Abstract
Since the initial observations made at the beginning of the last century, it has been established that solid tumors contain regions of low oxygenation (hypoxia). Tumor cells can survive in these hypoxic conditions and are a major factor in tumor radioresistance. This significance has resulted in hypoxia becoming the most cited biological topic in translational radiation oncology. Identifying hypoxic cells in human tumors has become paramount, and the ability to do this has been improved by the help of new imaging techniques and the use of predictive gene profiles. Substantial data confirm the presence of hypoxia in many types of human tumors, although with considerable heterogeneity among individual tumors. Various approaches have been investigated for eliminating the hypoxic population. These include increasing oxygen availability, directly radiosensitizing or killing the hypoxic cells, indirectly affecting them by targeting the tumor vascular supply, increasing the radiation dose to this resistant population, or by using radiation with a high linear energy transfer, for which hypoxia is believed to be less of an issue. Many of these approaches have undergone controlled clinical trials during the last 50 years, and the results have shown that hypoxic radiation resistance can indeed be overcome. Thus, ample data exists to support a high level of evidence for the benefit of hypoxic modification. However, such hypoxic modification still has no impact on general clinical practice. In this review we summarize the biological rationale, and the current activities and trials, related to identifying and overcoming hypoxia in modern radiotherapy.
Keywords: hypoxia, radiation, oxygen modifiers, radiosensitizers, hypoxic cell cytotoxins, vascular targeting agents
THE HYPOXIA PROBLEM
Significance of hypoxia
The first studies suggesting the importance of oxygen in radiotherapy were made as far back as the early 1900s. In 1909, Schwarz demonstrated that the radiation response of skin was decreased if the blood flow in the irradiated area was reduced by compression [1]. One year later, Müller reported that tissues in which the blood flow was stimulated by diathermia showed a more prominent response to radiation [2]. These indirect studies were followed by sporadic experimental and clinical observations indicating the importance of a sufficient blood supply for an adequate radiation response. Eventually this led Gray and co-workers, in the early 1950s, to both postulate that oxygen deficiency (hypoxia) was a major source of radiation resistance [3], and from histological data from patients with carcinoma of the bronchus to suggest that hypoxia can exist in human tumors as a result of a diffusion limitation of oxygen [4].
This concept of oxygen diffusing from blood vessels and being utilized by the tumor cells, thus resulting in diffusion gradients, with cells at the end of these gradients being oxygen-deprived or chronically hypoxic, was the working hypothesis for hypoxia until the 1980s. It was then suggested [5] and shown [6] that tumor hypoxia could also be acute, arising as a result of transient fluctuations in tumor blood flow. The current use of ‘chronic and acute’ to describe hypoxia in tumors is probably an oversimplification of the real situation [7]. Chronic hypoxia generally refers to prolonged and reduced oxygen concentrations that influence radiation response, but there is evidence that oxygen concentrations that are higher, yet below normal physiological levels, are often found [8], and these may influence malignant progression. Furthermore, reduced perfusion can be both partial as well as total [9], and while cells under the former condition would be oxygen deprived, with the latter they would be starved of oxygen and nutrients and as such their survival and response to therapy would be expected to be different.
Imaging tumor hypoxia
The difficulty has always been in applying definitive techniques that not only show that hypoxia exists in human tumors, but also that such measurements correlate with outcome from radiation therapy. Generally, these approaches involve indirect estimates (for review see [10, 11]). The earliest attempts at identifying tumor hypoxia focused on the vascular supply, since this was the oxygen supplier. Endpoints included: immunohistochemical estimates of intercapillary distance, vascular density, and distance from tumor cells to the nearest blood vessel; oxyhemoglobin saturation determined using cryophotometry, or non-invasively with near infrared spectroscopy or magnetic resonance imaging (MRI); or measurements of tumor perfusion using MRI, computed tomography, or positron emission tomography (PET). Hypoxia was later shown to upregulate gene/protein expression; thus, it was suggested that endogenous markers could be used to identify hypoxia. The principal markers have included hypoxia inducible factor 1 (HIF-1), carbonic anhydrase IX (CAIX), the glucose transporters GLUT-1 and GLUT-3, and osteopontin (OPN). These have been applied either individually or combined with other endogenous markers as gene signatures. More popular techniques involve measurements of the binding of exogenous markers. This can be achieved following immunohistological analysis of biopsied sections, using pimonidazole or EF5. It can also be done non-invasively with PET, single-photon emission computed tomography (SPECT), or MRI analysis of radioactively labeled nitroimidazoles (i.e. [18F]-labeled misonidazole or fluoroazomycin arabinoside (FAZA); [123I] labeled azomycin arabinoside); or PET imaging of [60–64Cu]-ATSM (copper (II)-diacetyl-bis(N4-methylthiosemicarbazone). The most direct method involves determining oxygen partial pressure (pO2) distributions with polarographic electrodes.
