Abstract
The current Zika virus (ZIKV) outbreak is associated with high numbers of human congenital birth defects, yet it is unclear how ZIKV infection during pregnancy causes these abnormalities. Three new mouse models now show that ZIKV crosses the placenta and replicates in the brains of fetal mice.
Zika virus (ZIKV) is a positive-stranded RNA virus of the Flaviviridae family, which includes the notable human pathogens Dengue (DENV), West Nile (WNV), and yellow fever (YFV) viruses. ZIKV was first isolated in 1947 from the blood of a febrile rhesus macaque in the Zika Forest of Uganda. There have been relatively few documented cases of human ZIKV infection prior to the recent outbreaks in Micronesia and French Polynesia and pandemic spread throughout Central and South America. Most ZIKV infections are acquired through the bite of an Aedes species mosquito and the broadening global distribution of these vectors increases the risk of further spread into non-immune populations, such as North America. Currently there are no licensed vaccines or therapeutics against ZIKV.
Infection with ZIKV is asymptomatic and self-limiting in most individuals, generally resolving within a week. Clinical symptoms, when present, can include fever, rash, arthritis, myalgia, and conjunctivitis. Recent ZIKV outbreaks have been linked to a number of more severe neurological sequelae, including Guillain-Barré Syndrome and meningoencephalitis. Increasing amounts of data indicate that infection of pregnant women with ZIKV can result in devastating fetal abnormalities, including microcephaly (significantly smaller head size than normal infants), ocular deformation, and spontaneous abortion. The underlying cause(s) for these disease manifestations are incompletely understood. Because many flaviviruses exhibit neurotropism, and ZIKV has been isolated in the brain of infected human fetuses (Martines et al., 2016; Mlakar et al., 2016), understanding how the virus is vertically transmitted and gains access into the developing nervous system of the fetus is of critical importance.
Due to the historical paucity of human infection with ZIKV, there have been no animal models developed to study ZIKV pathogenesis or vaccine-generated protective immunity. While the most common scientifically utilized strain of mice (C57BL/6) does not support widespread ZIKV replication post-infection (p.i.) and does not exhibit clinical symptoms, several groups have recently reported murine models which are permissive for viral replication in multiple organs including the brain (Aliota et al., 2016; Dowall et al., 2016; Lazear et al., 2016; Rossi et al., 2016; Zmurko et al., 2016). Three recent reports in Nature (Cugola et al., 2016), Cell (Miner et al., 2016) and Cell Stem Cell (Li et al., 2016) have extended these important studies to describe mouse models where ZIKV infection results in fetal birth defects stemming from viral replication in the placenta and brain (Figure 1).
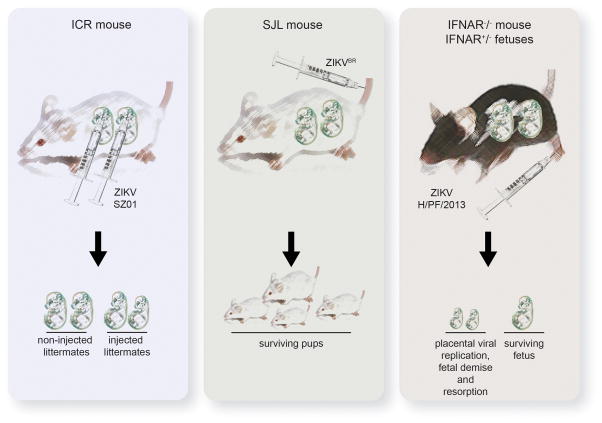
Figure 1. Current Murine Models for ZIKV-Induced Fetal Abnormalities.
Left panel) ICR fetuses carefully injected intracerebrally exhibit viral infection of the brain and reduced brain size by 5 days post-infection. Middle panel) SJL mice infected with a Brazilian ZIKV isolate give birth to pups with whole-body growth delay and IUGR. Viral replication can be detected in fetal brains. Right panel) After ZIKV-infection of IFNAR−/− dams carrying IFNAR+/− heterozygous fetuses, the virus replicates in placental trophoblasts and neurons. Most fetuses undergo demise and resorption, while surviving fetuses exhibit IUGR.
To understand the capacity for ZIKV to infect embryonic neurons, Li et al. meticulously injected ~105 PFU (plaque forming units) of ZIKV strain SZ01 (an Asian isolate) directly into the cerebroventricular space of ICR strain mouse fetuses (bypassing maternal immunity) at embryonic day (d) 13.5 (Li et al., 2016). ZIKV infection of neurons was detected by both immunohistochemistry and PCR, with a 300-fold increase in viral RNA by d3 p.i. After 5d, the brains of infected fetuses were smaller than those of mock-infected littermates with prominent cell death in infected brains. Thus, ZIKV can replicate in the embryonic brain and decrease brain size once the virus gains access to this compartment.
