Abstract
The embryonic brain is radiation-sensitive, with cognitive deficits being observed after exposure to low radiation doses. Exposure of neonates to radiation can cause intracranial carcinogenesis. To gain insight into the basis underlying these outcomes, we examined the response of the embryonic, neonatal and adult brain to low-dose radiation, focusing on the neural stem cell compartments. This review summarizes our recent findings. At E13.5–14.5 the embryonic neocortex encompasses rapidly proliferating stem and progenitor cells. Exploiting mice with a hypomorphic mutation in DNA ligase IV (Lig4Y288C), we found a high level of DNA double-strand breaks (DSBs) at E14.5, which we attribute to the rapid proliferation. We observed endogenous apoptosis in Lig4Y288C embryos and in WT embryos following exposure to low radiation doses. An examination of DSB levels and apoptosis in adult neural stem cell compartments, the subventricular zone (SVZ) and the subgranular zone (SGZ) revealed low DSB levels in Lig4Y288C mice, comparable with the levels in differentiated neuronal tissues. We conclude that the adult SVZ does not incur high levels of DNA breakage, but sensitively activates apoptosis; apoptosis was less sensitively activated in the SGZ, and differentiated neuronal tissues did not activate apoptosis. P5/P15 mice showed intermediate DSB levels, suggesting that DSBs generated in the embryo can be transmitted to neonates and undergo slow repair. Interestingly, this analysis revealed a stage of high endogenous apoptosis in the neonatal SVZ. Collectively, these studies reveal that the adult neural stem cell compartment, like the embryonic counterpart, can sensitively activate apoptosis.
Keywords: neural stem cells, DNA damage response, low-dose radiation, DNA double-strand break repair, radiation sensitivity
INTRODUCTION
A significant lesion induced by ionizing radiation (IR) is a DNA double-strand break (DSB). DSBs can arise directly from radiation exposure or indirectly by, for example, attempts to replicate past base damage or single-strand breaks, lesions also induced by IR. The DNA damage response (DDR) to DSBs involves DSB repair processes as well as signal transduction responses, which transduce signals to a range of endpoints, including apoptosis, cell cycle checkpoint arrest and senescence. The two major DSB repair processes are DNA non-homologous end-joining (NHEJ) and homologous recombination (HR) [1]. The major role of HR lies in promoting recovery from lesions encountered during replication, and it exerts only a minor role in the repair of DSBs directly induced by IR. [2]. The damage response kinase, ataxia telangiectasia mutated (ATM), lies at the heart of DSB-induced DDR signaling [3]. Although NHEJ is predominantly ATM-independent, ATM influences NHEJ by regulating chromatin structure and activating processes such as cell cycle checkpoint arrest. Substantial insight has been gained into the mechanism of NHEJ, but a significant outstanding question concerns the interplay between NHEJ and damage response signaling. Moreover, there is increasing recognition that the outcome of DSB generation can differ between cell types and tissues. Indeed, while some tissues are exceedingly radiation sensitive, others are radioresistant, with the response not necessarily reflecting the tissue's DSB repair capability [4]. There is mounting realization that the response of stem cells can be distinct from that of differentiated cells and, further, that the tissue response can be determined by the response of its stem cells [5]. Recent studies have also highlighted the distinct responses of cells and tissues to low versus high radiation doses [6]. The response of tissues and their respective stem cells to low-dose radiation is an issue of mounting concern given the potential cancer risk caused by increasing use of diagnostic procedures involving exposure to IR [7]. This review will summarize the findings from recent papers from our own laboratory, focusing on the response of the embryonic and adult neural stem cell compartments to low radiation doses and discuss the results in the context of other findings using in vivo irradiated stem cells.
Human disorders with defects in NHEJ components have been described [8]. Significantly, microcephaly at birth is a feature of most of the disorders caused by NHEJ defects. Generally, microcephaly is detectable at birth and is rarely or mildly progressive post birth [8, 9]. The embryonic brain is known to be highly sensitive to radiation exposure [10, 11]. Collectively, these findings suggest that there is a stage during neurogenesis sensitive to NHEJ deficiency. DNA ligase IV (LIG4) functions uniquely during NHEJ. LIG4 Syndrome represents the human disorder caused by mutations in DNA ligase IV (LIG4), and LIG4 Syndrome patients display microcephaly [12]. They also display immunodeficiency, arising as a consequence of NHEJ's function during Variable, Diversity and Joining V(D)J recombination. A second goal of this work was to gain insight into the basis underlying the microcephaly in LIG4 Syndrome patients.