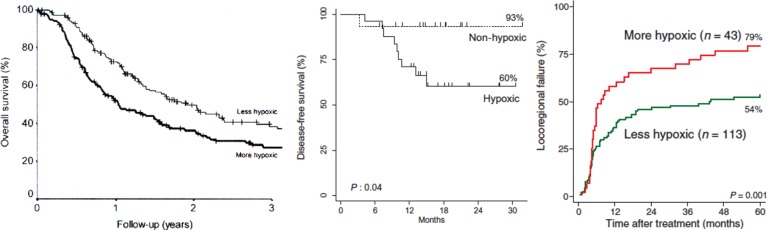
Each technique has its limitations. Although oxygen is delivered to the tumor cells via the vasculature, deficiencies in either vascular development, the oxygen-carrying capacity of the blood, or perfusion are not the only reasons why hypoxia develops [7]. Endogenous markers can be upregulated in response to stress factors that are not hypoxia related; even under normoxic conditions, expression has been reported to be induced by reactive oxygen species, cancer cell–specific mutations, and activation of signal transduction pathways [12]. Exogenous markers require time to identify hypoxic regions so probably only detect chronic hypoxia, not acute, and there are clear resolution issues with the various imaging approaches [11]. The techniques that can produce direct estimates of tumor oxygenation are generally invasive, thus not suitable for all tumors and are unlikely to be used on a routine clinical basis [10, 11]. Nevertheless, data using all the different approaches have not only shown that hypoxia exists in tumors, but that its presence can have a significant negative influence on patient outcome following radiation therapy [11]. This is illustrated in Fig. 1 for Eppendorf oxygen electrode measurements, PET-FAZA scans, and gene signature estimates in patients with head and neck squamous cell carcinomas. Such results clearly support the concept of finding clinically applicable approaches to overcoming tumor hypoxia.
Fig. 1.
Demonstration that the presence of hypoxia measured in head and neck squamous cell carcinomas prior to radiation therapy had a negative influence on outcome. (A) Overall survival in 397 patients in which oxygen estimates were obtained with the Eppendorf electrode. The patients were divided into two groups based on whether the percentage of oxygen values ≤2.5 mmHg were below (less hypoxic than) or above (more hypoxic than) the median value of 19%. (B) Disease-free survival in 40 patients that had received an injection of [18F]-labeled FAZA 2 h prior to PET imaging. They were separated into having hypoxic or non-hypoxic tumors based on whether the tumor-to-muscle ratio was above or below 1.4, respectively. (C) Locoregional failure in 156 patients in which a 15-gene hypoxia classifier was applied to biopsy material to separate the tumors into more or less hypoxic. Composite figure from a number of sources [13–15].
APPROACHES FOR DEALING WITH HYPOXIA
Increasing oxygen delivery
The most obvious approach to the hypoxia issue is to increase tumor oxygen delivery. Indeed, this was applied relatively early in patients by allowing them to breathe high-oxygen-content gas under hyperbaric (typically 3 atmospheres) conditions [16]: hyperbaric oxygen was expected to saturate the blood with oxygen more than normobaric conditions. Positive clinical outcomes were obtained, as shown in Table 1. However, the complexity of the procedure and patient compliance led to this approach being stopped [17]. Pre-clinical studies had also shown that the radiosensitizations produced by normobaric oxygen and carbogen (95% oxygen + 5% carbon dioxide) were quite substantial [18, 19], so clinical trials using carbogen were initiated. The early studies failed to show any dramatic improvement in outcome [20, 21], which may have resulted from a failure to achieve the optimum pre-irradiation gas breathing time; experimental studies have shown this to be critical for enhancing radiation response and that it varies from tumor to tumor [19, 22, 23]. Later studies in which short pre-irradiation breathing times were applied gave conflicting results, with either a benefit [24] or no improvement obtained [25]. The failure in the latter study may have been the result of a size limitation, whereas the former positive study may have been due to the fact that nicotinamide was included in the treatment regime. While carbogen breathing will most likely affect chronic hypoxia it is likely to have limited influence on acute hypoxia. Pre-clinical studies have now demonstrated that nicotinamide is an effective treatment for preventing transient fluctuations in tumor blood flow and thus reduces acute hypoxia, although the mechanism is not known [26]. Those results led to the suggestion and subsequent demonstration that effective hypoxic modification would be possible by combining nicotinamide (to target acute hypoxia) with a modifier of chronic hypoxia [27]. Those studies and others eventually led to the clinical evaluation of carbogen with nicotinamide in patients with bladder cancer in the BCON (Bladder, CarbOgen, and Nicotinamide) trial [28], and in head and neck cancer patients in the ARCON (Accelerated Radiation, CarbOgen, and Nicotinamide) trial [29]; both studies reported positive improvements in outcome.
Table 1.
Medical Research Council (MRC) multicenter randomized trials with hyperbaric oxygen (HBO)
| Site and study | No. of patients | Endpoint | Response |
Statistical significance | |
|---|---|---|---|---|---|
| HBO | Air | ||||
| Head and neck carcinoma | |||||
| MRC 1st trial (1977) | 294 | Control (5 years) | 53% | 30% | P < 0.01 |
| MRC 2nd trial (1986) | 106 | Control (5 years) | 60% | 41% | P < 0.05 |
| Uterine cervix carcinoma | |||||
| MRC (1978) | 320 | Control (5 years) | 67% | 47% | P < 0.001 |
| MRC (1978) | 320 | Survival (5 years) | 37% | 25% | P < 0.01 |
| Bronchogenic carcinoma | |||||
| MRC (1978) | 51 | Survival (2 years) | 15% | 8% | n.s. |
| MRC (1978) | 123 | Survival (2 years) | 25% | 12% | P < 0.05 |
| Carcinoma of the bladder | |||||
| MRC (1978) | 241 | Survival (2 years) | 28% | 30% | n.s. |
Endpoints were Control (locoregional control) or Survival; n.s. = not significant. Modified from [17].
