Abstract
The early incorporation of exposure assessment can be invaluable to help design, prioritize, and interpret toxicological studies or outcomes. The sum total of the exposure assessment findings combined with preliminary toxicology results allows for exposure-informed toxicological study design and the findings can then be integrated, together with available epidemiologic data, to provide health effect relevance. With regard to engineered nanomaterial inhalation toxicology in particular, a single type of material (e.g. carbon nanotube, graphene) can have a vast array of physicochemical characteristics resulting in the potential for varying toxicities. To compound the matter, the methodologies necessary to establish a material adequate for in vivo exposure testing raises questions on the applicability of the outcomes. From insights gained from evaluating carbon nanotubes, we recommend the following integrated approach involving exposure-informed hazard assessment and hazard-informed exposure assessment especially for materials as diverse as engineered nanomaterials: 1) market-informed identification of potential hazards and potentially exposed populations, 2) initial toxicity screening to drive prioritized assessments of exposure, 3) development of exposure assessment-informed chronic and sub-chronic in vivo studies, and 4) conduct of exposure- and hazard-informed epidemiological studies.
Keywords: Exposure assessment, Inhalation, Carbon nanotube, Toxicology, Nanomaterial, Epidemiology
1. Introduction
As formulated in the National Academy of Sciences/National Research Council for risk assessment/risk management (NRC, 1983, 2009), risk assessment itself has four integral parts including hazard identification, dose-response assessment, and exposure assessment that lead to risk characterization. In schematic representations of the paradigm, the dose-response and exposure assessments contribute to the risk characterization but are oftentimes treated independently. For well-defined xenobiotics this would seem adequate, but with regard to the complexity of the physicochemical characteristics of engineered nanomaterials, an integration between exposure and toxicological assessments is a necessity. An early review of nanotoxicology as an emerging discipline indicated that exposure assessment could be informative for dose-response assessments (Oberdorster, Oberdorster, & Oberdorster, 2005).
2. Risk and exposure assessments
2.1. Knowledge-of-exposure and knowledge-of-hazard influence the relevance and reliability of risk assessments
Risk, in reference to particle toxicology, is an evaluation of the relative hazard of a material taking into account the exposure, or more specifically, the delivered dose. If little to no knowledge exists for the hazard and exposure then the risk will be poorly understood. Having only thorough knowledge of the hazard without any exposure data will also limit the interpretation of the findings. Conversely, knowing all facets of the exposure with little hazard information provides no indication of the risk. Once both detailed exposure assessments are performed in association with properly designed and executed toxicological evaluations using relevant exposure metrics then assessments of risk are likely to be valid (Fig. 1). An additional need is for an understanding of the factors involved in transferring risk observed from animal toxicology studies to human exposures and health effects (NIOSH, 2013). Ideally, epidemiologic studies would be available as a source of hazard identification or dose-response information, or to corroborate risk projections from toxicology and exposure assessment studies and to serve, potentially, as an additional data source for risk assessment (Vermeulen et al., 2014).
Fig. 1.
View of the influence of knowledge-of-exposure and knowledge-of-hazard on the relevance and reliability of risk assessments. Adapted from the approach previously described (Hoover et al., 2014, 2015).
2.2. A framework to integrate exposure and toxicity assessments for engineered nanomaterials
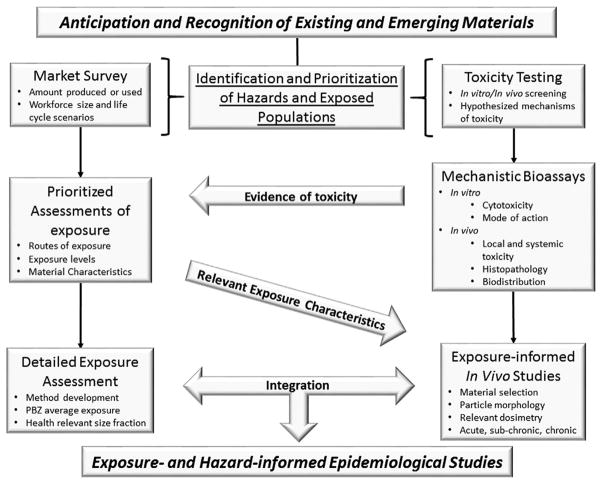
In the adaptive risk assessment paradigm, risk characterization arises from hazard identification and subsequent dose-response assessments as well as exposure assessments. With regard to engineered nanomaterial inhalation toxicology, a single type of material (e.g. carbon nanotube, graphene) can have a vast array of physicochemical characteristics resulting in the potential for varying toxicities. To compound the matter, the methodologies necessary to establish a material adequate for in vivo exposure testing raises questions on the applicability of the outcomes. The early incorporation of exposure assessment can be invaluable to help design, prioritize, and interpret toxicological studies or outcomes (Fig. 2). Initially there needs to be an identification and prioritization of hazards and exposed populations (Schubauer-Berigan, Dahm, & Yencken, 2011). The decision should be market-informed with a reasonable anticipation of potential toxicity. Toxicity screening then drives prioritized assessments of exposure. The exposure assessments provide information about routes of exposure, levels of exposure, and material characteristics. When feasible, there should be congruence among exposure metrics being used, or reasonable extrapolations from the workplace to toxicology studies may be unreliable. While surface area of particles has significant relevance when considering toxicological outcomes, particularly for engineered nanomaterials, there is no reliable way to measure this metric in the workplace for materials such as carbon nanotubes (Dahm, Evans, Schubauer-Berigan, Birch, & Deddens, 2013). In addition, a recent study of various graphite nanoplates showed an inverse relationship between surface area and toxicity.