THE SYSTEM UNDER STUDY
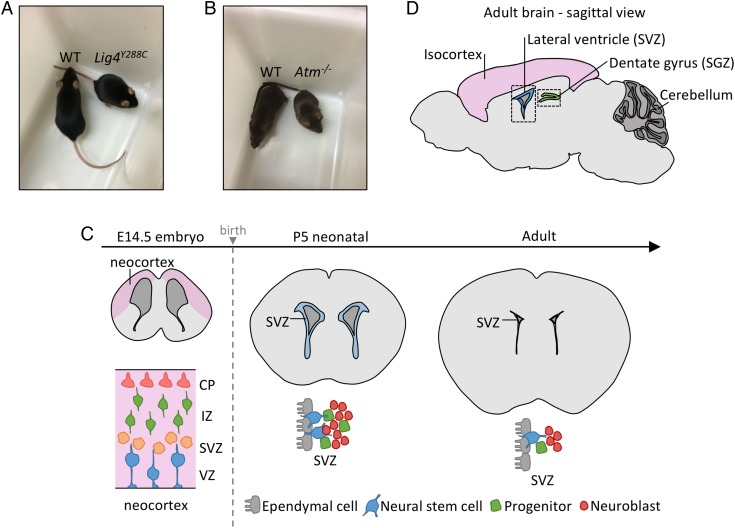
Since LIG4 is essential for embryogenesis, LIG4 Syndrome patients harbor hypomorphic mutations in LIG4 [13, 14]. Lig4Y288C is a mouse strain derived from a mutagenesis screen and has a homozygous hypomorphic mutation in the mouse DNA ligase IV gene (Lig4) [15]. Lig4Y288C mice resemble LIG4 Syndrome patients in displaying small growth and immunodeficiency (Fig. 1A). Significantly, an impact of the Y288C substitution (which lies in the Lig4 catalytic domain) during neurogenesis has been demonstrated [15]. Mouse embryonic fibroblasts (MEFs) derived from Lig4Y288C mice show slow DSB repair, a feature also observed in LIG4 Syndrome patient fibroblasts [12, 15, 16]. We exploited Lig4Y288C mice to assess DSB formation during neurogenesis, making the assumption that enhanced DNA breakage would manifest as increased DSB levels in these repair-deficient mice. We also used the Lig4Y288C mice to examine the response to endogenously arising DSBs, focusing on apoptosis, which has been shown to correlate with the premature lethality of Lig4 null embryos [13, 14]. We also examined mice lacking ATM (Atm−/−), which like Lig4Y288C mice are small at birth, to assess ATM's role in apoptosis and to evaluate DSB levels (Fig. 1B). More limited analysis was also undertaken using double mutant Atm−/−/Lig4Y288C mice. All animal experiments were carried out in accordance with accepted standards of animal welfare approved by the UK Home Office and complied with the Animals (Scientific Procedures) Act 1986.
Fig. 1.
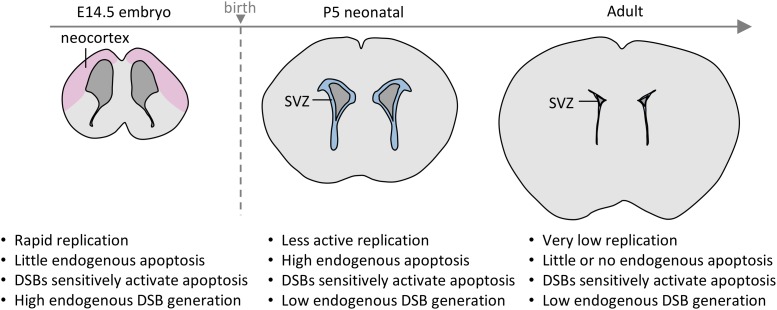
Description of the system under study. (A–B) Photographs of WT, Lig4Y288C and Atm−/− mice displaying their small size compared with WT littermates of the same gender. (C) A coronal depiction of the neural stem cell compartment at E14.5, P5 and 2–3 months (adult). At E14.5, ventricular/subventricular zones (VZ/SVZ) occupy around half of the forebrain. The IZ and CP encompass the progenitor and more differentiated cells, respectively. From E11.5 to E16.5, the VZ/SVZ cells are undergoing rapid proliferation. At P5, the SVZ occupies a smaller region of the forebrain but is still actively dividing and in a dynamic state. By adulthood, the SVZ is much smaller and the neural stem and progenitor cells are less numerous and less actively dividing. (D) Sagittal representation of the adult brain showing the main regions of postnatal neurogenesis: the SVZ located close to the lateral ventricles, and the SGZ, which lies within the dentate gyrus of the hippocampus.
The embryonic forebrain (referred to as the neocortex) houses the ventricular and subventricular zones (VZ/SVZ), which encompass the neural stem and early progenitor cells [17–19]. The VZ/SVZ lies adjacent to the ventricle, providing a positional localization (Fig. 1C). From E11.5–16.5, VZ cells proliferate rapidly, initially symmetrically generating two stem cells and subsequently asymmetrically, forming stem and progenitor daughters (Fig. 1C) [20]. At E13.5, the VZ/SVZ represents the major forebrain region. By E14.5, asymmetric division generates the intermediate zone (IZ), encompassing the early-differentiated progenitors. From E14.5 onwards, the cortical plate (CP), housing the differentiated neurons, enlarges as the VZ/SVZ diminishes in size. Procedures were developed to optimize DSB detection and repair, with 53BP1 foci per cell providing the best results [16, 21]. Apoptosis was optimally monitored using TUNEL staining [22]. 53BP1 foci in tissues were detected at approximately one-third of the level observed in cultured cells (i.e. at 30 min post 100 mGy approximately one 53BP1 focus per cell is observed in tissues compared with approximately three foci per cell in cultured fibroblasts). This lower detection is reasonable given that cells in vivo are spherical and scoring the entire depth is difficult, whereas cultured fibroblasts are flat. Apoptosis is monitored per area, per section or as a total cell percentage, which allows intertissue comparisons. Analysis of an entire tissue section enhances detection levels, an advantage for assessing the response to low doses [22].
Subsequently, analysis was undertaken in neonatal and adult brains. The adult brain has two neural stem cell regions: the SVZ (which lies adjacent to the lateral ventricle) and the subgranular zone (SGZ; located in the dentate gyrus of the hippocampus) (Fig. 1D) [23]. To compare responses in neural stem and differentiated cells, we examined the SVZ and isocortex of neonatal mice, which are derived from the embryonic VZ/SVZ. At this stage, the SVZ has actively proliferating cells but is undergoing a reduction in size to form the adult SVZ [24, 25] (Fig. 1C). The isocortex encompasses predominantly differentiated cells. The neonatal brain was included in our analysis since it is a stage sensitive to radiation-induced carcinogenesis [7, 26, 27].