Transport of oxygen in the blood supply is via hemoglobin; thus considerable attention has been applied to finding various methods to target hemoglobin, thereby improving oxygen delivery to tumors. The most obvious approach is to increase hemoglobin levels. Attempts to do this using transfusion produced conflicting results, with either an increase [30] or no effect [31] on radiation response reported. Increasing hemoglobin concentration by stimulation with erythropoietin (EPO) has also been investigated [32]. Pre-clinical studies showed that this was an effective method for overcoming anemia and for improving radiation response; however, although it was also successful in correcting anemia in patients, those that received EPO and radiation had a poorer outcome than patients who were irradiated without EPO. This negative outcome has been attributed to the fact that EPO is a growth factor and thus probably stimulated tumor growth.
Other approaches for improving oxygen delivery that have been investigated include the use of artificial blood substitutes that can carry more oxygen than hemoglobin [33] and manipulators of the oxygen unloading capacity of blood by modifying the oxy-hemoglobin dissociation curve [34]. Although these approaches improved tumor oxygenation status and radiation response in pre-clinical studies, none reached controlled clinical testing. More recent studies suggest the potential of increasing the oxygen diffusion distance by inhibiting cellular oxygen consumption with metformin [35]. Although this agent is already used clinically in treating diabetes and may be associated with decreased rates of some cancer types [36], it is still too early to say whether this will be effective at decreasing tumor hypoxia in patients.
Targeting hypoxic cells
The most extensively investigated approach to the hypoxia problem is the use of agents that specifically target the hypoxic cells. This has been achieved using agents that either directly sensitize the hypoxic cells to radiation or preferentially kill them. In the early 1960s it was shown that the efficacy of hypoxic radiosensitisation was directly related to electron-affinity [37], and that led to in vitro studies demonstrating that highly electron-affinic nitroaromatic compounds could preferentially radiosensitise hypoxic cells [38]. These compounds were also found to be effective at enhancing tumor radiation response [39]; these agents are considered to be oxygen mimetics, but unlike oxygen they are not rapidly metabolized by the tumor cells through which they diffuse and thus reach all the cells in tumors, especially the hypoxic cells.
Clinical evaluation was started very early with metronidazole in brain tumors [40], but it was soon replaced by misonidazole and a large number of clinical trials were undertaken [17, 39]. Unfortunately, most misonidazole trials were unable to generate significant improvements in radiation response, although a benefit was seen in some trials, particularly the Danish Head and Neck Cancer (DAHANCA 2) study [41], as shown in Table 2. Part of the failure to see any benefit was attributed to the fact that the drug doses necessary for effective radiosensitization also produced substantial dose-limiting clinical toxicity. Further clinical studies focussed on identifying more efficient or less toxic hypoxic sensitizers (Table 2). The first of these was a European trial with pimonidazole in uterine cervical cancer, but the preliminary results were disappointing [42]. Etanidazole was then tested in two other multicenter trials in head and neck cancer, but the results showed no benefit [43, 44]. Additional studies with nimorazole, a less efficient sensitizer but less toxic drug, in head and neck cancer patients (DAHANCA 5) showed a highly significant benefit in terms of improved locoregional tumor control and disease-free survival [31]. A more recent International Atomic Energy Agency (IAEA) trial with the 3-nitrotriazole compound sanazole (AK-2123) in uterine cervical cancer also demonstrated a significant improvement in both local tumor control and overall survival [45], while a Japanese randomized trial with the 2-nitroimidazole doranidazole (PR-350) in locally advanced pancreatic cancer reported a significant increase in long-term survival [46]. To date, only one of these drugs has been incorporated into standard radiotherapy treatment—nimorazole in head and neck cancer—and that is only in Denmark, although additional clinical testing is ongoing elsewhere.
Table 2.
Selected multicenter randomized trials with nitroimidazole radiosensitizers
| Site and study | No. of patients | Drug | Endpoint | Response |
Statistical significance | |
|---|---|---|---|---|---|---|
| RT + drug | RT | |||||
| Uterine cervix carcinoma | ||||||
| MRC (1983) | 183 | Pimo | Control (4 years) | 64% | 80% | P < 0.01 |
| Survival (4 years) | 36% | 54% | P < 0.05 | |||
| IAEA (2007) | 326 | Sana | Control (5 years) | 61% | 46% | P = 0.005 |
| Survival (5 years) | 57% | 41% | P = 0.01 | |||
| Head and neck carcinoma | ||||||
| DAHANCA 2 (1989) | 626 | Miso | Control (5 years) | 41% | 34% | P < 0.05 |
| RTOG 85–27 (1995) | 521 | Eta | Control (2 years) | 40% | 40% | n.s. |
| Survival (2 years) | 43% | 41% | n.s. | |||
| EORTC (1978) | 374 | Eta | Control (2 years) | 53% | 53% | n.s. |
| Survival (2 years) | 54% | 54% | n.s. | |||
| DAHANCA 5 (1998) | 414 | Nim | Control (5 years) | 49% | 33% | P = 0.002 |
| Survival (5 years) | 52% | 41% | P = 0.01 | |||
| Pancreatic carcinoma | ||||||
| JAPAN (2008) | 46 | Dora | Survival (3 years) | 23% | 0% | P = 0.02 |
Endpoints were Control or Survival; n.s. = not significant; RT = radiotherapy. The trials were the Medical Research Council (MRC) trial with pimonidazole (Pimo) [42], the International Atomic Energy Agency (IAEA) trial with sanazol (Sana) [45], the Danish Head and Neck Cancer (DAHANCA) trials with misonidazole (Miso) [41] and nimorazole (Nim) [31], the North American Radiation Therapy Oncology Group (RTOG) [43], the European Organisation for Research and Treatment of cancer (EORTC) [44] trials with etanidazole (Eta), and the Japanese trials with doranidazole (Dora) [46].
