Abstract
The proportions of new cancer cases and deaths that are caused by exposure to risk factors and that could be prevented are key statistics for public health policy and planning. This paper summarizes the methodologies for estimating, challenges in the analysis of, and utility of, population attributable and preventable fractions for cancers caused by major risk factors such as tobacco smoking, dietary factors, high body fat, physical inactivity, alcohol consumption, infectious agents, occupational exposure, air pollution, sun exposure, and insufficient breastfeeding. For population attributable and preventable fractions, evidence of a causal relationship between a risk factor and cancer, outcome (such as incidence and mortality), exposure distribution, relative risk, theoretical-minimum-risk, and counterfactual scenarios need to be clearly defined and congruent. Despite limitations of the methodology and the data used for estimations, the population attributable and preventable fractions are a useful tool for public health policy and planning.
Keywords: Risk, Neoplasms, Etiology, Prevention and control, Mortality, Incidence, Population Attributable Fraction
Introduction
The impacts of behavioral and environmental risk factors on disease have long been studied, and quantifying such impacts has been a major public health objective in order to guide prevention and policy [1, 2]; specifically, for cancer [3]. The proportion of an outcome that would have not occurred in a population over a given period of time by decreasing a population‘s exposure to a risk factor, firstly to a theoretical-minimum-risk, and secondly to an attainable level, are known respectively as the population attributable fraction (PAF) and the population preventable fraction (PPF) [4]. The attributable fraction was developed by Levin in 1953 for estimation of the “maximum proportion of lung cancer attributable to smoking” [2]. It was further elaborated on in the 1970s [5–8] when the etiologic fraction (a similar but conceptually distinct fraction) was developed [1]. At the same time, the concept of a preventable fraction, defined as the potential proportion of outcomes that was prevented by a protective risk factor, was introduced [1]; however, the the current definition of a preventable fraction differs from this original definition [9]. The methodologies to estimate cancer PAFs and PPFs continue to develop, differ by subtype, and are dependent on etiology [10, 11]. Accordingly, this review aims to provide updated and globally applicable methodologies for linking risk factors to cancer incidence and mortality.
Population attributable and preventable fraction studies
Numerous, national, regional, and international PAF and PPF estimation studies have estimated the attributable and/or preventable cancers due to either a specific risk factor or multiple risk factors. Risk factors are selected based on the level of evidence for a causal relationship, relevancy of the risk factors for population health, availability and quality of population-representative data, and if the risk factors are avoidable [10–12]. The Comparative Risk Assessment (CRA) studies require sufficient causal evidence [13]; see also [14, 15] for an outline of the different levels of causal evidence. Commonly studied factors include tobacco smoking, diet, alcohol consumption, infectious agents, occupational exposure, air pollution, sun exposure (ultraviolet radiation (UVR)), insufficient or decreased duration of breastfeeding, body mass index (BMI) (a measure of body fat), and physical inactivity [10, 11]. Among infectious agents, relevancy is a large consideration: for example, in a United Kingdom study [10], infections of clonorchis sinensis, opisthorchis viverrini and schistosoma haematobium were excluded because of their low infection rate [16]. Other risk factors, including oral contraceptive use, hormone replacement therapy (HRT), lack of aspirin use, and medical radiation exposure [10, 11] have also been assessed; however, interpretation of the burden caused by these factors is challenging due to the effects of these risk factors on other aspects of health [10, 11]. Lastly, risk factors for cancer such as number of births and age at first birth [17] are generally not modelled as policies changing these risk factors are not politically acceptable in many countries [18].
Methods to estimate the population attributable and preventable fractions
The PAFs and PPFs are estimated by comparing the risk of cancer for populations under past and/or current conditions as compared to a counterfactual scenario [19]. For the PAFs, the population risk under the counterfactual scenario is the theoretical-minimum-risk (see Box 1 for a definition). However, for the PPFs, the population risk under the counterfactual scenario is the risk exposure distribution that is attainable (either through the achievement of a health target (see [20]) or through the implementation of one or various policies [21]). The main differences between PAFs and PPFs are outlined in Table 1. See Box 1 for an overview of PAF and PPF terminology.
Box 1. Population attributable fraction (PAF) and population preventable fraction (PPF) terminology.
Attributable case (excess case): A case that would not have occurred if the person was not exposed to a given risk factor.
Attributable benefit: The fraction by which the occurrence of a disease of interest would be increased under an alternative exposure distribution.
Comparative Risk Assessment: The systematic evaluation of changes in population health which result from modifying the population distribution of exposure to a risk factor or a group of risk factors.
Counterfactual: An alternative scenario for a given place and time that has already past, whereby conditions of the scenario that has already occurred are altered (such as a cancer risk exposure which then results in a different number of cancer incident and mortality cases).
Excess risk model: A method of estimating the attributable or preventable fraction based on the number of observed cases as compared to the number of expected cases (based on either a predictive model or data from a reference population). This method is employed for smoking-attributable fractions and for sun exposure-attributable fractions, and is approximated using the following formula:
where casesobs represents the cases under the factual scenario and casesexpected represents the expected number of cases.
Impact risk: The success rate in changing the risk factor through an intervention program.
Impact number: The relative efficacy of the intervention, that is, the extent to which a successful change in a risk factor results in a risk reduction to the risk level of persons never exposed.
Population attributable fraction: The fraction by which the occurrence of a disease of interest would be reduced under an alternative exposure distribution (or counterfactual) during a given period in a given population. This exposure distribution is the theoretical-minimum-risk.
Population preventable fraction: The fraction by which the occurrence of a disease of interest would be reduced under an alternative exposure distribution (or counterfactual) during a given period and in a given population. This exposure distribution is achieved through the implementation of interventions or through achieving health targets.
PAF and PPF formulas:
Categorical exposure based PAF or PPF:
Continuous exposure based PAF:
Case based (for adjusted RR) PAF:
Combination of multiple PAFs:
where P represents the prevalence, PD represents the prevalence of cases where there was exposure to a risk factor and RR represents the relative risk given either xc or xcf which represents the current exposure distribution and the counterfactual exposure distribution respectively (either categorically or continuously). For the combination formula, T represents the total PAF or PPF, and n represents a risk factor-specific PAF or PPF. See [68] for other commonly used PAF formulas.
Theoretical-minimum-risk: The level of exposure to a risk factor that would result in the lowest overall burden.
Table 1.