HIGH DSB FORMATION AND SENSITIVE ACTIVATION OF APOPTOSIS IN THE EMBRYONIC NEOCORTEX
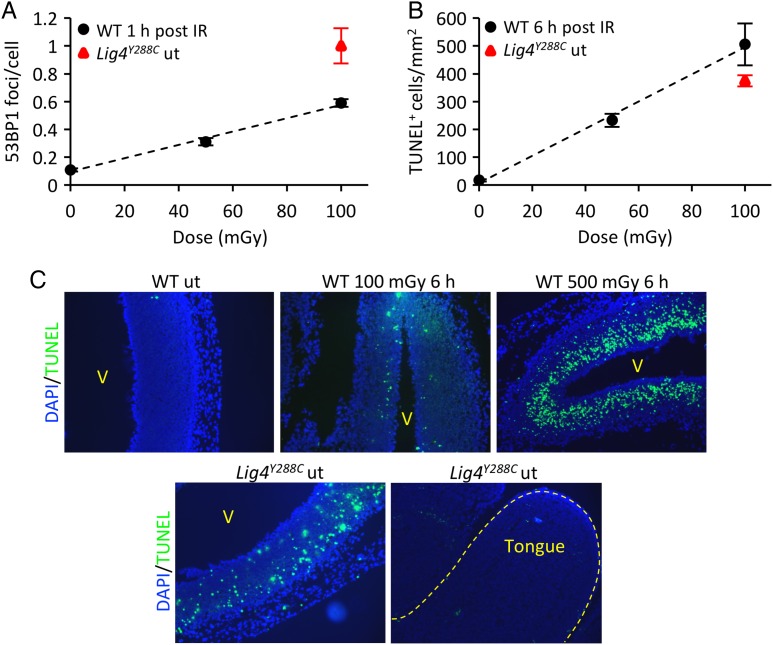
DSB levels in the embryonic neocortex of wild-type (WT) embryos following low-dose radiation exposure were compared with the endogenous level in Lig4Y288C embryos [21]. Pregnant mice (E13.5) were exposed to X-rays (50–200 mGy) and embryos analyzed at various times post IR. DSB levels in cultured cells (assessed as γH2AX or 53BP1 foci) are maximal at 15–60 min post exposure [28]. From 5–15 min, γH2AX foci increase in size; beyond 60 min foci numbers diminish as repair ensues. Although the rate of repair appears similar when assessed by γH2AX foci analysis or by physical methods, a delay of 1–2 h is observed by foci scoring, since foci must be dismantled before ‘repair’ is observed; this step takes longer than the rejoining event. The process in vivo appears similar. DSBs were sensitively detected in the neocortex, with a linear dose response after 10–200 mGy and statistically significant elevated foci after 10 mGy [22] (Fig. 2A for E13.5 embryos). Significantly, we observed increased endogenous 53BP1 foci in the Lig4Y288C neocortex at E14.5, with foci numbers being slightly greater than at 1 h post exposure of WT E13.5 embryos to 100 mGy (Fig. 2A; see legend for further discussion). This is substantially greater than the level observed in other embryonic tissues, such as the tongue (Fig. 2C).
Fig. 2.
Endogenous and IR-induced DNA damage and apoptosis in the embryonic neocortex. (A) 53BP1 foci dose response in E13.5 WT embryos 1 h after exposure to 50–100 mGy X-rays (black circles). Endogenous level of 53BP1 foci in E14.5 Lig4Y288C embryos (red triangle). (B) Dose-dependent increase in apoptosis in the neocortex of E13.5 WT embryos 6 h after exposure to 50–100 mGy X-rays (black circles). Endogenous level of apoptosis in E14.5 Lig4Y288C embryos (red triangle) [16, 22]. (C) Representative sagittal sections of E14.5 embryonic forebrains showing the level of apoptosis (TUNEL staining, green) in the WT neocortex after 100 and 500 mGy X-rays. Increased endogenous apoptosis is observed in the Lig4Y228C untreated neocortex; in contrast, other embryonic tissues (e.g. tongue) show very little or no endogenous apoptosis. Note that the analysis for WT embryos was carried out at E13.5, whereas for Lig4Y288C, E14.5 embryos were used. For 53BP1 foci analysis, the foci numbers are more efficiently enumerated at E14.5, where the IZ occupies a greater part of the forebrain [22]. 53BP1 foci numbers at E14.5 are still slightly elevated in Lig4Y288C embryos compared with WT E14.5 embryos exposed to 100 mGy [21]. For apoptosis, the level is substantially greater at E13.5 compared with E14.5 [22]. Thus, at E14.5, the level of apoptosis in Lig4Y288C embryos is greater than in WT embryos exposed to 100 mGy (as evident in Fig. 2C) [21]. ut stands for untreated. [These figures are based on original data presented in references 16, 21 and 22].
We also quantified apoptosis in the neocortex of E13.5 WT embryos at 6 h post low X-ray doses (the optimal time for scoring apoptosis) and in unirradiated Lig4Y288C embryos (Fig. 2B and C) [21]. In WT embryos, sensitive activation of apoptosis was observed, with a linear dose response from 10–200 mGy [22] (Fig. 2B, E13.5 embryos; Fig. 2C, E14.5 embryos). Consistent with the elevated endogenous DSBs in Lig4Y288C embryos, we observed a level of endogenous apoptosis in Lig4Y288C embryos similar to that induced in WT embryos by 100 mGy (Fig. 2B and C; E14.5 embryos). Apoptosis in WT embryos was greatest in the VZ/SVZ, slightly less in the IZ and much reduced in the cortical plate, strongly suggesting that the stem cell compartment has the greatest sensitivity for activation of apoptosis, which declines as cells differentiate (Fig. 2C). Intriguingly, endogenous apoptosis in Lig4Y288C embryos was highest in the IZ, potentially a consequence of DSB persistence and movement of cells with DSBs from the VZ/SVZ into the IZ (Fig. 2C).