Preferentially killing hypoxic cells is another direct approach to targeting hypoxia that has become popular. This has been achieved using non-toxic prodrugs that undergo enzymatic reduction to a cytotoxin under hypoxic conditions. Three basic classes of bioreductive compounds have been developed. These are quinones, nitroaromatics and N-oxides [47, 48]. The first compound recognized to possess bioreductive activity was mitomycin C [49]. Clinical studies designed to test its potential to overcome hypoxia produced conflicting results, with either a benefit or no benefit obtained. A lack of response was probably not surprising because mitomycin C shows only a small differential in cell killing between hypoxic and aerobic cells. Attempts to find more efficient quinones led to the development of porfiromycin and EO9, but again no real additional benefit has been found clinically. Nitroimidazole radiosensitizers such as misonidazole have also been found to be metabolized to cytotoxic products selectively in hypoxic tumor cells [50]. However, these agents were only weakly cytotoxic and moderately selective for hypoxic cells [51]. Nevertheless, the basic concept of using nitroimidazole compounds has led to other agents being developed, including RSU1069, PR-104 and TH-302, the latter compound showing significant selectivity for hypoxic cells [52]; it is currently in clinical development. The lead aromatic N-oxide developed was tirapazamine, and it had a hypoxic-aerobic differential of up to 200 in murine cells and 50 in human cell lines [53]. Although results from Phase II trials generally showed promise, randomized trials were somewhat disappointing. However, the potential of such agents has led to the development of related compounds, such as chlorambucil N-oxide and banoxantrone [47].
Probably one of the best approaches for targeting hypoxic cells is the use of hyperthermia, since heat can both sensitize and kill hypoxic cells [54]. Pre-clinical studies have clearly shown that irradiating tumors and at the same time heating them to temperatures up to 43°C can substantially enhance radiation response [54]. This has been attributed to a sensitization affect mediated through an inhibition of radiation-induced DNA repair [55, 56]. However, if a time interval is introduced between the radiation and heat treatments, sensitization is lost, yet an enhancement of radiation response is still observed [54]. This effect has been attributed to heat simply killing those cells that are hypoxic [57, 58]. Regardless of the mechanism for the heat effect on radiation, there is now good clinical evidence that hyperthermia can substantially improve tumor response to radiation in a number of different clinical sites, as illustrated in Table 3.
Table 3.
Meta-analysis of randomized clinical trials comparing radiation only with radiation and hyperthermia
| Tumor site | No. of trials | No. of patients | Response |
Odds ratio (95% CI) | |
|---|---|---|---|---|---|
| RT + HT | RT | ||||
| Advanced breast | 2 | 143 | 68% | 67% | 1.06 (0.52–2.14) |
| Prostate | 1 | 49 | 81% | 79% | 1.16 (0.28–4.77) |
| Mixed | 3 | 442 | 39% | 34% | 1.24 (0.84–1.82) |
| Head and neck | 5 | 274 | 51% | 33% | 2.08 (1.28–3.39) |
| Rectum | 2 | 258 | 19% | 9% | 2.27 (1.08–4.76) |
| Chest wall | 4 | 276 | 59% | 38% | 2.37 (1.46–3.86) |
| Bladder | 1 | 101 | 73% | 51% | 2.61 (1.14–5.98) |
| Melanoma | 1 | 128 | 56% | 31% | 2.81 (1.36–5.80) |
| Cervix | 4 | 248 | 77% | 52% | 3.05 (1.77–5.27) |
| All trials | 23 | 1919 | 52% | 38% | 1.80 (1.50–2.16) |
Endpoints were all locoregional control, RT = radiation, HT = hyperthermia, CI = confidence intervals. Modified from [54].
Vascular targeting agents
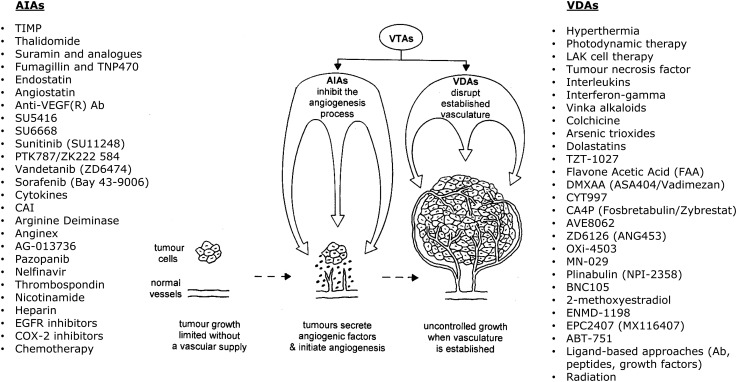
The growth and development of solid tumors requires they develop their own functional vascular supply, which they do from the normal host vasculature by the process of angiogenesis. This importance of the tumor vascular supply makes it an attractive target for therapy, and two major groups of vascular targeting agents (VTAs) have emerged [59], as shown in Fig. 2. One approach involves preventing the development of the tumor vasculature by inhibiting various steps in the angiogenic process (angiogenesis inhibiting agents; AIAs). An alternative method is to use agents that damage the already established tumor vessels (vascular disrupting agents; VDAs). Although both AIAs and VDAs show antitumor effects, they never result in tumor control, even when used in combination [61, 62]. This has led to the suggestion that their potential clinical application would be when combined with more conventional treatments, especially radiation [63]. In fact, numerous pre-clinical studies have now shown that the response of tumors to radiation can be significantly improved when animals are treated with either AIAs or VDAs [61].
