Abstract
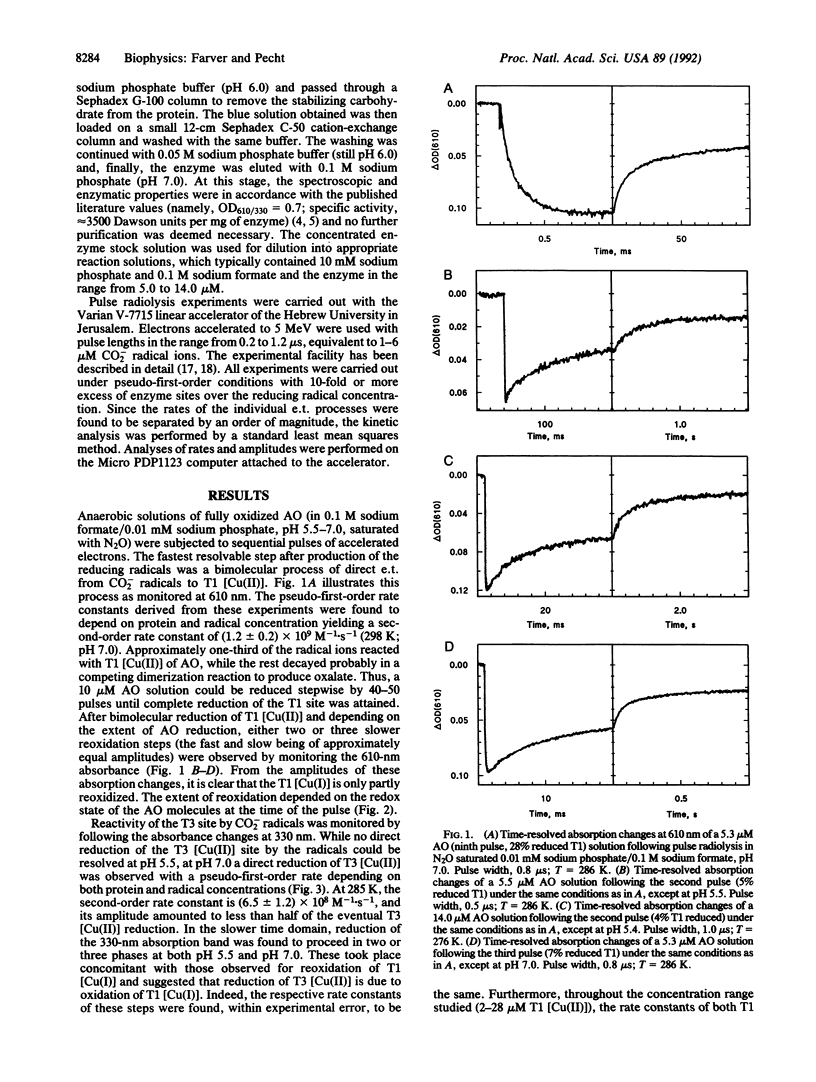
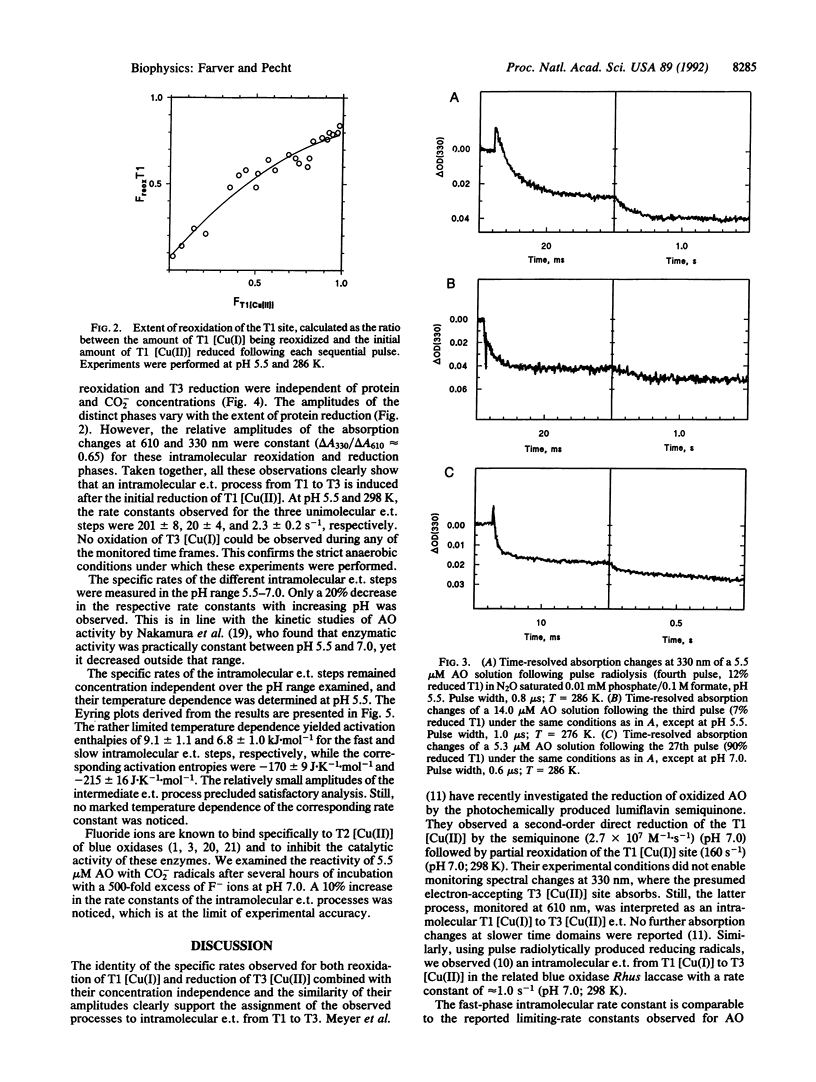
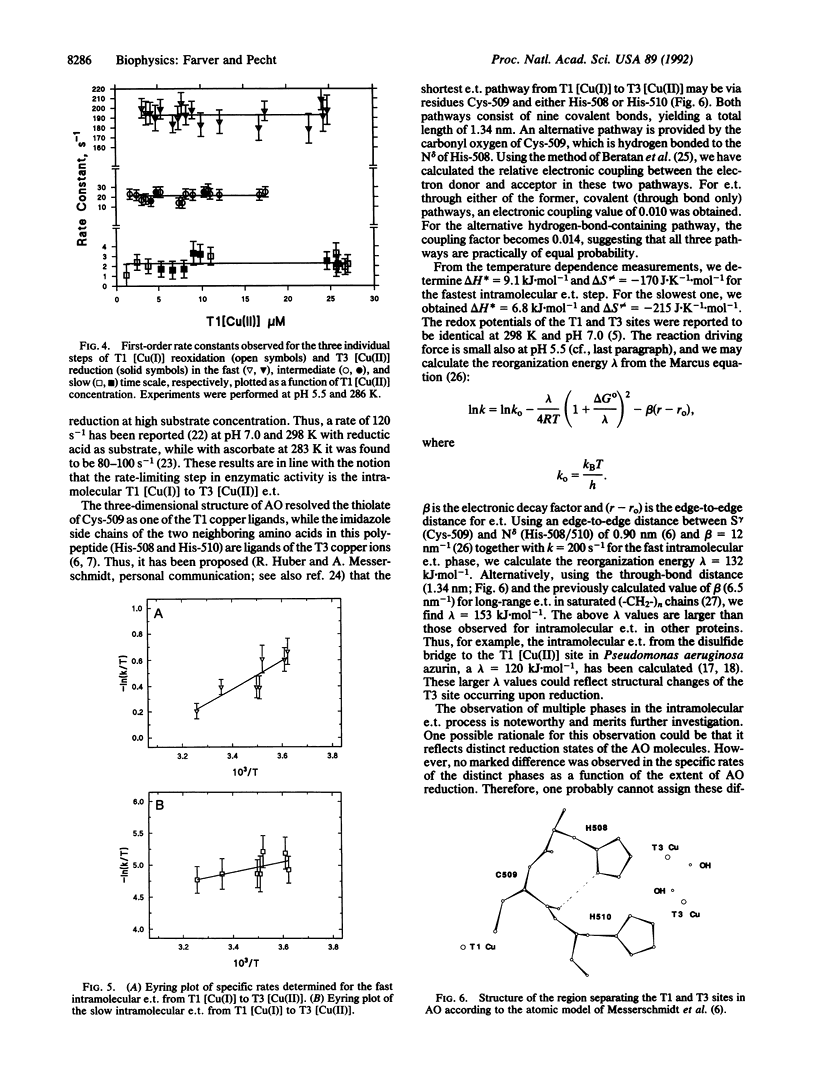
Anaerobic reduction kinetics of the zucchini squash ascorbate oxidase (AO; L-ascorbate:oxygen oxidoreductase, EC 1.10.3.3) by pulse radiolytically produced CO2- radical ions were investigated. Changes in the absorption bands of type 1 [Cu(II)] (610 nm) and type 3 [Cu(II)] (330 nm) were monitored over a range of reactant concentrations, pH, and temperature. The direct bimolecular reduction of type 1 [Cu(II)] [(1.2 +/- 0.2) x 10(9) M-1.s-1] was followed by its subsequent reoxidation in three distinct phases, all found to be unimolecular processes with the respective specific rates of 201 +/- 8, 20 +/- 4, and 2.3 +/- 0.2 s-1 at pH 5.5 and 298 K. While at this pH no direct bimolecular reduction was resolved in the 330-nm band, at pH 7.0 such a direct process was observed [(6.5 +/- 1.2) x 10(8) M-1.s-1]. In the same slower time domains where type 1 [Cu(I)] reoxidation was monitored, reduction of type 3 [Cu(II)] was observed, which was also concentration independent and with identical rate constants and amplitudes commensurate with those of type 1 [Cu(II)] reoxidation. These results show that after electron uptake by type 1 [Cu(II)], its reoxidation takes place by intramolecular electron transfer to type 3 [Cu(II)]. The observed specific rates are similar to values reported for the limiting-rate constants of AO reduction by excess substrate, suggesting that internal electron transfer is the rate-determining step of AO activity. The temperature dependence of the intramolecular electron transfer rate constants was measured from 275 to 308 K at pH 5.5 and, from the Eyring plots, low activation enthalpies were calculated--namely, 9.1 +/- 1.1 and 6.8 +/- 1.0 kJ.mol-1 for the fastest and slowest phases, respectively. The activation entropies observed for these respective phases were -170 +/- 9 and -215 +/- 16 J.K-1.mol-1. The exceptionally low enthalpy barriers imply the involvement of highly optimized electron transfer pathways for internal electron transfer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H. Copper in biological systems. A report from the 6th Manziana Conference, September 23-27, 1990. J Inorg Biochem. 1991 Nov 15;44(3):173–218. doi: 10.1016/0162-0134(91)80054-l. [DOI] [PubMed] [Google Scholar]