Collectively, these findings expose three features: (i) they demonstrate high endogenous DSB formation in the embryonic neocortex. This high level is likely a consequence of the rapid stem cell proliferation at this stage. At E17.5, when replication has ceased in the VZ/SVZ, DSB levels in Lig4Y288C embryos are slightly decreased [16, 21]. (ii) IR-induced apoptosis is sensitively activated in the VZ/SVZ of the WT neocortex, whereas the IZ is less sensitive. (iii) There is endogenous apoptosis in Lig4Y288C embryos, matching the increased DSB levels. Thus, we posit that the microcephaly observed in LIG4 Syndrome patients arises as a consequence of these features (high endogenous breakage and sensitive activation of apoptosis), coupled with the failure to replenish lost neurons post-natally since the stem cell compartment diminishes in neurogenic activity and size with aging.
LOW DSB FORMATION BUT SENSITIVE ACTIVATION OF APOPTOSIS IN THE ADULT SVZ
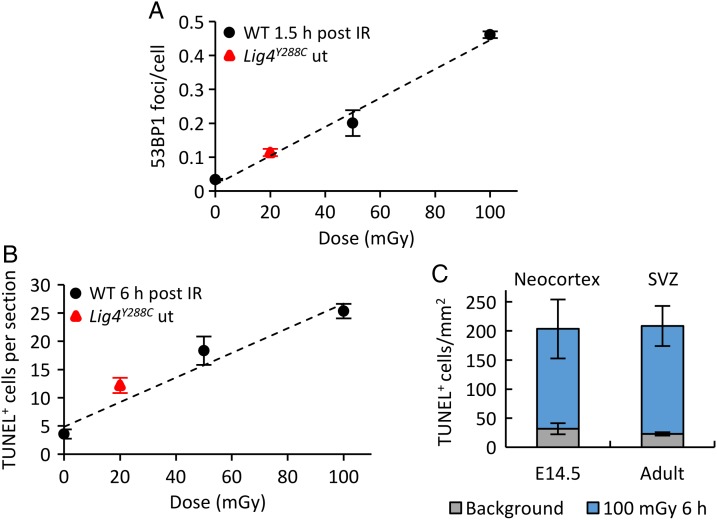
A similar analysis was undertaken in the SVZ and SGZ of 2–3-month-old adult WT and Lig4Y288C mice [16]. 53BP1 foci were detectable in the WT adult SVZ after 50–100 mGy X-rays, with a linear response (Fig. 3A). Endogenous DSB levels in the WT SVZ and SGZ were low, as expected (Fig. 3A for the SVZ; SGZ not shown). 53BP1 foci levels were elevated in the SVZ and SGZ of Lig4Y288C mice, at a level similar to that observed in differentiated neuronal tissues of Lig4Y288C mice and to that expected from exposing WT mice to ∼ 15–20 mGy, ∼8-fold lower than observed in the Lig4Y288C embryonic neocortex (Figs 2A and 3A for the SVZ) [16]. The level of apoptosis in the adult SVZ of WT mice exposed to 50 mGy X-rays was similar to that observed in the embryonic neocortex following the same dose (Fig. 3B and C). Apoptosis in the SGZ was only observed after 500 mGy, however, and was not seen at all in differentiated neuronal tissues [16]. Similarly, enhanced endogenous apoptosis was seen in the Lig4Y288C SVZ that was equivalent to levels in the WT SVZ induced by 15–20 mGy (assessed by extrapolation) (Fig. 3B). The Lig4Y288C SGZ did not display elevated endogenous apoptosis, consistent with the diminished sensitivity to activation of apoptosis in IR-exposed WT mice [16]. Significantly, the WT SVZ, SGZ and differentiated neuronal tissues showed a similar rate of DSB repair [16]. Thus, sensitive activation of apoptosis in the SVZ is not a consequence of a failure to repair DSBs.
Fig. 3.
Endogenous and IR-induced DNA damage and apoptosis in the adult SVZ of 2–3 month-old mice. (A) 53BP1 foci dose response in the SVZ of WT mice 1.5 h after exposure to 50–100 mGy X-rays (black circles). Endogenous level of 53BP1 foci in the SVZ of Lig4Y288C mice (red triangle). (B) Dose-dependent increase in apoptosis in the SVZ of adult WT mice 6 h after exposure to 50–100 mGy X-rays (black circles). Endogenous level of apoptosis in the SVZ of Lig4Y288C mice (red triangle). Importantly, 53BP1 foci and TUNEL levels in the adult Lig4Y288C SVZ are substantially reduced compared with that at E14.5 [16]. (C) Comparison of endogenous and IR-induced apoptosis in the E14.5 embryonic neocortex and in the adult SVZ. The embryonic sensitivity to apoptosis is maintained in the adult SVZ, although the response of specific subsets of stem cells has not yet been examined. ut stands for untreated. [These figures are based on original data described in reference 16.].
Collectively, these findings demonstrate that the adult SVZ, as distinct from the embryonic neocortex, is not characterized by high DSB formation, most likely due to the lower level of proliferating cells and/or slower rate of proliferation. Strikingly, however, apoptosis is sensitively activated in the adult SVZ. Thus, sensitive activation of apoptosis is not a direct consequence of rapid proliferation.