Fig. 2.
Schematic illustration that the growth and development of solid tumors requires they form their own functional vasculature to supply essential oxygen and nutrients. Tumors achieve this from the normal host vessels by the process of angiogenesis. Therapeutic targeting of the tumor vasculature can be achieved using various vascular targeting agents (VTAs). These are either angiogenesis-inhibiting agents (AIAs), which can inhibit any one of the steps in the angiogenesis process, or vascular-disrupting agents (VDAs), which damage the already established vasculature. Examples of both types of VTAs are listed. Redrawn from [60].
With both AIAs and VDAs, hypoxia has been implicated in the mechanism for this enhancement of radiation response. For AIAs, the consensus of opinion is that the improvement in radiation response found in pre-clinical studies is the consequence of normalization of the tumor vasculature resulting in a decrease in tumor hypoxia [64]. While there are certainly pre-clinical studies showing an improved tumor oxygenation status with such treatments, there are just as many studies showing no change and even a decrease in tumor oxygenation [61]. These findings not only make it unclear as to the role of vessel normalization in influencing the combination of angiogenesis inhibitors with radiation, they also suggest that timing and sequencing of the two modalities may be critical for an optimal benefit. With VDAs the situation is less controversial. VDAs damage tumor vasculature and as a result cause a severe reduction in tumor blood flow, leading to ischemia and cell death [61]. The cells that die first are most likely the hypoxic cells that are already oxygen and nutrient deprived, and the improved antitumor responses observed when such agents are combined with radiotherapy probably reflects an additive tumor response resulting from the VDA eliminating treatment-resistant hypoxic tumor cells while the radiotherapy acts against the aerobic tumor cell population. However, there are indications that VDAs may induce hypoxia in cells that were not previously hypoxic and that such cells survive this treatment [61, 65]. This supports the concept that timing of VDA therapy relative to radiation treatment is critical, and that for the best affect the drugs should be given soon after irradiating.
Radiation-based approaches
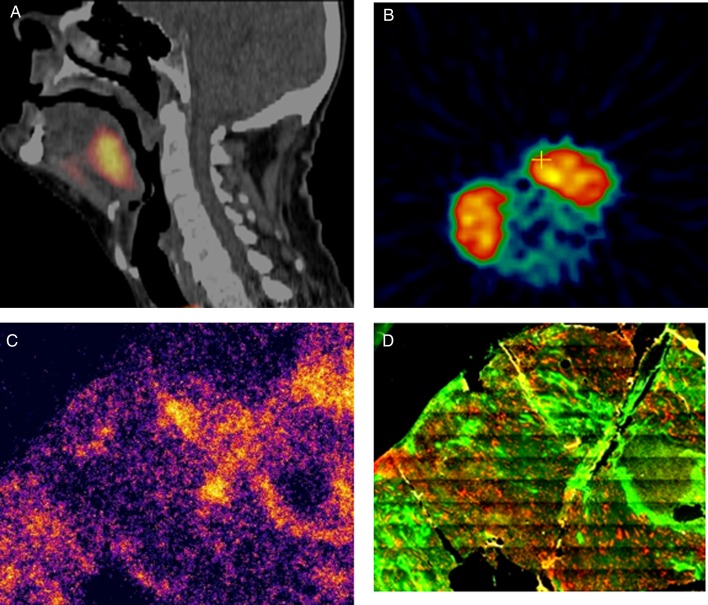
It has been suggested that rather than add some form of modifier to eliminate hypoxic cells, it may be possible to overcome hypoxia by using radiation itself. If we can identify the hypoxic regions in tumors, we could then simply increase the radiation to these areas, an approach that is referred to as ‘dose painting’ [66]. The problem with this approach is in truly identifying the hypoxic regions with current technology. This is illustrated in Fig. 3. Using histological analysis of hypoxic markers, we are able to separate clearly hypoxic and non-hypoxic areas [67]. However, from PET scans of tumors, whether animal or human, the hypoxic region appears as a solid mass; this is simply because the region identified by each individual voxel is large due to resolution issues. As a consequence, areas are being detected that could contain both hypoxic and non-hypoxic cells. Furthermore, the lack of hypoxic imaging in other areas does not mean that hypoxia is not present, rather that the amount of hypoxia in a particular voxel is not large enough to raise the overall threshold value. It has also been suggested that tumor hypoxia is dynamic rather than static, thus hypoxia measured prior to the start of therapy may not be the same as that during therapy. This was indicated in a PET study in head and neck cancer patients in which considerable variability in intratumoral uptake of [18F] misonidazole was reported between repeated scans [68]. However, a more recent pre-clinical study using FAZA-PET [69] and another clinical study in head and neck cancer patients using [18F] misonidazole reported that the uptake between repeated scans was highly reproducible [70]. There is also the question of how much the radiation dose should be increased to have a definitive effect on response. Based on these issues it would seem more pertinent to simply increase the dose to the entire tumor in which hypoxia has been identified and see whether this actually makes a difference to outcome [11].
Fig. 3.
