multi-institutional analysis. Eur Urol 2016;69:9–12. [DOI] [PubMed] [Google Scholar]
- 68.Lo SS, Sloan AE, Machtay M. Stereotactic radiosurgery for more than four brain metastases. Lancet Oncol 2014;15:362–3. [DOI] [PubMed] [Google Scholar]
- 69.Lussier YA, Xing HR, Salama KK et al. MicroRNA expression characterizes oligometastases. PloS One 2011;6:e28650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lussier YA, Khodarev NN, Regan K et al. Oligo- and polymetastatic progression in lung metastases patients is associated with specific microRNAs. PloS One 2012;7:e50141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Solanki AA, Weichselbaum RR, Appelbaum D et al. The utility of FDG-PET for assessing outcomes in oligometastatic cancer patients treated with stereotactic body radiotherapy: a cohort study. Radiat Oncol 2012;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cristofanilli M, Budd GT, Ellis MJ et al. Circulating tumor cells, disease progression and survival in metastatic breast cancer. N Engl J Med 2004;351:781–91. [DOI] [PubMed] [Google Scholar]