Population attributable fractions compared to population preventable fractions as applied to cancer risk factor surveillance and to cancer policy projection
| Input/ Output | Population attributable fraction | Population preventable fraction |
|---|---|---|
| Reference exposure scenario | The current or historical exposure distribution in the entire population |
The current or historical exposure distribution in the entire population |
| Reference exposure group (reference group for the RR used) |
Theoretical-minimum-risk | Theoretical-minimum-risk |
| Counterfactual scenario | Everyone at the theoretical- minimum-risk |
Counterfactual scenario of an attainable decrease in risk factor exposure (e.g., an exposure decrease through the implementation of an intervention or through the achievement of a health target) |
| Outcomes | Deaths, years of life lost, years lived with disability, Disability Adjusted Life Years, Quality Adjusted Life Years, monetary units, health resources (such as hospital stays) |
Deaths, years of life lost, years lived with disability, Disability Adjusted Life Years, Quality Adjusted Life Years, monetary units, health resources (such as hospital stays) |
| Main aim | Estimate the proportion of an outcome that would not be present in a given population under the assumption that everyone had the theoretical-minimum-risk for a given risk factor during a specified time period |
Assess the impact of implementing interventions and/or reaching health targets on an outcome for a given population during a specified time period |
| Also known as* | Attributable proportion Attributable risk percent [A]etiologic[al] fraction** Excess fraction |
Avoidable fraction Impact fraction*** Prevented fraction |
These terms also appear with the word “population” preceding the term to denote that the fraction/proportion are estimated for a given population, whereas without the term “population” the term can refer to the cancer cases within a cohort or case series attributable to a given risk factor.
Etiological fraction has been previously used as the proportion of cases that would have occurred by a certain time even in the absence of exposure, but, with exposure, occurred earlier than they otherwise would have. Although distinct conceptually from the attributable fraction, based on this definition, all attributable cases are etiologic cases, but not vice versa [73].
This term also appears with the word “theoretical” preceding the term to denote that the fraction/proportion is based on a theoretical scenario.
The PAFs and PPFs can be estimated, firstly, using cohort or case-control data from population representative studies, secondly, using exposure data among cases (where the cases are representative of the population) combined with relative risk (RR) data, or, thirdly, through separate data sources on the prevalence of exposure to a risk factor and the corresponding RR (most common method). This review focuses on the third method to estimate PAFs and PPFs. In addition to these methods, other risk factor-specific methods (see section on smoking, UVR and infections) are also briefly summarized.
Specifying the theoretical-minimum-risk and the counterfactual scenario
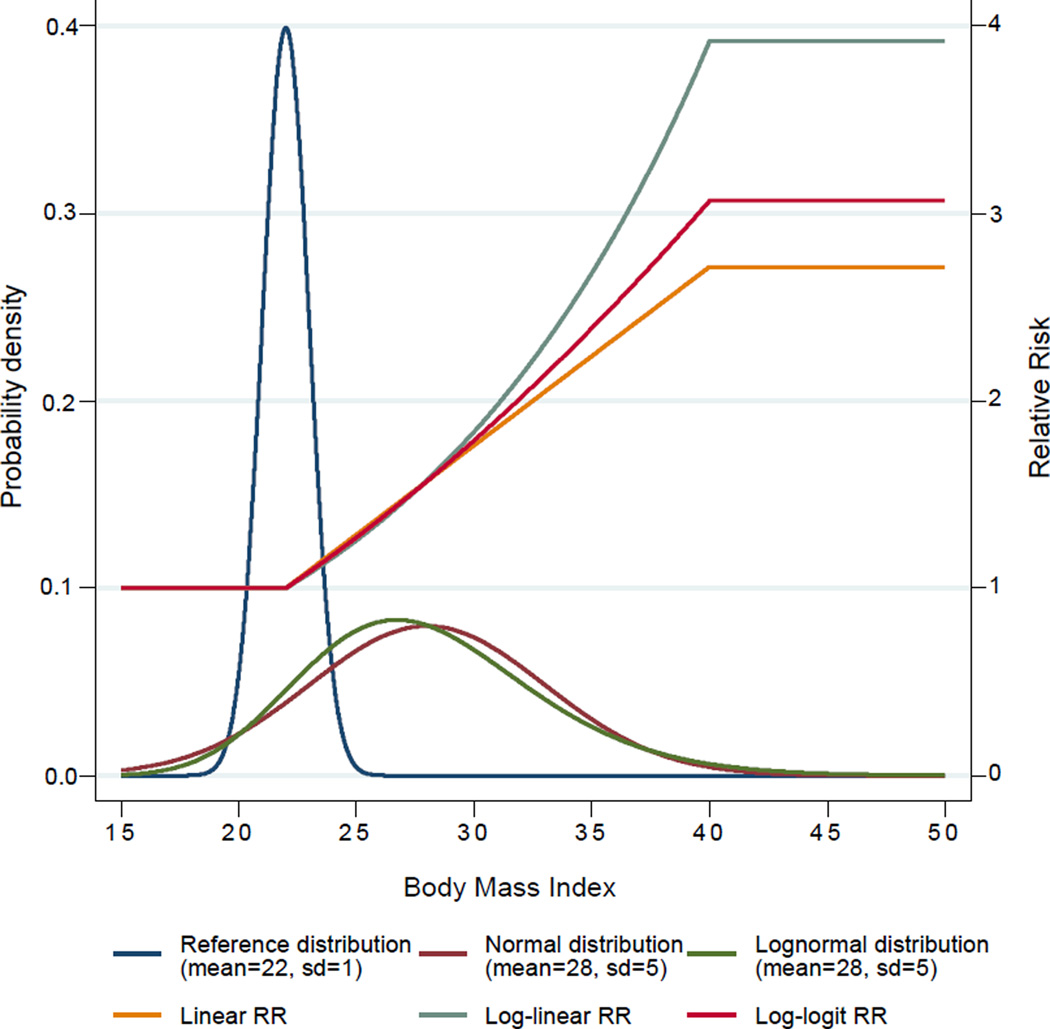
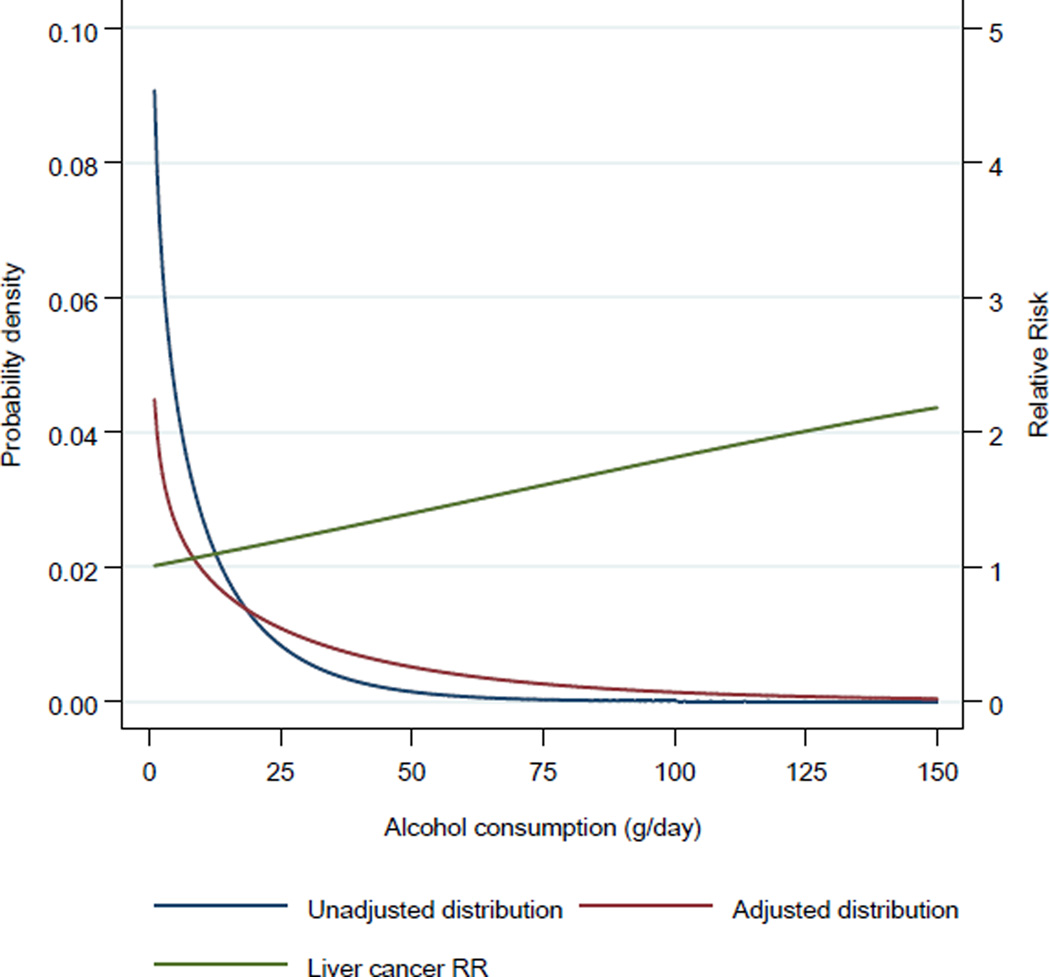
For the PAFs, the counterfactual scenario is based on everyone having a theoretical-minimum-risk exposure. The theoretical-minimum-risk exposure is usually defined as the exposure distribution leading to the lowest population risk of morbidity and/or mortality [22], which is generally no exposure to that risk factor, e.g., lifetime never smokers. However, there are exceptions: for example, for BMI, a BMI of zero is not possible, hence the theoretical-minimum-risk is a BMI distribution that leads to the lowest cancer risk (see Figure 1). For factors such as physical inactivity or fibre consumption, the counterfactual is often to have everyone physically active or consuming a sufficient amount of fibre [23]. Finally, for alcohol, the theoretical-minimum-risk exposure is generally lifetime abstention, even though low levels of alcohol consumption have an overall protective effect on total mortality (See Figure 2) [24].
Figure 1.