Fig. 2.
A framework for integrating exposure assessment and toxicity testing to design, prioritize, and interpret exposure- and hazard-informed epidemiological studies. PBZ=personal breathing zone.
To make informed interpretations between toxicological and exposure assessments the evaluation of the toxicant should be categorically representative. This is important for engineered nanomaterials that may have varying levels of toxicity within a single class of material. For example, a particular multi-walled carbon nanotube (MWCNT), Mitsui-7 or MWCNT-7, has a count mean width of 49 nm for individual fibers (Porter et al., 2010) and during inhalation exposures has a count median width of 100.3 nm and a count median length of 3.04 μm during inhalation exposure (Chen et al., 2012). MWCNT-7 causes fibrosis, promotes lung tumorigenesis, and translocates to the pleural cavity and extrathoracic organs following an inhalation exposure designed to administer a nearly 100% respirable fraction (Chen et al., 2012; Grosse et al., 2014; Mercer, Scabilloni, Hubbs, Battelli et al., 2013; Mercer, Scabilloni, Hubbs, Wang, et al., 2013; Sargent et al., 2014). Conversely, exposure assessment studies have indicated that MWCNT used in U.S. facilities are more often smaller in diameter, resulting in significantly more nanotube agglomeration, which should decrease the likelihood of extrathoracic translocation (Dahm, Evans, Schubauer-Berigan, Birch, & Fernback, 2012; Dahm et al., 2015). These factors may create different outcomes than described for MWCNT-7 and perhaps alter the risk characterization.
There is no clear answer on the exposure assessment side for how many evaluations would be representative of a single industry but caution should be used when designing toxicological studies or interpreting findings to human health relevance based on one or two observations. For example, personal breathing zone measurements for a facility manufacturing carbon nanofibers (CNF) had exposures of 45 and 80 μg/m3 of elemental carbon in the respirable size range (Birch, Ku, Evans, & Ruda-Eberenz, 2011). However, in a recently published study by Dahm et al. (2015) the authors found inhalable CNF personal breathing zone exposures of 4.2 and 7.5 μg/m3 of elemental carbon at a downstream manufacturing facility. Exposures at the respirable size fraction were not collected in this instance, but it can be assumed that respirable portion would only comprise a small percentage of the exposure based on transmission electron microscopy (TEM) and dustiness data (Dahm et al., 2015; Erdely et al., 2013; Evans, Turkevich, Roettgers, Deye, & Baron, 2013). Since these are the only two CNF facilities where mass based measurements of elemental carbon has been collected, it is still relatively unclear whether these measurements are representative of the industry or just these particular facilities. Much more information is becoming available about exposure levels for single-walled (SW) CNT and MWCNT, with a conclusion being reached that exposures are generally low outside of powder-handling tasks (Dahm et al., 2015).
The sum total of the exposure assessment findings combined with preliminary toxicology results allows for exposure-informed toxicological study design and the findings can then be integrated, together with available epidemiologic data, to provide health effect relevance. While the reality of toxicological science for engineered nanomaterials may have in vivo and in vitro studies proceeding without any exposure assessment guidance, every effort should be made to push for detailed exposure assessment to provide timely context to the toxicology studies.