- Beratan D. N., Betts J. N., Onuchic J. N. Protein electron transfer rates set by the bridging secondary and tertiary structure. Science. 1991 May 31;252(5010):1285–1288. doi: 10.1126/science.1656523. [DOI] [PubMed] [Google Scholar]
- Brändén R., Malmström B. G., Vänngård T. The effect of fluoride on the spectral and catalytic properties of the three copper-containing oxidases. Eur J Biochem. 1973 Jul 2;36(1):195–200. doi: 10.1111/j.1432-1033.1973.tb02901.x. [DOI] [PubMed] [Google Scholar]
- Farver O., Goldberg M., Pecht I. A circular dichroism study of the reactions of Rhus laccase with dioxygen. Eur J Biochem. 1980 Feb;104(1):71–77. doi: 10.1111/j.1432-1033.1980.tb04401.x. [DOI] [PubMed] [Google Scholar]
- Farver O., Goldberg M., Wherland S., Pecht I. Reductant-dependent electron distribution among redox sites of laccase. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5245–5249. doi: 10.1073/pnas.75.11.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farver O., Pecht I. Long-range intramolecular electron transfer in azurins. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6968–6972. doi: 10.1073/pnas.86.18.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M., Farver O., Pecht I. Interaction of Rhus laccase with dioxygen and its reduction intermediates. J Biol Chem. 1980 Aug 10;255(15):7353–7361. [PubMed] [Google Scholar]
- Goldberg M., Pecht I. The reaction of "blue" copper oxidases with O2: a pulse radiolysis study. Biophys J. 1978 Oct;24(1):371–373. doi: 10.1016/S0006-3495(78)85384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guissani A., Henry Y., Gilles L. Radical scavenging and electron-transfer reactions in Polyporus versicolor laccase a pulse radiolysis study. Biophys Chem. 1982 Jun;15(3):177–190. doi: 10.1016/0301-4622(82)80001-x. [DOI] [PubMed] [Google Scholar]