An example of the exposure distribution of Body Mass Index (based on the mean and standard deviation (SD)) and the corresponding continuous generic Relative Risks (RRs) (Figure from [28])
Figure 2.
Example of the exposure distribution of alcohol consumption among current drinkers (in grams of pure alcohol per day) before and after adjustment for undercoverage (for French men 35 to 44 years of age in 2005 as obtained from the Baromètre [99] and the Global Information System on Alcohol and Health [95]) and the Relative Risks (RRs) for liver cancer [100]
For the PPFs, the counterfactual scenario is determined based on an attainable exposure distribution (see [19, 25]). These exposure distributions are achieved either through intervention(s), such as an increase in taxation (for alcohol and tobacco) or vaccination programs (for infectious agents), or through the exposure distribution reaching a predetermined target (e.g., Global Monitoring Framework for non-communicable diseases targets [26]). Attainable targets can also be defined as the level of an exposure that is observed in other populations [27], or in the same population at a different point in time [28]. Exposure changes due to interventions are usually modelled based on the results of studies that examine how these interventions affect the exposure distribution (which should take into account the potential participation rate if applicable [29]) [30, 31]. Another utility of the PPF is its use in assessing cost-effectiveness, and, to determine the cost-effective minimum, the intervention(s) that produce the maximum health gain for the lowest economic cost. In high-income countries, the cost effectiveness can be assessed using the cost of saving one Quality Adjusted Life Year (QALY) according to NICE guidelines (20,000 to 30,000 British pounds or an equivalent per QALY saved) [32].
Specifying the outcome
Incidence, mortality, years of life lost due to premature mortality, and years lived with disability are the most common outcomes modelled using PAFs and PPFs [33]. The PAFs and PPFs can also be used to estimate attributable and preventable Disability Adjusted Life Years (DALYs) [34], QALYs, and monetary costs by applying the PAFs and PPFs to lower level components of these measures. Other outcomes include resources (such as hospital days and/or stays) [33]. These statistics are important when determining public policy and allocating health resources; however, the choice of the outcome(s) modelled is dependent on data availability as well as the goal of the project [33].
Specifying the exposure
When estimating the PAFs and PPFs, the time between exposure and outcome i.e., the biological latency period (time lag) should be considered. For example, in the case of tobacco smoking, there is a time lag of approximately 30 years between exposure and the diagnosis of cancer [35]. Furthermore, for occupational exposure and the incidence of solid tumors, there is a 20- to 50-year time lag [36–38], while for occupational exposures and the incidence of hematological cancer, there is a 10- to 20-year time lag [39, 40]. Conversely, for HRT (estrogen plus progestin) use and the incidence of breast cancer, there is assumed to be a very short time lag [41, 42]. In some cases, no time lag is used to facilitate comparisons between different diseases [43]. Therefore, there is a need to carefully consider the issue of latency, and also to clearly document the latency period chosen to enable comparisons between studies.
The population exposure can be modelled using a categorical or continuous distribution. Categorical exposure estimates are sometimes used when population surveys report exposures within categories, or when RR estimates are reported for categorical measures of exposure. The precision of PAFs and PPFs when using categorical estimates is dependent on the number of categories used. For BMI and cancer, a previous study showed small differences in estimations of cancers attributable to a high BMI when using a categorical distribution as compared to a continuous distribution [28, 44]. The impact on other risk factors need to be further determined. Additionally, when a continuous exposure distribution is used, the distribution may need to be truncated or bounded to prevent the modelling of exposure at unrealistic levels (see [45]).