(A) FAZA-PET scan from a patient with a head and neck tumor; high FAZA activity was detected 2 h after injection and is shown by the bright spot in the neck region. (B) FAZA activity in two SCCVII squamous cell tumors on the flanks of a C3H mouse; FAZA activity, measured 2 h after injection is again shown by the two large bright areas and was recorded using an animal dedicated micro PET. (C) Autoradiography section of one of the SCCVII mouse tumors showing microregional areas of FAZA activity, which are unlike two large areas in Fig. 3B. (D) The same section as in C, but now stained for binding of the hypoxic cell marker pimonidazole, which was injected 2 h prior to excision; note that the bright areas showing pimonidazole binding are the same as those in the FAZA autoradiography image. Modified from [14, 67].
It has been established that as the linear energy transfer (LET) for radiation increases, so the oxygen enhancement ratio (OER) decreases [71, 72]. Based on historical data, the consensus of opinion has generally been that if radiation of a sufficiently high LET is applied then hypoxia ceases to be an issue. However, a more recent comprehensive summary of results from a range of different in vitro experiments [72] has shown that the OER effect only disappears when the LET value is ∼500 keV/µm or greater, which is beyond what we can achieve clinically. Thus, although the use of high-LET radiation can certainly reduce the impact of hypoxia, it is unlikely that using current technology it will ever be possible to completely eliminate hypoxia, suggesting that even with high-LET radiation some form of modifier needs to be included in the treatment regime.
CONCLUSIONS
Current interest in cancer therapy focuses on the application of targeted therapies. In that context, hypoxia must be considered the ultimate target. It is a characteristic feature of most human tumors that has a major negative influence on determining tumor response to conventional therapy, as well as being an important factor in influencing malignant progression, both in terms of the aggressive growth of the primary tumor and its ability for metastatic spread. Numerous clinically applicable techniques have been developed that allow us to identify hypoxia in human tumors. Both pre-clinical and clinical studies have shown that hypoxia can be reduced to improve the outcome of radiation therapy. However, despite the fact that the potential of hypoxia to modify radiation response was first identified 100 years ago, that we have known for some 60 years as to why hypoxia influences radiation response, and that almost 40 years of clinical trials have clearly shown we can successfully modify hypoxia, hypoxic modification has still not been established as a standard treatment with radiotherapy. Why this should be so is unclear, but from a patient point of view this must be considered a totally unacceptable situation that should be addressed.
FUNDING
Financial support from the Danish Cancer Society, the Danish Council for Independent Research: Medical Sciences, and CIRRO (the Lundbeck Foundation Center for Intervention Research in Radiation Oncology and the Danish Council for Strategic Research) is gratefully acknowledged. Funding to pay the Open Access publication charges for this article was provided by funds from the Department of Experimental Clinical Oncology.
ACKNOWLEDGEMENTS
This research was presented at the 15th International Congress of Radiation Research (ICRR2015), 25–29 May 2015, Kyoto Japan.
REFERENCES
- 1.Schwarz G. über Desensibiliserung gegen Röntgen- und Radiumstrahlen. Munchener Medizinische Wochenschrift 1909;24:1–2. [Google Scholar]
- 2.Müller C. Eine neue Behandlungsmethode bösartiger Geschwülste. Munchener Medizinische Wochenschrift 1910;28:1490–3. [Google Scholar]
- 3.Gray LH, Conger AD, Ebert M et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638–48. [DOI] [PubMed] [Google Scholar]
- 4.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955;9:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol 1979;52:650–6. [DOI] [PubMed] [Google Scholar]
- 6.Chaplin DJ, Olive PL, Durand RE. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res 1987;47:597–601. [PubMed] [Google Scholar]
- 7.Bayer C, Shi K, Astner ST et al. Acute versus chronic hypoxia: why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys 2011;80:965–8. [DOI] [PubMed] [Google Scholar]
- 8.Helmlinger G, Yuan F, Dellian M et al. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 1997;3:177–82. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Braun RD, Ong ET et al. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res 1996;56:5522–8. [PubMed] [Google Scholar]
- 10.Horsman MR. Measurement of tumor oxygenation. Int J Radiat Oncol Biol Phys 1998;42:701–4. [DOI] [PubMed] [Google Scholar]
- 11.Horsman MR, Mortensen LS, Petersen JB et al. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 2012;9:674–87. [DOI] [PubMed] [Google Scholar]
- 12.Span PN, Bussink J. Biology of hypoxia. Semin Nucl Med 2015;45:101–9. [DOI] [PubMed] [Google Scholar]