3. Insights from the carbon nanotube experience
3.1. Initial market projections and toxicity studies
Unprecedented global investment in innovative nanoscale science and engineering has led to the production and utilization of novel materials in expanding fields of electronics, medicine, and composites. However, health and environmental implications of these new critical nanomaterials have raised serious issues. Engineered nanomaterials, such as carbon nanotubes (CNT), have caused toxicity in experimental models. Generally, limited data exists for human exposures (Liou, Tsai, Pelclova, Schubauer-Berigan, & Schulte, 2015), the physicochemical properties most prevalent in exposure scenarios, and health outcomes. These deficiencies make interpretation of experimental findings to human relevance difficult.
Early projections had CNT production becoming a multi-billion dollar industry suggesting the potential for exposure risk. The projections implied the workforce handling CNT would rapidly expand and the CNT market would reach the everyday consumer in the very near future. This expected growth led increased in vivo and in vitro toxicology assessment of CNT world-wide. The initial in vivo studies evaluating the toxicity of SWCNT clearly showed pulmonary toxicity that included inflammation and rapid onset fibrosis following exposure (Lam, James, McCluskey, & Hunter, 2004; Mangum et al., 2006; Shvedova et al., 2005; Warheit et al., 2004). Additional studies on the more market prevalent MWCNT found similar results (Ma-Hock et al., 2009; Pauluhn, 2010). These findings were consistent with the mode of action for biopersistent fibrous shaped particles. Additional studies provide evidence of adverse extrathoracic effects following pulmonary exposure, including cardiovascular and immunological responses as well as translocation (Erdely et al., 2009; Li et al., 2007; Mercer, Scabilloni, Hubbs, Wang, et al., 2013; Mitchell, Lauer, Burchiel, & McDonald, 2009). While early CNT studies clearly illustrated a hazard, there was incomplete extrapolation to relevant human health effects. Furthermore, materials prominently tested in early studies (e.g., MWCNT-7) were of unknown relevance to actual market use. This was not a fault of these studies but an illustration of the lack of data from detailed workplace exposure assessments. To date, thousands of articles have been published concerning CNT (includes toxicology and application), but very few examine human exposure scenarios and health effects.
3.2. Field assessments
The adverse outcomes of the initial toxicology studies combined with market projections created the need to assess exposures and the feasibility of human health effect studies (Schubauer-Berigan et al., 2011). Initial exposure assessment studies relied on gravimetric area measurements, extrapolations from catalyst, and short duration worst case scenario measurements (Erdely et al., 2013). These early exposure assessment studies on CNT provided some useful insight while also highlighting needs for future more detailed studies. More recent workplace exposure assessments, such as the work by Dahm et al. (2012, 2015), provided significant impact for toxicological assessments. Five critical areas of exposure assessment became apparent when extrapolating relevance to experimental studies of CNT toxicity: 1) What is the level of exposure with health relevant size fractions? 2) What are the physicochemical characteristics of the material with the potential for exposure (e.g. CNT are mostly agglomerated in human exposure settings)? 3) What are the most common brands and types of CNT being utilized in industry, including secondary manipulations of CNT such as surface coatings or functionalization? 4) What is the area of future interest with potential large scale applications and other downstream uses? and 5) How long is the average cumulative exposure of the workforce handling CNT? Recent elucidation of these critical areas greatly contributes to the overall interpretation of already published toxicology studies, while contributing to future toxicological and epidemiological study design and predictions of human health risks.
3.3. Bridging the gap between exposure assessment and inhalation toxicology
Evaluation of background-corrected elemental carbon from all personal breathing zone (PBZ) collections from 8 MWCNT facilities found an average inhalable concentration of 10.6 μg/m3 (arithmetic mean). The field measurements also contributed to an estimated mass median aerodynamic diameter derived from the respirable to inhalable ratio that was used to predict an alveolar deposition fraction. These measures allowed for a prediction of toxicological effects with regard to average exposures seen in the workplace. Following MWCNT inhalation, general effects were evident up to 28 days post-exposure at a deposition with estimated human equivalence of 7.6 years at 10.6 μg/m3 (Erdely et al., 2013). The utility of our extrapolations were supported by a recent study exploring human health effects from a single facility in Korea (Lee et al., 2015). Lee et al. (2015) indicated increased markers of oxidative stress from exhaled breath condensate from workers with an average personal sampling of elemental carbon at 8.34 μg/m3 and an average of 4 years of exposure. These results were in very close agreement with our extrapolations (Erdely et al., 2013) and showed the immense utility of combining detailed exposure assessment with ongoing in vivo toxicology studies, together with available epidemiologic data, to make a reasonable conclusion concerning human relevance.