HIGH ENDOGENOUS APOPTOSIS IN THE NEONATAL SVZ
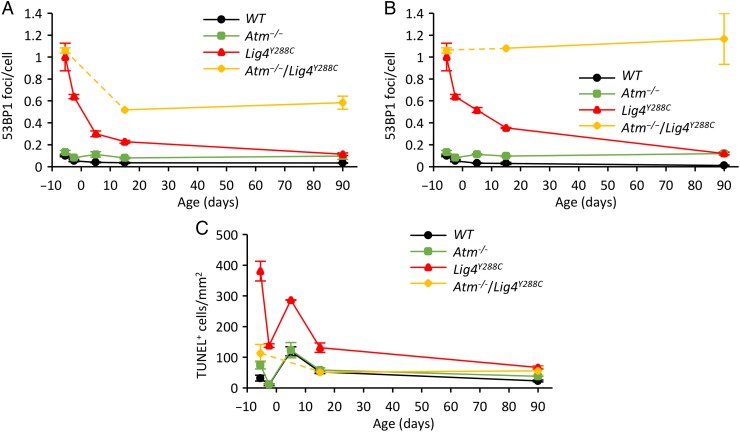
Exposure of the brain of neonatal mice and children to radiation can lead to radiation-induced carcinogenesis [7, 26, 27]. To gain insight into the causal factors and to examine the fate of cells with DSBs in the embryonic neocortex, we undertook a temporal analysis, examining DSB levels and apoptosis in P5 and P15 neonates [16]. Additionally, we compared these endpoints in the neonatal SVZ and isocortex, a differentiated zone also derived from the embryonic VZ/SVZ. 53BP1 foci levels in Lig4Y288C neonates (P5 and P15) were intermediate between the high level in the embryonic neocortex at E14.5 and the lower level observed in the adult brain (Fig. 4A and B), a finding consistent with the notion that cells with DSBs can be transmitted to neonates and undergo slow repair. An alternative explanation, which is difficult to eliminate, is that DSBs continue to form in neonatal mice. A similar trend is observed in WT mice, although the difference is less marked due to efficient DSB repair (Fig. 4A). A striking observation is that DSB levels in Lig4Y288C mice were lower in the SVZ compared to those in the isocortex at P5 and P15 (compare Fig. 4A and B) [16]. A similar finding was observed in Atm−/−/Lig4Y288C mice (discussed below).
Fig. 4.
Endogenous level of DNA damage and apoptosis at different ages. (A) In vivo time course analysis of endogenous 53BP1 foci formation in the SVZ from E14.5 (5.5 days before birth), E17.5, P5, P15 and into adulthood (90 days post birth). Note that time 0 is the time of birth and minus numbers represent embryonic times before birth. In WT and Lig4Y228C mice, the level of DSBs diminishes with age, reaching a steady state level 2–3 months after birth, in contrast with the trend shown in mice deficient in Atm. (B) As for ‘A’, except that the analysis is carried out in the isocortex at times P5 and P15, and in adult mice. (C) In vivo time-course analysis of the endogenous level of apoptosis from the embryonic neocortex to the adult SVZ, showing the presence in the neonatal SVZ of a developmentally regulated ATM-independent apoptotic process occurring at P5. [The data is taken from reference 16.].
Examination of apoptosis in neonatal mice additionally revealed high endogenous apoptosis in the SVZ at this stage (Fig. 4C). Moreover, this endogenous apoptosis was ATM-independent, whereas most radiation-induced apoptosis in the SVZ is ATM-dependent [16].
Collectively, these findings reveal the presence of a process that diminishes DSBs in the neonatal SVZ compared with in its differentiated counterpart (Fig. 4A and B) [16]. They also reveal an ATM-independent process of endogenous apoptosis in the neonatal SVZ.
ANALYSIS OF ATM−/−/LIG4Y288C DOUBLE MUTANT MICE
ATM plays the predominant role in activating radiation-induced apoptosis (at doses less than 500 mGy) in the neural embryonic and adult stem cell compartments. ATM, however, is dispensable for the endogenous apoptosis at P5/P15 (Fig. 4C). Atm−/− mice display a small increase in 53BP1 foci in some tissues (Fig. 4B). In fibroblasts, ATM is dispensable for most DSB repair, but is essential for the repair of a ∼15% subset of DSBs induced by X-rays, which represent those DSBs repaired with slow kinetics [29]. ATM also influences repair by activating checkpoint arrest and apoptosis [30]. To further an understanding of ATM's role in neuronal cells, we generated Atm−/−/Lig4Y288C double mutant mice. Atm−/− /Lig4Y288C mice are born at sub-Mendelian ratios, substantially restricting the extent of analysis. At E14.5 Atm−/−/Lig4Y288C embryos had similar 53BP1 foci levels to Lig4Y288C embryos, suggesting that they incur a similar induction of DSBs during the phase of rapid proliferation (Fig. 4A). Endogenous apoptosis at E14.5 in Atm−/−/Lig4Y288C was substantially less than that seen in Lig4Y288C embryos, consistent with the notion that this apoptosis is predominantly ATM-dependent (Fig. 4C). However, whereas in Lig4Y288C mice, 53BP1 foci numbers diminish from E17.5, P5, P15 to a low number at 2–3 months, which we assume arises from diminished DSB formation coupled with slow repair, foci numbers in Atm−/−/Lig4Y288C mice remain high at P15 and 2–3 months, suggesting an impeded capacity to repair the DSBs (Fig. 4A and B). Indeed, even in adult Atm−/−/Lig4Y288C mice, 53BP1 foci numbers in most tissues were similar to that observed at E14.5 (Fig. 4B). Strikingly, similar to the situation with Lig4Y288C mice, lower numbers of 53BP1 foci were observed in the SVZ compared with in the isocortex. However, whereas in the Lig4Y288C mice no marked difference was seen when comparing 53BP1 foci in the adult SVZ and isocortex, a substantial difference remained at this stage in the Atm−/−/Lig4Y288C mice, where foci numbers in the SVZ were lower than in the isocortex (Fig. 4A and B).