Specifying the relative risk
When estimating PAFs based on different data sources, where possible, the risk data should be from meta-analyses (or large cohort studies), and consistent with the available exposure and outcome data in terms of the categories of exposure or the units of measurement used (for the exposure and RR data) and the outcome measured (for the RR and the outcome). This is a problem for environmental exposure PAF and PPF estimations, in particular where RRs for high exposures are commonly applied to people with low exposures [46]. Furthermore, RRs affected by population level genetics should use population-specific RRs where possible. For example, in the case of excess BMI and breast cancer, the RR is below 1 for Caucasian women and above one for Asian women [47].
The reference category for the RR measures, the PAF estimations, is the theoretical-minimum-risk, whereas in the PPF estimations, the RR reference category can either be the theoretical-minimum-risk or a risk category with the preventative target exposure status. Similar to the population exposure, RRs can be modelled using categorical or continuous exposure distributions. If only RR point estimates are provided, the continuous functions can be modelled using either a linear RR, a log-linear RR, or a log-logit RR (see Figure 2 for an example). The choice of which model to use is dependent on the biological relationship, and on the methods used to estimate the RRs (or the methods used to estimate the underlying RRs upon which a meta-analysis is based). An alternative to these approaches is to model the exposure based on weighted observations from a survey, such that the estimation of the counterfactual is done at the survey participant level (see [48] for an example).
Differences in the risks of cancer among various sub-populations and across cancer subtypes need to be accounted for, if possible. For example, the RR for an exposure can vary by the histological subtype of cancer at a particular organ site (e.g., for esophageal cancer, obesity is only a cause of adenocarcinomas [49], whereas alcohol consumption is only a cause of squamous cell carcinomas [50]); however, due to the rarity of certain cancers, RRs are commonly reported for aggregate cancer sites. The effects of various risk factors on cancer incidence may deviate by sex, age [51], or life stage (e.g., there is an increased breast cancer risk caused by risk factor exposure between menarche and menopause due to the susceptibility of undifferentiated nulliparous breast tissue to carcinogens [52]). There also may be differences in RRs among people with genetic variations (e.g., people with the ALDH 2 genotype have a much greater risk of cancer due to alcohol consumption [53]), with the size of the effect of these genetic variations dependent upon the size of the effect modification and the prevalence of the genetic variation in the population. Lastly, risk reversibility and risk accumulation also may need to be taken into account when modelling PAFs and PPFs [54].
Other methods of estimating the population attributable and preventable fractions
Tobacco smoking
The PAF for smoking can be modelled using the Lopez and Peto methodology [55], where lung cancer mortality rates are used as a proxy for tobacco smoking. The Lopez and Peto method assesses differences in lung cancer mortality between a population of interest and a counterfactual population of never smokers. The difference in mortality is then assumed to be attributable to smoking and is used as a proxy for exposure to tobacco smoking when estimating the PAFs for other cancers. Many studies have used the American Cancer Prevention Study (CPS) phase II cohort as the counterfactual population when using this method. Lung cancer rates of never smokers between countries are, for the most part, quite similar where data are available [56]. The exception to this observation was found in countries where other strong risk factors for lung cancer are present, such as in China where indoor and outdoor air pollution greatly affect lung cancer rates, in particular among women [57, 58]. To adjust for these other strong lung cancer risk factors [59], correction factors have been applied, but, generally, it is perceived that the Lopez and Peto method is not valid in settings where smoking prevalence is low, i.e., in many developing countries. Furthermore, the choice of the correction factor to be applied to adjust for other confounding factors has been a contentious issue. In the original Lopez and Peto paper, the correction factor was 50%, which has changed several times since the method was first published [55].
Preston and colleagues present an alternative method of estimating the PAF for tobacco smoking and total mortality, based on lung cancer mortality rates relative to total mortality rates [60]. This method applies a regression model to estimate the relationship between lung cancer mortality and total mortality by age, sex, and year to derive the PAF of total mortality due to tobacco smoking. This method avoids the utilization of RRs, and, thus, provides more flexibility. However, this method can only be applied to data from countries with similar distributions of causes of death to those countries used in the Preston and colleagues‘ study, and where there are no other strong lung cancer risk factors besides smoking (such as air pollution). Accordingly, this method is not suitable for PAF estimations for developing countries.
Infectious Agents
The PAFs and PPFs for infectious agents are based on an estimation of the proportion of cancer cases that would not have occurred if all or some of the infections had been avoided or successfully treated before oncogenesis [16]. These fractions are estimated through three different methods. Firstly, for HTLV-1 in adult T-cell lymphoma, and KSHV in Kaposi sarcoma, the infections are necessary causes of cancer and, thus, all of these cancers are attributable to the infections [16]. HPV in cervical cancer is also included in this model as non-HPV-related cervical cancers are rare. Secondly, for HPV (at other cancer sites) and Epstein-Barr virus-related cancers, these infectious agents are sufficient causes of cancer, with very high RRs (see [16]) and, thus, the prevalence of transcriptional viral gene products in tumor cells is used as a direct measure of the PAFs [16]. Lastly, helicobacter pylori, and Hepatitis B and C viruses are neither sufficient nor necessary cancer causes and, thus, the PAFs for cancers caused by these infectious agents are estimated based on the prevalence of transcriptional viral gene products in tumor cells and the RR of cancer given infection [16]. The infection status among cancer cases is obtained from pathological tests, and, therefore, depends largely on the techniques used, the infectious agent, and the cancer examined (see [16] for a more detailed explanation).
Sun exposure (ultraviolet radiation)
The PAF and PPF fractions for skin melanoma due to sun UVR are usually estimated using a direct method based on differences of skin melanoma rates between populations [61, 62] (for artificial UVR exposure such as sunbeds, traditional PAF and PPF estimation methods are used). For the sun exposure-PAFs, the theoretical-minimum-risk exposure has previously been estimated using the incidence of melanoma at body sites which are not exposed to UVR [62]. For the sun exposure-PPFs, which are considered to be more relevant due to the absence of an ‘unexposed’ population [61], the incidence of melanoma in reference areas or during a time period, (e.g., a historical cohort of lower sun exposure) is used as a counterfactual [61, 62]. This latter method is seen as the most relevant, as it provides PPFs which are contextually appropriate for population comparisons. Lastly, the PPFs of skin melanoma and keratinocyte cancers due to sun exposure, assuming an increase in sunscreen use, can be modelled by combining data on sunscreen use, the RR of melanoma and keratinocyte cancers for people who use sunscreen compared to those who do not, and the number of preventable or attributable cancers estimated using one of the previously described methodologies [63].