- 13.Nordsmark M, Bentzen SM, Rudat V et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol 2005;77:18–24. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen LS, Johansen J, Kallehauge J et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol 2012;105:14–20. [DOI] [PubMed] [Google Scholar]
- 15.Toustrup K, Sørensen BS, Nordsmark M et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 2011;71:5923–31. [DOI] [PubMed] [Google Scholar]
- 16.Churchill-Davidson I. The oxygen effect in radiotherapy – historical review. Frontiers Radiat Ther Oncol 1968;1:1–15. [Google Scholar]
- 17.Overgaard J. Sensitization of hypoxic tumour cells – clinical experience. Int J Radiat Biol 1989;56:801–11. [DOI] [PubMed] [Google Scholar]
- 18.Du Sault LA. The effect of oxygen on the response of spontaneous tumours in mice to radiotherapy. Br J Radiol 1963;36:749–54. [DOI] [PubMed] [Google Scholar]
- 19.Suit HD, Marshall N, Woerner D. Oxygen, oxygen plus carbon dioxide, and radiation therapy of a mouse mammary carcinoma. Cancer 1972;30:1154–8. [DOI] [PubMed] [Google Scholar]
- 20.Bergsjø P, Kolstad P. Clinical trial with atmospheric oxygen breathing during radiotherapy of cancer of the cervix. Scand J Clin Lab Invest 1968;106 (suppl.);167–71. [PubMed] [Google Scholar]
- 21.Rubin P, Hanley J, Keys HM et al. Carbogen breathing during radiation therapy. The RTOG study. Int J Radiat Oncol Biol Phys 1979;5:1963–70. [DOI] [PubMed] [Google Scholar]
- 22.Siemann DW, Hill RP, Bush RS. The importance of the pre-irradiation breathing times of oxygen and carbogen (5% CO2; 95% O2) on the in vivo radiation response of a murine sarcoma. Int J Radiat Oncol Biol Phys 1977;2:903–11. [DOI] [PubMed] [Google Scholar]
- 23.Chaplin DJ, Horsman MR, Siemann DW. Further evaluation of nicotinamide and carbogen as a strategy to reoxygenate hypoxic cells in vivo: importance of nicotinamide dose and pre-irradiation breathing time. Br J Cancer 1993;68:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol 2002;3:728–37. [DOI] [PubMed] [Google Scholar]
- 25.Mendenhall WM, Morris CG, Amdur RJ et al. Radiotherapy alone or combined with carbogen breathing for squamous cell carcinoma of the head and neck: a prospective randomized trial. Cancer 2005;104:333–7. [DOI] [PubMed] [Google Scholar]
- 26.Horsman MR. Nicotinamide and other benzamide analogs as agents for overcoming hypoxic cell radiation resistance in tumours. Acta Oncol 1995;34:571–87. [DOI] [PubMed] [Google Scholar]
- 27.Horsman MR, Chaplin DJ, Overgaard J. Combination of nicotinamide and hyperthermia to eliminate radioresistant chronically and acutely hypoxic tumour cells. Cancer Res 1990;50:7430–6. [PubMed] [Google Scholar]
- 28.Hoskin PJ, Rojas AM, Bentzen SM et al. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol 2010;28:4912–8. [DOI] [PubMed] [Google Scholar]
- 29.Janssens GO, Rademakers SE, Terhaard CH et al. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 2012;30:1777–83. [DOI] [PubMed] [Google Scholar]
- 30.Grogan M, Thomas GM, Melamed I et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999;86:1528–36. [DOI] [PubMed] [Google Scholar]
- 31.Overgaard J, Sand Hansen H, Overgaard M et al. A randomised double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish head and neck cancer study (DAHANCA) protocol 5–85. Radiother Oncol 1998;46:135–46. [DOI] [PubMed] [Google Scholar]
- 32.Hoff CM. Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncol 2012;51:419–32. [DOI] [PubMed] [Google Scholar]
- 33.Rockwell S. Use of a perfluorochemical emulsion to improve oxygenation in a solid tumor. Int J Radiat Oncol Biol Phys 1985;11:97–103. [DOI] [PubMed] [Google Scholar]
- 34.Siemann DW, Macler LM. Tumor radiosensitization through reductions in hemoglobin affinity. Int J Radiat Oncol Biol Phys 1986;12:1295–7. [DOI] [PubMed] [Google Scholar]
- 35.Zannella VE, Dal Pra A, Muaddi H et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res 2013;19:6741–50. [DOI] [PubMed] [Google Scholar]
- 36.Zhang P, Li H, Tan X et al. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 2013;37:201–18. [DOI] [PubMed] [Google Scholar]
- 37.Adams GE, Cooke MS. Electron–affinic sensitization. I. A structural basis for chemical radiosensitizers in bacteria. Int J Radiat Biol 1969;15:457–71. [DOI] [PubMed] [Google Scholar]
- 38.Adams GE, Flockhart IR, Smithen CE et al. Electron–affinic sensitization. VII. A correlation between structures, one-electron reduction potentials and efficiencies of some nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res 1976;67:9–20. [PubMed] [Google Scholar]
- 39.Overgaard J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncol Res 1994;6:509–18. [PubMed] [Google Scholar]
- 40.Urtasun R, Band P, Chapman JD et al. Radiation and high-dose metronidazole in supratentorial glioblastomas. NEJM 1976;294:1364–7. [DOI] [PubMed] [Google Scholar]
- 41.Overgaard J, Sand Hansen H, Andersen AP et al. Misonidazole combined with split-course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys 1989;16:1065–8. [DOI] [PubMed] [Google Scholar]
- 42.Dische S, Machin D, Chassagne D. A trial of Ro 03–8799 (pimonidazole) in carcinoma of the uterine cervix: an interim report from the Medical Research Council Working Party on advanced carcinoma of the cervix. Radiother Oncol 1993;26:93–103. [PubMed] [Google Scholar]