These findings suggest either that Atm−/−/Lig4Y288C mice have a more dramatic defect in DSB repair compared with Lig4Y288C mice or that more DSBs arise during adulthood, possibly due to enhanced oxidative stress. We were unable to assess the DSB repair capacity of Atm−/−/Lig4Y288C mice in vivo due to the low number of viable mice. However, DSB repair measurements in confluent arrested Lig4Y288C MEFs with or without treatment with an ATM inhibitor (ATMi) showed that ATMi addition reduced the DSB repair rate, albeit to a much lesser extent than observed in vivo [16]. However, failure to activate apoptosis and/or checkpoint arrest in the absence of ATM could underlie the greater impact in vivo. Although the precise explanation underlying the findings with Atm−/−/Lig4Y288C mice requires further analysis, the striking observation is that DSB levels are diminished in the neonatal and adult SVZ compared with those in the differentiated counterpart.
SENSITIVE ACTIVATION OF APOPTOSIS IN STEM CELL COMPARTMENTS
The embryonic brain, the hematopoietic system and the small intestinal crypt are highly radiosensitive tissues [10, 11, 31, 32]. Significantly, apoptosis can be readily activated by low-dose radiation in these tissues [4, 21, 33]. Differentiated cells in the colon are located in folds called villi and are lost by shedding at the tip of the villi [34]. The stem and progenitor cells are located at the base of the villi, called the crypt. Detailed studies of cycling intestinal stem cells (ISCs) have shown that they readily activate apoptosis [35–37]. The crypt base columnar (CBC) stem cells are found at the crypt base and stain positively for Lgr5, an intestinal stem cell marker [38]. Only ∼10–30% of the Lgr5+ stem cells are proliferating at any one time [38, 39]. A quiescent subcomponent of Lgr5+ CBCs, which are believed to be located at a position four cell layers above the bottom of the crypt (designated the +4 position), in contrast to proliferating stem cells, are resistant to apoptosis [35, 40, 41]. This suggests a model whereby the dormant stem cells are resistant to apoptosis, but once they enter the cell cycle, they become able to sensitively activate apoptosis [42].
The hematopoietic system, like the intestinal crypt, has a high turnover rate and the stem cells can be dormant or proliferating [43, 44]. The stem cells with long-term reconstituting potential, the long-term reconstituting hematopoietic stem cells (LT-HSCs), are in a distinct niche and in a quiescent state [45]. Short-term reconstituting HSCs (ST-HSCs) have reduced reconstituting potential. Multipotent progenitor cells (MPPs) and common myeloid progenitors (CMG) have even more restricted proliferation capacity. Although the hematopoietic system has long been known to activate radiation-induced apoptosis, recent studies have shown that LT-HSCs do not undergo apoptosis nor activate p53 but do activate p21. ST-HSCs show a more intermediate response but are also relatively resistant to apoptosis, whereas MPPs and CMPs are dramatically sensitive to X-ray–induced apoptosis [46]. Significantly, LT-HSCs can enter the cell cycle and expand following radiation damage. Thus, like the crypt stem cells, the dormant stem cells appear resistant to apoptosis, whereas the progenitors are exceptionally sensitive.
The embryonic neural VZ/SVZ is rapidly proliferating and has few quiescent cells. The VZ/SVZ cells sensitively activate apoptosis, but the relative sensitivity of the VZ versus the SVZ cells has not been examined (see Fig. 5 for a summary of this and the following discussion). The first differentiated layer, the IZ, has reduced sensitivity but still activates apoptosis; apoptosis is rarely activated in the differentiated cortical plate. The adult SVZ has a low turnover rate, with most stem cells being quiescent, although there are proliferating stem and progenitor cells. Our findings show that apoptosis is also sensitively activated in this compartment, with the overall level being similar to that in the embryo. However, the distinct responses of quiescent versus proliferating or progenitor stem cells has not been examined, and it is possible that resistant and sensitive subpopulations exist. One study reported the resistance of quiescent neural stem cells to 4 Gy X-rays, in contrast to the marked sensitivity of the progenitor cells [47]. Further work is required to consolidate whether, as in the crypt and HSCs, quiescent neural stem cells are resistant to apoptosis in contrast to the sensitive progenitor cells. It is also noteworthy that the embryonic brain fails to activate a G1/S checkpoint arrest, which may correlate with the sensitive apoptotic activation [48]. It will also be important to understand the basis underlying the sensitive activation of apoptosis in the progenitor stem cells versus the resistance of the quiescent stem cells. In the HSCs, this has been attributed to p21 activation, but Bcl2 expression may also play a significant role [46].
Fig. 5.
A graphical summary of our findings examining the response of the neural stem cell compartments (from the embryonic stage, P5 and adult) to endogenous damage and that created by low-dose radiation exposure. SVZ represents the subventricular zone.
CONSEQUENCES FOR LIG4 SYNDROME PATIENTS
The embryonic brain is characterized by high DSB formation (most likely a consequence of rapid proliferation) as well as sensitive activation of apoptosis. In the LIG4 Syndrome mouse model, the cortical plate is thinned relative to WT mice, which could be a consequence of cell loss by apoptosis [21]. However, the magnitude of endogenous apoptosis is low and it is unclear if the markedly smaller neocortex can be entirely attributed to apoptosis. Indeed, it is possible that unrepaired DSBs can influence other endpoints, such as a switch from symmetric to asymmetric cell division, which is essential for establishing sufficient stem cells for normal neuron generation [9]. Our findings support the notion that neural stem and differentiated cells with DSBs can be transmitted from the embryo to the neonatal mouse brain, however, the possibility that further DSBs arise post birth cannot be eliminated. Although the role of the adult SVZ is unclear, it represents a slowly proliferating compartment and fails to fully replenish neurons that do not develop during embryogenesis. Consequently, the microcephaly evident at birth is not redeemed post birth. Indeed, there is evidence that microcephaly could be progressive post birth in LIG4 Syndrome patients [9]. This could be a consequence of our finding that the adult SVZ in Lig4Y288C mice also has elevated spontaneous DSBs and apoptosis. Further studies are required to establish whether elevated apoptosis is the sole causal factor underlying microcephaly in Lig4Y288C mice and LIG4 Syndrome patients. It is also unclear whether such elevated apoptosis in the adult SVZ can cause neural stem cell depletion with age.