Other considerations
Relative Risks
Current PAF and PPF formulas assume the use of a RR; however, studies often report odds ratios. An odds ratio can be used as an approximation of the risk ratio; however, it may overestimate the effect size of the RR (i.e., the distance by which the RR deviates from the null (i.e., 1)) when the incidence of an outcome of interest is not rare within the exposure group [64]. In such cases, a conversion of the odds ratio to a RR is desirable [64].
Commonly, risk factors are not found to be independent of one another [65]. For example, for cancer it is common to find a clustering of lifestyle unhealthy lifestyles [66], especially among a portion of the population, e.g., lower socio-economic strata [67]. However, the original attributable fraction formula described by Levin is considered valid only in the absence of confounding and/or effect modification [68, 69]. In cases of confounding, estimates of the attributable cancer burden based on an adjusted RR due to a risk factor of interest are likely to misestimate the true PAFs, the extent to which is dependent on the magnitude of confounding. In the case of effect modification, the estimation of PAFs is more complex. For example, moderate cigarette smoking or moderate alcohol consumption alone have a negligible effect on esophageal cancer risk; however, in combination they have a synergistically larger effect on esophageal cancer risk [70]. To account for the correlation of risk factor exposure, ideally the multivariate distribution of exposure to all risk factors can be used when estimating the PAFs. Additionally, in the presence of confounding or effect modification, the PAFs can be presented for people of different groups stratified by the confounding or effect modifying factors [28]. However, most data sources do not provide data on the joint exposure distribution. Alternatively, in the presence of confounding, the formula presented in Box 1 can also be used; however, this formula requires knowledge of the exposure among cases [68, 71]. Therefore, given the lack of data needed to adjust the PAFs for risk factor correlation, PAFs are generally estimated not taking confounding into account [72].
Cancers attributable to multiple risk factors
The PAFs and PPFs for multiple risk factors cannot be estimated through the simple addition of the PAFs and PPFs for the individual risk factors [73]. The PAFs and PPFs due to multiple risk factors can be estimated using the standard PAF functions using the prevalence distribution and RRs for co-occurring risk factors. However, this method requires data on co-occurrence of the risk factors, and the relationship between risk factors [74]. Alternatively, assuming that risk factors are independent of each other, a PAF for multiple risk factors can be estimated using case exposure data [75], or the formula presented by Miettinen [1] (see Box 1).
Uncertainty estimation
Due to the use of data from multiple sources to estimate the PAFs and PPFs, the uncertainty of these estimates is often determined using a Monte Carlo approach, where a set of the lowest level parameters used in these estimations is generated from their respective uncertainty distributions (taking into account variation between the parameters). These lowest level parameters are then used to estimate the uncertainty distribution of the PAFs and PPFs [76].
Limitations or challenges
As previously mentioned, the time lag between exposure and outcome in some cases is as long as 50 years. Besides the challenge of retrieving high quality historical data that are consistently measured across time (which is particularly problematic for disease classification, especially malignant lymphomas), the cancer risk factors may affect the risk of competing causes of death [77]; however, current methods rarely account for changes in mortality rates among those who are exposed to different sets of risk factors.
PAF and PPF estimates are restricted by time and population and depend on the quality and representativeness of the exposure and risk data. Data for risk factor exposure usually are obtained from population surveys [78] that often may not be representative of the population due to recruitment and participation biases [79, 80] and exclusion of subsets of the general population, such as the institutionalized and homeless [79, 80]. Furthermore, the questions on risk exposure may be limited by the respondents‘ comprehension of the questions asked, recall of the information, and possible deliberate misreporting of information (especially for socially negative behaviors [81, 82]). In some cases, the exposure as measured by a survey can be adjusted to match the population level exposure (as is the case with alcohol consumption [83]) (see Figure 2). For RR estimations, a source of exposure measurement bias is the potential for misclassification within the theoretical-minimum-risk reference group [84]. For example, Zeisser and colleagues showed that meta-analyses of the relationship between breast cancer and alcohol are affected by misclassification of occasional drinkers as lifetime abstainers, which leads to a small, yet significant, decrease in the RR estimates [85].
Discussion
The estimation of the proportion of cancer incidence and mortality attributable to various risk factors, as well as the proportion that could be prevented, provides useful information for health planning and setting health priorities by creating a hierarchy of cancer risk factors and interventions [86]. Current and previous PAF and PPF studies [10, 11, 28, 87, 88] have provided important information to set cancer prevention priorities, such as the new United Nations sustainable goals [89] or the national cancer prevention campaign [90, 91]. Furthermore, these studies can inform policy through establishing future health targets by calculating PPFs using data from prediction models [20, 92] or established automated tools [30, 93].
The accuracy of PAFs and PPFs greatly depends on the quality of the underlying exposure and RR data. Therefore, there is a need to support initiatives at the national and international level in order to improve both risk factor and cancer surveillance systems, as these data are required for planning, implementing, and evaluating cancer prevention and control efforts [94]. Current multi-national risk factor surveillance systems include the Global Information System on Alcohol and Health [95] and the CAREX survey database for monitoring occupational exposures to carcinogens [96]. Furthermore, to strengthen cancer surveillance, the Global Initiative for Cancer Registry Development in low- and middle-income countries (GICR, http://gicr.iarc.fr) provides the necessary technical support and training to cancer registries to ultimately increase global coverage of high quality population-based cancer incidence data.
Many of the risk factors for cancer are also risk factors for other diseases, conditions and injuries [57], and changes in these risk factors may also have social and monetary effects. Therefore, it is imperative to consider the effects of changes in these risk factors on health conditions other than cancer and on social factors [77]. For example, to re-enforce the effects of prevention strategies, the estimation of the effects of prevention strategies on multiple disease may be desirable. Furthermore, changes in risk factors that are taxable products (such as tobacco and alcohol) to preventable targets may result in decreases in state revenues; however, decreases in health care expenditures and increases in productivity achieved through the reduction of these risk factors are likely to far outweigh losses in state revenues [97, 98].
Conclusion
When performing PAF and PPF studies, it is important to clearly describe the methods used, including data sources and assumptions made, to ensure replicability and transparency, and to highlight the limitations of these estimates in their applicability to health policy. Studies estimating attributable and preventable cancer burdens also provide an opportunity for cross-disciplinary collaboration in order to ensure this translational research is reflected in public health policies.