Our extrapolations (Erdely et al., 2013) and supporting confirmation in a single facility human study (Lee et al., 2015) provided a dosing regimen for screening similar manufacturing-relevant carbon-based nanomaterials. A screening process representing facility-relevant exposures (4 μg deposition) and a high dose which confers pathology (40 μg deposition), have been utilized by ongoing nanomaterial studies (e.g. CNT, CNF, graphite nanoplates) to evaluate relative potency between and within classes. The premise is very similar to that proposed by Landsiedel et al. for using short term inhalation studies (5 day) as an early tier screen for nanomaterial potency (Landsiedel et al., 2014). In many cases inhalation is simply not feasible and instillation studies may be necessary, but the overall premise of screening materials with standardized outcomes is an extremely valuable first step.
Exposure assessment studies (Dahm et al., 2012, 2015) and accompanying human health effects studies (Lee et al., 2015; Schubauer-Berigan et al., 2011) provided insight into types of CNT utilized by industry as well as the downstream applications and products containing these materials. These studies provided data on specific preference of materials, level of exposure, years handling a given material, and future direction of the market. Material selection for new toxicity testing is facilitated by this life cycle knowledge of particles with regard to surface coating or functionalization followed by product incorporation.
Exposure assessments with personal breathing zone measures also provided representation of the morphology of the CNT exposure. Recent studies show the majority of the CNT exposure is in the inhalable or thoracic size range (i.e., aerodynamic diameter less than about 100 μm) rather than the respirable size range (i.e., aerodynamic diameter less than about 10 μm). This was shown for both elemental carbon mass and by TEM (Dahm et al., 2012, 2015; Erdely et al., 2013). These field findings can guide in vivo exposures so that a generated aerosol or instillate reflects workforce or consumer exposures. This is crucial for intratracheal instillation or oropharyngeal aspiration studies. Altering the physicochemical characteristics of nanomaterials often influences toxicity, complicating data interpretation. This may be further confounded when determining potency for similar classes of materials that respond differently to the same dispersion method or a single material with varying toxicity depending on the dispersion method (Baisch et al., 2014; Sager et al., 2015). In ongoing studies, TEM images from personal breathing zone collections of CNT or CNF exposed workers (Dahm et al., 2012, 2015) were compared to TEM images of the same material dispersed for ongoing in vivo studies to ensure relevance of the in vivo exposures. While the differences in size of the rodent and human respiratory tract must be considered, the goal should be to expose rodents to particles with characteristics relevant to human exposures. Therefore, extraordinary measures to disperse particles may not produce toxicological findings relevant to humans. Establishing similarities between laboratory and field exposures provides some evidence that the in vivo studies are representative of human health outcomes.
4. Conclusion
Toxicity assessments and estimates of risk to develop exposure limits can generally proceed without exposure assessment. However risk characterization is more informed when exposure assessment is available. For engineered nanomaterials specifically, detailed exposure assessment fills a large void in relating toxicity findings to human health relevance from a dosimetry perspective. In conclusion, we recommend the following integrated approach between exposure assessment and toxicity testing especially for materials as diverse as engineered nanomaterials:
Market-informed identification of potential hazards and potentially exposed populations.
Initial toxicity screening to drive prioritized assessments of exposure.
Development of exposure assessment-informed chronic and sub-chronic in vivo studies.
Conduct of exposure- and hazard-informed epidemiological studies.