REDUCED DSB LEVELS IN THE STEM CELL COMPARTMENT
DSB levels in Lig4Y288C mice were high in the embryonic VZ/SVZ at E14.5 and declined slowly post-natally. From 2–3 months, DSB levels remained at a slightly increased level relative to WT mice. Although it is difficult to eliminate the possibility that endogenous DSBs arise at a higher frequency in neonatal mice and slowly decline, our findings appear more consistent with the notion that cells with DSBs can be transmitted to the offspring, and undergo slow repair. Strikingly, we observed lower DSBs levels in the SVZ of P5 and P15 Lig4Y288C neonatal mice compared with those in the differentiated isocortex, which, like the SVZ, is derived from the embryonic VZ/SVZ. This difference is not evident by 2–3 months, when most DSBs have undergone repair. These findings raise the possibility that there is a process that promotes elimination of cells with DSBs or reduces the average DSB levels in the SVZ during the neonatal period. However, the neonatal SVZ and isocortex showed identical DSB repair capacity. Significantly, we observed high endogenous apoptosis at P5–P15. We suggest that the compartmental size reduction at P5/P15 enhances selection for stem cells with the least level of breakage; in other words, the process selects for the ‘fittest’ stem cells. This elimination of the more damaged stem cells could result from apoptosis or differentiation, or could also arise as a consequence of proliferation, which is higher at this stage compared with in the adult SVZ. Significantly, a similar and more marked finding is seen in Atm−/−/Lig4Y288C mice, where the DSB levels in differentiated tissues remain similar to the embryonic levels, in contrast to those in the SVZ.
FUTURE PERSPECTIVE
In summary, these studies with low-dose radiation and Lig4Y288C mice have demonstrated the exquisite sensitivity to activation of apoptosis in both the embryonic and adult neural stem cell compartments. Translating these results to the situation in LIG4 Syndrome patients, we obtain insight into how unrepaired DSBs in neural stem cells can influence development. Further studies are required in order to assess the long-term consequences of low endogenous apoptosis or low-dose radiation-induced apoptosis in the adult brain, including whether it can promote neural stem cell depletion. Additional questions remain: for example, are all stem cells equally susceptible to apoptosis or do the dormant and proliferating stem cells differ in their response, as observed in the crypt and HSC. It is also important to assess whether this exquisite sensitivity is a general feature of all stem cells or defines the response of the more radiosensitive tissues. However, it will be critical to undertake these experiments in vivo since increasing evidence suggests that the environmental niche and surrounding tissue may influence the response. Finally, studies are also required to establish the signalling cascade that underlies the exquisite sensitivity to activation of apoptosis in the SVZ compartment.
FUNDING
This work was supported by the EU Seventh Framework Programme (FP7) Risk, Stem Cells and Tissue Kinetics-Ionising Radiation (RISK-IR) project (grant agreement number 323267).
ACKNOWLEDGEMENTS
This data has been presented at the International Conference on Radiation Research at Kyoto, Japan, 25–29 May 2015.
REFERENCES
- 1.Jeggo PA, Lobrich M. How cancer cells hijack DNA double-strand break repair pathways to gain genomic instability. Biochem J 2015;471:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Petermann E, Helleday T. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol 2010;11:683–7. [DOI] [PubMed] [Google Scholar]
- 3.Jeggo P, Lavin MF. Cellular radiosensitivity: how much better do we understand it. Int J Radiat Biol 2009;85:1061–81. [DOI] [PubMed] [Google Scholar]
- 4.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 1977;269:518–21. [DOI] [PubMed] [Google Scholar]
- 5.Mandal PK, Blanpain C, Rossi DJ. DNA damage response in adult stem cells: pathways and consequences. Nat Rev Mol Cell Biol 2011;12:198–202. [DOI] [PubMed] [Google Scholar]
- 6.El-Saghire H, Thierens H, Monsieurs P et al. Gene set enrichment analysis highlights different gene expression profiles in whole blood samples X-irradiated with low and high doses. Int J Radiat Biol 2013;89:628–38. [DOI] [PubMed] [Google Scholar]
- 7.Pearce MS, Salotti JA, Little MP et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012;380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodbine L, Gennery AR, Jeggo PA. The clinical impact of deficiency in DNA non-homologous end-joining. DNA Repair 2014;16C:84–96. [DOI] [PubMed] [Google Scholar]
- 9.Murray JE, Bicknell LS, Yigit G et al. Extreme growth failure is a common presentation of ligase IV deficiency. Hum Mutat 2014;35:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino K, Kameyama Y. Developmental-stage-dependent radiosensitivity of neural cells in the ventricular zone of telencephalon in mouse and rat fetuses. Teratology 1988;37:257–62. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino K, Kameyama Y, Inouye M. Split-dose effect of X-irradiation on the induction of cell death in the fetal mouse brain. J Radiat Res 1991;32:23–7. [DOI] [PubMed] [Google Scholar]
- 12.O'Driscoll M, Cerosaletti KM, Girard P-M et al. DNA Ligase IV mutations identified in patients exhibiting development delay and immunodeficiency. Mol Cell 2001;8:1175–85. [DOI] [PubMed] [Google Scholar]
- 13.Barnes DE, Stamp G, Rosewell I et al. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr Biol 1998;8:1395–8. [DOI] [PubMed] [Google Scholar]