Acknowledgments
Funding sources: French National Cancer Institute [contract number 2015-002]
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Kevin D. Shield, D. Maxwell Parkin, David C. Whiteman, Jürgen Rehm, Vivian Viallon, Claire Marant Micallef, Paolo Vineis, Lesley Rushton, Freddie Bray, Isabelle Soerjomataram declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance •• Of major importance
- 1.Miettinen OS. Proportion of disease caused or prevented by a given exposure, trait or intervention. Am J Epidemiol. 1974;99(5):325–332. doi: 10.1093/oxfordjournals.aje.a121617. [DOI] [PubMed] [Google Scholar]
- 2.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 3.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1192–1308. [PubMed] [Google Scholar]
- 4.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia, USA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 5.Cole P, MacMahon B. Attributable risk percent in case-control studies. Br J Prev Soc Med. 1971;25(4):242–244. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lilienfeld A. Epidemiology of infectious and noninfectious disease: some comparisons. Am J Epidemiol. 97(1973):135–147. doi: 10.1093/oxfordjournals.aje.a121494. [DOI] [PubMed] [Google Scholar]
- 7.Walter SD. Prevention for multifactorial diseases. Am J Epidemiol. 1980;112(3):409–416. doi: 10.1093/oxfordjournals.aje.a113007. [DOI] [PubMed] [Google Scholar]
- 8.Walter S. The estimation and interpretation of attributable risk in health research. Biometrics. 1976:829–849. [PubMed] [Google Scholar]
- 9.Morgenstern H, Bursic ES. A method for using epidemiologic data to estimate the potential impact of an intervention on the health status of a target population. J Community Health. 1982;7(4):292–309. doi: 10.1007/BF01318961. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM. 1. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S2–S5. doi: 10.1038/bjc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whiteman DC, Webb PM, Green AC, Neale RE, Fritschi L, Bain CJ, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health. 2015;39(5):477–484. doi: 10.1111/1753-6405.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland S. Concepts and pitfalls in measuring and interpreting attributable fractions, prevented fractions, and causation probabilities. Ann Epidemiol. 2015;25(3):155–161. doi: 10.1016/j.annepidem.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–2323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Cancer Research Fund and American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: A Global perspective. Washington DC, USA: American Institute for Cancer Research; 2008. [Google Scholar]
- 15.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans: preamble. Lyon, France: International Agency for Research on Cancer; 2006. [PMC free article] [PubMed] [Google Scholar]
- 16.De Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13(6):607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 17.Soerjomataram I, Pukkala E, Brenner H, Coebergh JWW. On the avoidability of breast cancer in industrialized societies: older mean age at first birth as an indicator of excess breast cancer risk. Breast Cancer Res Treat. 2008;111(2):297–302. doi: 10.1007/s10549-007-9778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack V, Boffetta P. Today's lifestyles, tomorrow's cancers: trends in lifestyle risk factors for cancer in low-and middle-income countries. Ann Oncol. 2011 doi: 10.1093/annonc/mdq763. mdq763. [DOI] [PubMed] [Google Scholar]
- 19.Murray CJ, Lopez AD. On the comparable quantification of health risks: lessons from the Global Burden of Disease Study. Epidemiology. 1999;10(5):594–605. [PubMed] [Google Scholar]
- 20.Kontis V, Mathers CD, Rehm J, Stevens GA, Shield KD, Bonita R, et al. Contribution of six risk factors to achieving the 25× 25 non-communicable disease mortality reduction target: a modelling study. Lancet. 2014;384(9941):427–437. doi: 10.1016/S0140-6736(14)60616-4. [DOI] [PubMed] [Google Scholar]
- 21.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370(9604):2044–2053. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 22.Ezzati M, Vander Hoorn S, Rodgers A, Lopez AD, Mathers CD, Murray CJ. Estimates of global and regional potentil health gains from reducing muliple major risk factors. Lancet. 2003;362(9380):271–280. doi: 10.1016/s0140-6736(03)13968-2. [DOI] [PubMed] [Google Scholar]
- 23.Olsen CM, Wilson LF, Nagle CM, Kendall BJ, Bain CJ, Pandeya N, et al. Cancers in Australia in 2010 attributable to insufficient physical activity. Aust N Z J Public Health. 2015;39(5):458–463. doi: 10.1111/1753-6405.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shield KD, Parry C, Rehm J. Focus On: Chronic Diseases and Conditions Related to Alcohol Use. Alcohol Res. 2013;85:2. doi: 10.35946/arcr.v35.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehm J, Taylor B, Patra J, Gmel G. Avoidable burden of disease: conceptual and methodological issues in substance abuse epidemiology. Int J Methods Psychiatr Res. 2006;15(4):191–111. doi: 10.1002/mpr.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Geneva, Switzerland: World Health Organization; 2013. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. [Google Scholar]
- 27.Soerjomataram I, De Vries E, Pukkala E, Coebergh JW. Excess of cancers in Europe: a study of eleven major cancers amenable to lifestyle change. Int J Cancer. 2007;120(6):1336–1343. doi: 10.1002/ijc.22459. [DOI] [PubMed] [Google Scholar]
- 28.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulik MC, Nusselder WJ, Boshuizen HC, Lhachimi SK, Fernández E, Baili P, et al. Comparison of tobacco control scenarios: quantifying estimates of long-term health impact using the DYNAMO-HIA modeling tool. PLoS One. 2012;7(2):e32363. doi: 10.1371/journal.pone.0032363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy DT, Ellis JA, Mays D, Huang A-T. Smoking-related deaths averted due to three years of policy progress. Bull World Health Organ. 2013;91(7):509–518. doi: 10.2471/BLT.12.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soerjomataram I, De Vries E, Engholm G, Paludan-Müller G, Brønnum-Hansen H, Storm HH, et al. Impact of a smoking and alcohol intervention programme on lung and breast cancer incidence in Denmark: An example of dynamic modelling with Prevent. Eur J Cancer. 2010;46(14):2617–2624. doi: 10.1016/j.ejca.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 32.Appleby J, Devlin N, Parkin D. NICE's cost effectiveness threshold. Br Med J. 2007;7616:358. doi: 10.1136/bmj.39308.560069.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray CJ, Salomon JA, Mathers CD, Lopez AD. Summary measures of population health: concepts, ethics, measurement and applications. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 34.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72(3):429. [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss W. Cigarette smoking and lung cancer trends. A light at the end of the tunnel? Chest. 1997;111(5):1414–1416. doi: 10.1378/chest.111.5.1414. [DOI] [PubMed] [Google Scholar]