- 43.Lee D-J, Cosmatos D, Marcial VA et al. Results of an RTOG phase III trial (RTOG 85–27) comparing radiotherapy plus etanidazole (SR-2508) with radiotherapy alone for locally advanced head and neck carcinomas. Int J Radiat Oncol Biol Phys 1995;32:567–76. [DOI] [PubMed] [Google Scholar]
- 44.Eschwége F, Sancho-Garnier H, Chassagne D et al. Results of a European randomized trial of etanidazole combined with radiotherapy in head and neck carcinomas. Int J Radiat Oncol Biol Phys 1997;39:275–81. [DOI] [PubMed] [Google Scholar]
- 45.Dobrowsky W, Huigol NG, Jayatilake RS et al. AK-2123 (sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer: results of an IAEA multicentre randomized trial. Radiother Oncol 2007;82:24–9. [DOI] [PubMed] [Google Scholar]
- 46.Karasawa K, Sunamura M, Okamoto A et al. Efficacy of novel hypoxic cell sensitiser doranidazole in the treatment of locally advanced pancreatic cancer: long-term results of a placebo-controlled randomized study. Radiother Oncol 2008;87:326–30. [DOI] [PubMed] [Google Scholar]
- 47.McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clin Oncol 2007;19:427–42. [DOI] [PubMed] [Google Scholar]
- 48.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393–410. [DOI] [PubMed] [Google Scholar]
- 49.Rockwell S. Effect of some proliferative and environmental factors on the toxicity of mitomycin C to tumor cells in vitro. Int J Cancer 1986;38:229–35. [DOI] [PubMed] [Google Scholar]
- 50.Rauth AM, McClelland RA, Michaels HB et al. The oxygen dependence of the reduction of nitroimidazoles in a radiolytic model system. Int J Radiat Oncol Biol Phys 1984;10:1323–6. [DOI] [PubMed] [Google Scholar]
- 51.Sutherland RM, Keng P, Conroy PJ et al. In vitro hypoxic cytotoxicity of nitroimidazoles: uptake and cell cycle phase specificity. Int J Radiat Oncol Biol Phys 1982;8:745–8. [DOI] [PubMed] [Google Scholar]
- 52.Meng F, Evans JW, Bhupathi D et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther 2012;11:740–51. [DOI] [PubMed] [Google Scholar]
- 53.Zeman EM, Brown JM, Lemmon MJ et al. SR-4233: a new bioreductive agent with high selective toxicity for human mammalian cells. Int J Radiat Oncol Biol Phys 1986;12:1239–42. [DOI] [PubMed] [Google Scholar]
- 54.Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol 2007;19:418–26. [DOI] [PubMed] [Google Scholar]
- 55.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevanc. Int J Radiat Biol 2001;77:399–408. [DOI] [PubMed] [Google Scholar]
- 56.Roti Roti JL. Introduction: radiosensitization by hyperthermia. Int J Hyperthermia 2004;20:109–14. [DOI] [PubMed] [Google Scholar]
- 57.Overgaard J, Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology 1977;123:511–4. [DOI] [PubMed] [Google Scholar]
- 58.Gerweck LE, Nygaard TG, Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res 1979;39:966–72. [PubMed] [Google Scholar]
- 59.Siemann DW, Bibby MC, Dark G et al. Differentiation and definition of vascular-targeted therapies. Clin Cancer Res 2005;11:416–20. [PubMed] [Google Scholar]
- 60.Horsman MR. Angiogenesis and vascular targeting: relevance for hyperthermia. Int J Hyperthermia 2008;24:57–65. [DOI] [PubMed] [Google Scholar]
- 61.Horsman MR, Siemann DW. Pathophysiological effects of vascular targeting agents and the implications for combination therapies. Cancer Res 2006;66:11520–39. [DOI] [PubMed] [Google Scholar]
- 62.Siemann DW, Horsman MR. Vascular targeted therapies in oncology. Cell Tissue Res 2009;335:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siemann DW, Warrington KH, Horsman MR. Vascular targeting agents: adjuvants to radiation therapy. Radiother Oncol 2000;57:5–12. [DOI] [PubMed] [Google Scholar]
- 64.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001;7:987–9. [DOI] [PubMed] [Google Scholar]
- 65.Iversen AB, Busk M, Horsman MR. Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol 2013;52:1320–6. [DOI] [PubMed] [Google Scholar]
- 66.Ling CC, Humm J, Larson S et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 2000;47:551–60. [DOI] [PubMed] [Google Scholar]
- 67.Busk M, Horsman MR, Jakobsen S et al. Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, pO2-polarography and fluorescence microscopy. Int J Radiat Oncol Biol Phys 2008;70:1202–12. [DOI] [PubMed] [Google Scholar]
- 68.Nehmeh SA, Lee NY, Schröder H et al. Reproducibility of intratumor distribution of 18F-Fluoromisonidazole in head and neck cancer. Int J Radiat Oncol Biol Phys 2008;70:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Busk M, Mortensen LS, Nordsmark M et al. PET hypoxia imaging with FAZA: reproducibility at baseline and during fractionated radiotherapy in tumor-bearing mice. Eur J Nucl Med Mol Imaging 2013;40:186–97. [DOI] [PubMed] [Google Scholar]
- 70.Okamoto S, Shiga T, Yasuda K et al. High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET in head and neck cancer. J Nucl Med 2013;54:201–7. [DOI] [PubMed] [Google Scholar]
- 71.Barendsen GW. Responses of cultured cells, tumours and normal tissues to radiations of different linear energy transfer. Curr Topics Radiat Res Q 1968;4:293–356. [Google Scholar]
- 72.Wenzl T, Wilkens JJ. Modelling of the oxygen enhancement ratio for ion beam radiation therapy. Phys Med Biol 2011;56:3251–68. [DOI] [PubMed] [Google Scholar]