- Malmström B. G. Enzymology of oxygen. Annu Rev Biochem. 1982;51:21–59. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- Marchesini A., Kroneck P. M. Ascorbate oxidase from Cucurbita pepo medullosa. New method of purification and reinvestigation of properties. Eur J Biochem. 1979 Nov 1;101(1):65–76. doi: 10.1111/j.1432-1033.1979.tb04217.x. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990 Jan 26;187(2):341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A., Rossi A., Ladenstein R., Huber R., Bolognesi M., Gatti G., Marchesini A., Petruzzelli R., Finazzi-Agró A. X-ray crystal structure of the blue oxidase ascorbate oxidase from zucchini. Analysis of the polypeptide fold and a model of the copper sites and ligands. J Mol Biol. 1989 Apr 5;206(3):513–529. doi: 10.1016/0022-2836(89)90498-1. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Marchesini A., Cusanovich M. A., Tollin G. Direct measurement of intramolecular electron transfer between type I and type III copper centers in the multi-copper enzyme ascorbate oxidase and its type II copper-depleted and cyanide-inhibited forms. Biochemistry. 1991 May 7;30(18):4619–4623. doi: 10.1021/bi00232a037. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Makino N., Ogura Y. Purification and properties of ascorbate oxidase from cucumber. J Biochem. 1968 Aug;64(2):189–195. doi: 10.1093/oxfordjournals.jbchem.a128879. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ogura Y. Oxidation and reduction of copper proteins: note added to the previous report on the state and activity of copper atoms in copper proteins. J Biochem. 1968 Aug;64(2):267–270. doi: 10.1093/oxfordjournals.jbchem.a128890. [DOI] [PubMed] [Google Scholar]
- O'Neill P., Fielden E. M., Morpurgo L., Agostinelli E. Pulse-radiolysis studies on the interaction of one-electron reduced species with blue oxidases. Reduction of native and type-2-copper-depleted Vietnamese-lacquer-tree and Japanese-lacquer-tree laccases. Biochem J. 1984 Aug 15;222(1):71–76. doi: 10.1042/bj2220071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecht I., Faraggi M. Reduction of copper (II) in fungal laccase by hydrated electrons. Nat New Biol. 1971 Sep 22;233(38):116–118. doi: 10.1038/newbio233116a0. [DOI] [PubMed] [Google Scholar]