- 36.Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Environ Med. 1992;34(7):718–721. [PubMed] [Google Scholar]
- 37.Olsen J, Dragsted L, Autrup H. Cancer risk and occupational exposure to aflatoxins in Denmark. Br J Cancer. 1988;58(3):392. doi: 10.1038/bjc.1988.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Reilly KM, McLaughlin AM, Beckett WS, Sime PJ. Asbestos-related lung disease. Am Fam Physician. 2007;75(5):683–688. [PubMed] [Google Scholar]
- 39.Steenland K, Stayner L, Deddens J. Mortality analyses in a cohort of 18 235 ethylene oxide exposed workers: follow up extended from 1987 to 1998. Occup Environ Med. 2004;61(1):2–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Crump KS. Risk of benzene-induced leukemia: A sensitivity analysis of the pliofilm cohort with additional follow-up and new exposure estimates. J Toxicol Environ Health A. 1994;42(2):219–242. doi: 10.1080/15287399409531875. [DOI] [PubMed] [Google Scholar]
- 41.Narod SA. Hormone replacement therapy and the risk of breast cancer. Nat Rev Clin Oncol. 2011;8(11):669–676. doi: 10.1038/nrclinonc.2011.110. [DOI] [PubMed] [Google Scholar]
- 42.Chlebowski RT, Kuller LH, Prentice RL, Stefanick ML, Manson JE, Gass M, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–587. doi: 10.1056/NEJMoa0807684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briggs AD, Kehlbacher A, Tiffin R, Garnett T, Rayner M, Scarborough P. Assessing the impact on chronic disease of incorporating the societal cost of greenhouse gases into the price of food: an econometric and comparative risk assessment modelling study. BMJ Open. 2013;3(10):e003543. doi: 10.1136/bmjopen-2013-003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126(3):692–702. doi: 10.1002/ijc.24803. [DOI] [PubMed] [Google Scholar]
- 45.Gmel G, Shield KD, Kehoe-Chan TA, Rehm J. The effects of capping the alcohol consumption distribution and relative risk functions on the estimated number of deaths attributable to alcohol consumption in the European Union in 2004. BMC Med Res Methodol. 2013;13(1):1. doi: 10.1186/1471-2288-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saracci R, Vineis P. Disease proportions attributable to environment. Environ Health. 2007;6(38) doi: 10.1186/1476-069X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 48.Purshouse RC, Meier PS, Brennan A, Taylor KB, Rafia R. Estimated effect of alcohol pricing policies on health and health economic outcomes in England: an epidemiological model. Lancet. 2010;375(9723):1355–1364. doi: 10.1016/S0140-6736(10)60058-X. [DOI] [PubMed] [Google Scholar]
- 49.Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol. 2011;8(6):340–347. doi: 10.1038/nrgastro.2011.73. [DOI] [PubMed] [Google Scholar]
- 50.Pandeya N, Williams G, Green AC, Webb PM, Whiteman DC, Study AC. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136(4):1215–1224. e2. doi: 10.1053/j.gastro.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 51.Gauderman WJ, Morrison JL. Evidence for age-specific genetic relative risks in lung cancer. Am J Epidemiol. 2000;151(1):41–49. doi: 10.1093/oxfordjournals.aje.a010120. [DOI] [PubMed] [Google Scholar]
- 52.McPherson K, Steel C, Dixon J. Breast cancer—epidemiology, risk factors, and genetics. Br Med J. 2000;321(7261):624–628. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brennan P, Lewis S, Hashibe M, Bell DA, Boffetta P, Bouchardy C, et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: a HuGE review. Am J Epidemiol. 2004;159(1):1–16. doi: 10.1093/aje/kwh003. [DOI] [PubMed] [Google Scholar]
- 54.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 55.Peto R, Boreham J, Lopez AD, Thun M, Heath C. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339(8804):1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- 56.Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fajersztajn L, Veras M, Barrozo LV, Saldiva P. Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer. 2013;13(9):674–678. doi: 10.1038/nrc3572. [DOI] [PubMed] [Google Scholar]
- 58.Liu B-Q, Peto R, Chen Z-M, Boreham J, Wu Y-P, Li J-Y, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. Br Med J. 1998;317(7170):1411–1422. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ezzati M, Lopez AD. Measuring the accumulated hazards of smoking: global and regional estimates for 2000. Tob Control. 2003;12(1):79–85. doi: 10.1136/tc.12.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. Int J Epidemiol. 2010;39(2):430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parkin D, Mesher D, Sasieni P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105:S66–S69. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armstrong B, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res. 1993;3(6):395–402. doi: 10.1097/00008390-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Olsen CM, Wilson LF, Green AC, Bain CJ, Fritschi L, Neale RE, et al. Cancers in Australia attributable to exposure to solar ultraviolet radiation and prevented by regular sunscreen use. Aust N Z J Public Health. 2015;39(5):471–476. doi: 10.1111/1753-6405.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J, Kai FY. What's the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. J Am Med Assoc. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 65.Ebrahim S, Montaner D, Lawlor DA. Clustering of risk factors and social class in childhood and adulthood in British women's heart and health study: cross sectional analysis. Br Med J. 2004;328(7444):861. doi: 10.1136/bmj.38034.702836.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuit AJ, van Loon AJM, Tijhuis M, Ocké MC. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002;35(3):219–224. doi: 10.1006/pmed.2002.1064. [DOI] [PubMed] [Google Scholar]
- 67.Blakely T, Hales S, Kieft C, Wilson N, Woodward A. The global distribution of risk factors by poverty level. Bull World Health Organ. 2005;83(2):118–126. [PMC free article] [PubMed] [Google Scholar]
- 68.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88(1):15–19. doi: 10.2105/ajph.88.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flegal KM, Williamson DF, Graubard BI. Using adjusted relative risks to calculate attributable fractions. Am J Public Health. 2006;96(3):398-. doi: 10.2105/AJPH.2005.079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82(5):657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 71.Flegal KM, Panagiotou OA, Graubard BI. Estimating population attributable fractions to quantify the health burden of obesity. Ann Epidemiol. 2015;25(3):201–207. doi: 10.1016/j.annepidem.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenland S, Robins JM. Conceptual problems in the definition and interpretation of attributable fractions. Am J Epidemiol. 1988;128(6):1185–1197. doi: 10.1093/oxfordjournals.aje.a115073. [DOI] [PubMed] [Google Scholar]
- 74.Ezzati M, Vander Hoorn S, Rodgers A, Lopez AD, Mathers CD, Murray CJ. Potential health gains from reducing multiple risk factors. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva, Switzerland: World Health Organization; 2004. pp. 2167–2190. [Google Scholar]