While intracerebral inoculation yields an important proof-of-principle, would a more physiological route of infection result in ZIKV replication in the brain? To address this, Miner et al. developed two murine models of ZIKV-induced birth defects (Miner et al., 2016). In the first, the authors built upon their previous work demonstrating that adult mice deficient in the production of and response to type I interferon (IFNAR−/−) support high levels of ZIKV infection (Lazear et al., 2016). Extending these observations, IFNAR−/− mice were bred with wild-type male mice, generating IFNAR+/− heterozygous fetuses with intact interferon signaling. Pregnant dams were inoculated on gestational d 6.5 or 7.5 in the footpad with 103 PFU of a clinical isolate from French Polynesia ((H/PF/2013) genetically similar to contemporary Brazilian ZIKV isolates), and fetuses were examined prior to the onset of maternal symptoms. Importantly, by 7d p.i., most of the fetuses underwent demise and were resorbed, leaving only placental remnants. Surviving fetuses exhibited significant intrauterine growth restriction (IUGR) and infectious virus was isolated from both the placenta and fetal head. Intriguingly, the placenta exhibited 1000-fold greater ZIKV RNA than in the mother’s serum. The authors recapitulated these findings in a second model in which pregnant wild-type mice were treated with an IFNAR-blocking antibody prior to infection. Again, the authors observed significant IUGR and ZIKV RNA was detected in fetal heads. However, the highest level of viral RNA was again found in the placenta. Thus, the authors established preferential replication in the placenta using two separate models of murine ZIKV infection, suggesting that placental injury may represent the route in which the virus gains access to fetuses and contributes to IUGR.
In the third study, Cugola et al. first isolated a strain of ZIKV from a febrile patient in Brazil (ZIKVBR) and used it to infect pregnant adult SJL mice intravenously (i.v.) with an extraordinarily high inoculum of ZIKVBR (1011 PFU) from gestational d 10 (Cugola et al., 2016). Of note, SJL mice are immunocompetent animals commonly used in muscular dystrophy and autoimmune encephalomyelitis models; however SJL mice are more susceptible to other viral infections and exhibit known immune irregularities, including a lack of natural killer cells (Chastain et al., 2015; Hsu et al., 2012). Intriguingly, pups from infected mothers exhibited whole-body growth delay or IUGR. Although microcephaly did not occur, cortical malformations were present in surviving pups characteristic of microcephalic symptoms in humans. Viral replication in the brain in this model was demonstrated by PCR detection of viral genomic RNA. Thus, ZIKV can induce murine birth defects in the presence of a seemingly more intact maternal innate immune system.
Together, using 3 distinct methods, these studies show that ZIKV readily infects placental trophoblasts and neurons in vivo, and that murine fetuses with infected brains demonstrate decreased brain/head size, similar to human microcephaly. As with any important scientific advance, these reports raise a number of questions. First, are all ZIKV strains –including those of the African lineage –equally capable of infecting a fetus and inducing neurological birth defects? What role do interferons play in preventing placental infection, particularly during different stages of pregnancy? Is ZIKV-induced neuronal damage a direct result of infection, or does placental insufficiency along with an ensuing immune response contribute to pathogenesis?
Regardless of the answers, these new murine models are poised to answer a number of crucial questions regarding protective immunity to ZIKV infection and transmission to the unborn. For instance, what level of replication is needed to infect/breach the placenta and result in fetal growth restriction? Beyond potential utility of mouse models in the preclinical testing of vaccine candidates, the identification of a viral set point for vertical transmission in these models may inform human vaccine evaluation studies with the ultimate goal of preventing fetal infections. Furthermore, the systematic evaluation of these models will enable fundamental and translational research opportunities that inform strategies to blunt the global impact of this emerging pathogen.
Acknowledgments
This work was funded by the intramural program of the National Institute of Allergy and Infectious Disease to the Division of Intramural Research. We apologize for the many instances in which the important work of others is not cited due to formatting constraints of this article type.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain EM, Getts DR, Miller SD. Deficient Natural Killer Dendritic Cell Responses Underlay the Induction of Theiler’s Virus-Induced Autoimmunity. MBio. 2015;6:e01175. doi: 10.1128/mBio.01175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016 doi: 10.1038/nature18296. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl Trop Dis. 2016;10:e0004658. doi: 10.1371/journal.pntd.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TH, Althaus IW, Foreman O, Spindler KR. Contribution of a single host genetic locus to mouse adenovirus type 1 infection and encephalitis. MBio. 2012;3 doi: 10.1128/mBio.00131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, Zhang N, Shi L, Qin CF, Xu Z. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D, et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–160. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016 doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J. The Viral Polymerase Inhibitor 7-Deaza-2′-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl Trop Dis. 2016;10:e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]