- 14.Frank KM, Sekiguchi JM, Seidl KJ et al. Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature 1998;396:173–7. [DOI] [PubMed] [Google Scholar]
- 15.Nijnik A, Woodbine L, Marchetti C et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature 2007;447:686–90. [DOI] [PubMed] [Google Scholar]
- 16.Barazzuol L, Rickett N, Ju L et al. Endogenous and X-ray-induced DNA double strand breaks sensitively activate apoptosis in adult neural stem cells. J Cell Sci 2015;128:3579–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayer SA, Altman J, Dai XF et al. Planar differences in nuclear area and orientation in the subventricular and intermediate zones of the rat embryonic neocortex. J Comp Neurol 1991;307:487–98. [DOI] [PubMed] [Google Scholar]
- 18.Mitsuhashi T, Takahashi T. Genetic regulation of proliferation/differentiation characteristics of neural progenitor cells in the developing neocortex. Brain Dev 2009;31:553–7. [DOI] [PubMed] [Google Scholar]
- 19.Pontious A, Kowalczyk T, Englund C et al. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci 2008;30:24–32. [DOI] [PubMed] [Google Scholar]
- 20.Thornton GK, Woods CG. Primary microcephaly: do all roads lead to Rome. Trends Genet 2009;25:501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatz SA, Ju L, Gruber R et al. Requirement for DNA ligase IV during embryonic neuronal development. J Neurosci 2011;31:10088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha S, Woodbine L, Haines J et al. (10 September 2014) Increased apoptosis and DNA double-strand breaks in the embryonic mouse brain in response to very low-dose X-rays but not 50 Hz magnetic fields. J R Soc Interface 11, 10.1098/rsif.2014.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 2006;7:179–93. [DOI] [PubMed] [Google Scholar]
- 24.Luo J, Daniels SB, Lennington JB et al. The aging neurogenic subventricular zone. Aging Cell 2006;5:139–52. [DOI] [PubMed] [Google Scholar]
- 25.Capilla-Gonzalez V, Herranz-Perez V, Garcia-Verdugo JM. The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front Cell Neurosci 2015;9:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner D, Elliston C, Hall E et al. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roentgenol 2001;176:289–96. [DOI] [PubMed] [Google Scholar]
- 27.Pettorini BL, Park YS, Caldarelli M et al. Radiation-induced brain tumours after central nervous system irradiation in childhood: a review. Childs Nerv Syst 2008;24:793–805. [DOI] [PubMed] [Google Scholar]
- 28.Stiff T, O'Driscoll M, Rief N et al. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 2004;64:2390–96. [DOI] [PubMed] [Google Scholar]
- 29.Riballo E, Kuhne M, Rief N et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell 2004;16:715–24. [DOI] [PubMed] [Google Scholar]
- 30.Kastan MB, Zhan Q, El-Deiry WS et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 1992;71:587–97. [DOI] [PubMed] [Google Scholar]
- 31.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 2004;161:123–36. [DOI] [PubMed] [Google Scholar]
- 32.Mauch P, Constine L, Greenberger J et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319–39. [DOI] [PubMed] [Google Scholar]
- 33.Meng A, Wang Y, Brown SA et al. Ionizing radiation and busulfan inhibit murine bone marrow cell hematopoietic function via apoptosis-dependent and -independent mechanisms. Exp Haematol 2003;31:1348–56. [DOI] [PubMed] [Google Scholar]
- 34.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell 2013;154:274–84. [DOI] [PubMed] [Google Scholar]
- 35.Potten CS, Grant HK. The relationship between ionizing radiation–induced apoptosis and stem cells in the small and large intestine. Br J Cancer 1998;78:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potten CS, Gandara R, Mahida YR et al. The stem cells of small intestinal crypts: where are they? Cell Prolif 2009;42:731–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. BioEssays 2002;24:91–8. [DOI] [PubMed] [Google Scholar]
- 38.Barker N, van Es JH, Kuipers J et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]
- 39.Kozar S, Morrissey E, Nicholson AM et al. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 2013;13:626–33. [DOI] [PubMed] [Google Scholar]
- 40.Ijiri K, Potten CS. The re-establishment of hypersensitive cells in the crypts of irradiated mouse intestine. Int J Radiat Biol Relat Stud Phys Chem Med 1984;46:609–23. [DOI] [PubMed] [Google Scholar]
- 41.Hua G, Thin TH, Feldman R et al. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 2012;143:1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Huang YF, Kek C et al. Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell 2013;12:298–303. [DOI] [PubMed] [Google Scholar]
- 43.Babovic S, Eaves CJ. Hierarchical organization of fetal and adult hematopoietic stem cells. Exp Cell Res 2014;329:185–91. [DOI] [PubMed] [Google Scholar]
- 44.Wabik A, Jones PH. Switching roles: the functional plasticity of adult tissue stem cells. EMBO J 2015;34:1164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunisaki Y, Bruns I, Scheiermann C et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013;502:637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Insinga A, Cicalese A, Faretta M et al. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc Natl Acad Sci U S A 2013;110:3931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daynac M, Chicheportiche A, Pineda JR et al. Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem Cell Res 2013;11:516–28. [DOI] [PubMed] [Google Scholar]
- 48.Roque T, Haton C, Etienne O et al. Lack of a p21waf1/cip -dependent G1/S checkpoint in neural stem and progenitor cells after DNA damage in vivo. Stem Cells 2012;30:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]