- 75.Bruzzi P, Green S, Byar D, Brinton L, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122(5):904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization. Global Burden of Disease and Risk Factors. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 77.Kontis V, Mathers CD, Bonita R, Stevens GA, Rehm J, Shield KD, et al. Regional contributions of six preventable risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modelling study. Lancet Glob Healt. 2015;3(12):e746–e757. doi: 10.1016/S2214-109X(15)00179-5. [DOI] [PubMed] [Google Scholar]
- 78.Driscoll T, Steenland K, Prüss-Üstün A, Nelson Deborah I, Leigh J. Occupational carcinogens: assessing the environmental burden of disease at national and local levels. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 79.Shield KD, Rehm J. Difficulties with telephone-based surveys on alcohol consumption in high-income countries: the Canadian example. Int J Methods Psychiatr Res. 2012;21(1):17–28. doi: 10.1002/mpr.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groves RM. Survey errors and survey costs. New Jersey; USA: John Wiley & Sons; 2004. [Google Scholar]
- 81.Tourangeau R, Yan T. Sensitive questions in surveys. Psychol Bull. 2007;133(5):859. doi: 10.1037/0033-2909.133.5.859. [DOI] [PubMed] [Google Scholar]
- 82.Strack F, Martin LL. Thinking, judging, and communicating: A process account of context effects in attitude surveys. In: Schwarz N, Sudman S, editors. Social information processing and survey methodology. New York, USA: Springer-Verlag; 1987. pp. 123–148. [Google Scholar]
- 83.Rehm J, Baliunas D, Borges GL, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105(5):817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 85.Zeisser C, Stockwell TR, Chikritzhs T. Methodological biases in estimating the relationship between alcohol consumption and breast cancer: the role of drinker misclassification errors in meta-analytic results. Alcohol Clin Exp Res. 2014;38(8):2297–2306. doi: 10.1111/acer.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.World Health Organization. One Health Tool. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 87.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M Comparative Risk Assessment collaborating group. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 88.Attributable causes of cancer in France in the year 2000. Lyon, Fance: International Agency for Research on Cancer; 2007. International Agency for Research on Cancer. [Google Scholar]
- 89.United Nations. Sustainable development goals: 17 goals to transform our world. New York, USA: United Nations; 2016. [Google Scholar]
- 90.National Health Service. Change 4 life. London, United Kingdom: National Health Service; 2016. [Google Scholar]
- 91.Cancer Research UK. Statistics on preventable cancers. London, United Kingdom: Cancer Research UK; 2016. [Google Scholar]
- 92.Gartner CE, Barendregt JJ, Hall WD. Predicting the future prevalence of cigarette smoking in Australia: how low can we go and by when? Tob Control. 2009;18(3):183–189. doi: 10.1136/tc.2008.027615. [DOI] [PubMed] [Google Scholar]
- 93.Soerjomataram I, Barendregt JJ, Gartner C, Kunst A, Møller H, Avendano M. Reducing inequalities in lung cancer incidence through smoking policies. Lung Cancer. 2011;73(3):268–273. doi: 10.1016/j.lungcan.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Glaser SL, Clarke CA, Gomez SL, O‘Malley CD, Purdie DM, West DW. Cancer surveillance research: a vital subdiscipline of cancer epidemiology. Cancer Causes Control. 2005;16(9):1009–1019. doi: 10.1007/s10552-005-4501-2. [DOI] [PubMed] [Google Scholar]
- 95.World Health Organization. Global Information System on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 96.Kauppinen T, Toikkanen J, Pedersen D, Young R, Ahrens W, Boffetta P, et al. Occupational exposure to carcinogens in the European Union. Occup Environ Med. 2000;57(1):10–18. doi: 10.1136/oem.57.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Single E, Robson L, Xie X, Rehm J. The economic costs of alcohol, tobacco and illicit drugs in Canada, 1992. Addiction. 1998;93(7):991–1006. doi: 10.1046/j.1360-0443.1998.9379914.x. [DOI] [PubMed] [Google Scholar]
- 98.Fenoglio P, Parel V, Kopp P. The social cost of alcohol, tobacco and illicit drugs in France, 1997. Eur Addict Res. 2003;9(1):18–28. doi: 10.1159/000067730. [DOI] [PubMed] [Google Scholar]
- 99.Institut national de prevention et d‘éducation pour la santé. 2005 Baromètre. Saint Denis, France: Institut national de prevention et d‘éducation pour la santé; 2006. [Google Scholar]
- 100.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br J Cancer. 2014 doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Annotated References
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva, Switzerland: World Health Organization; 2013. This document establishes the current health priorities to reduce the burden caused by non-communicable diseases in the future. Among these priorities are reductions in cancer, tobacco smoking and alcohol consumption.
- Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46. doi: 10.1016/S1470-2045(14)71123-4. This manuscript estimates the global burden of cancer attributable to high Body Mass Index. This article is an exemplary example of a burden of disease study, and highlights that current upward trends in weight gain are expected to cause an increase in the burden of cancer.
- Praud D, Rota M, Rehm J, Shield K, Zatoński W, Hashibe M, et al. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. 2016;138(6):1380–1387. doi: 10.1002/ijc.29890. This manuscript estimates the burden of cancer attributable to alcohol consumption. This article is an exemplary example of a burden of disease study, and highlights the large and growing burden of cancer attributable to alcohol globally.
- Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(10010):2287–2323. doi: 10.1016/S0140-6736(15)00128-2. This manuscript outlines the most recent Comparative Risk Assessment study (part of the Global Burden of Disease study) comparing the burdens caused by various risk factors from 1990 to 2013.
- Kontis V, Mathers CD, Rehm J, Stevens GA, Shield KD, Bonita R, et al. Contribution of six risk factors to achieving the 25× 25 non-communicable disease mortality reduction target: a modelling study. Lancet. 2014;384(9941):427–437. doi: 10.1016/S0140-6736(14)60616-4. This manuscript outlines the effects of reductions in various risk factors to meet the 25×25 non-communicable disease mortality reduction targets.
- Whiteman DC, Webb PM, Green AC, Neale RE, Fritschi L, Bain CJ, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health. 2015;39(5):477–484. doi: 10.1111/1753-6405.12471. These manuscripts outline the cancers attributable to various risk factors in Australia and the United Kingdom, respectively, and act as exemplary examples of cancer comparative risk assessment studies.
- Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S77–S81. doi: 10.1038/bjc.2011.489. These manuscripts outline the cancers attributable to various risk factors in Australia and the United Kingdom, respectively, and act as exemplary examples of cancer comparative risk assessment studies.