Acknowledgments
Funding
This work was funded by the Nanotechnology Research Center of the National Institute for Occupational Safety and Health
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Baisch BL, Corson NM, Wade-Mercer P, Gelein R, Kennell AJ, Oberdorster G, Elder A. Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: The effect of dose rate on acute respiratory tract inflammation. Particle and Fibre Toxicology. 2014;11:5. doi: 10.1186/1743-8977-11-5. http://dx.doi.org/10.1186/1743-8977-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch ME, Ku BK, Evans DE, Ruda-Eberenz TA. Exposure and emissions monitoring during carbon nanofiber production – Part I: Elemental carbon and iron-soot aerosols. Annals of Occupational Hygiene. 2011;55(9):1016–1036. doi: 10.1093/annhyg/mer073. http://dx.doi.org/10.1093/annhyg/mer073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Schwegler-Berry D, McKinney W, Stone S, Cumpston JL, Friend S, Porter DW, Castranova V, Frazer DG. Multi-walled carbon nanotubes: Sampling criteria and aerosol characterization. Inhalation Toxicology. 2012;24(12):798–820. doi: 10.3109/08958378.2012.720741. http://dx.doi.org/10.3109/08958378.2012.720741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Fernback JE. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Annals of Occupational Hygiene. 2012;56(5):542–556. doi: 10.1093/annhyg/mer110. http://dx.doi.org/10.1093/annhyg/mer110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Deddens JA. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers: Mobile direct-reading sampling. Annals of Occupational Hygiene. 2013;57(3):328–344. doi: 10.1093/annhyg/mes079. http://dx.doi.org/10.1093/annhyg/mes079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon nanotube and nanofiber exposure assessments: An analysis of 14 site visits. Annals of Occupational Hygiene. 2015;59(6):705–723. doi: 10.1093/annhyg/mev020. http://dx.doi.org/10.1093/annhyg/mev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely A, Hulderman T, Salmen R, Liston A, Zeidler-Erdely PC, Schwegler-Berry D, Castranova V, Koyama S, Kim YA, Endo M, Simeonova PP. Cross-talk between lung and systemic circulation during carbon nanotube respiratory exposure. Potential biomarkers. Nano Letters. 2009;9(1):36–43. doi: 10.1021/nl801828z. http://dx.doi.org/10.1021/nl801828z. [DOI] [PubMed] [Google Scholar]
- Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML, Deddens JA, Hulderman T, Bilgesu SA, Battelli L, Schwegler-Berry D, Leonard HD, McKinney W, Frazer DG, Antonini JM, Porter DW, Castranova V, Schubauer-Berigan MK. Carbon nanotube dosimetry: From workplace exposure assessment to inhalation toxicology. Particle and Fibre Toxicology. 2013;10(1):53. doi: 10.1186/1743-8977-10-53. http://dx.doi.org/10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Turkevich LA, Roettgers CT, Deye GJ, Baron PA. Dustiness of fine and nanoscale powders. Annals of Occupational Hygiene. 2013;57(2):261–277. doi: 10.1093/annhyg/mes060. http://dx.doi.org/10.1093/annhyg/mes060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K International Agency for Research on Cancer Monograph Working G. Carcinogenicity of fluoroedenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncology. 2014;15(13):1427–1428. doi: 10.1016/S1470-2045(14)71109-X. http://dx.doi.org/10.1016/S1470-2045(14)71109-X. [DOI] [PubMed] [Google Scholar]
- Hoover MD, Cash LJ, Mathews SM, Feitshans IL, Iskander J, Harper SL. ‘Toxic’ and ‘nontoxic’: Confirming critical terminology concepts and context for clear communication. In: Wexler P, editor. Encyclopedia of toxicology. 3. Oxford: Elsevier; 2014. [Google Scholar]
- Hoover MD, Myers DS, Cash LJ, Guilmette RA, Kreyling WG, Oberdorster G, Smith R, Cassata JR, Boecker BB, Grissom MP. Application of an informatics-based decision-making framework and process to the assessment of radiation safety in nanotechnology. Health Physics. 2015;108(2):179–194. doi: 10.1097/HP.0000000000000250. http://dx.doi.org/10.1097/HP.0000000000000250. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicological Sciences. 2004;77(1):126–134. doi: 10.1093/toxsci/kfg243. http://dx.doi.org/10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Ma-Hock L, Hofmann T, Wiemann M, Strauss V, Treumann S, Wohlleben W, Groters S, Wiench K, van Ravenzwaay B. Application of short-term inhalation studies to assess the inhalation toxicity of nanomaterials. Particle and Fibre Toxicology. 2014;11:16. doi: 10.1186/1743-8977-11-16. http://dx.doi.org/10.1186/1743-8977-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Choi YC, Shin JH, Lee JH, Lee Y, Park SY, Baek JE, Park JD, Ahn K, Yu IJ. Health surveillance study of workers who manufacture multi-walled carbon nanotubes. Nanotoxicology. 2015;9(6):802–811. doi: 10.3109/17435390.2014.978404. http://dx.doi.org/10.3109/17435390.2014.978404. [DOI] [PubMed] [Google Scholar]
- Li Z, Hulderman T, Salmen R, Chapman R, Leonard SS, Young SH, Shvedova A, Luster MI, Simeonova PP. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environmental Health Perspectives. 2007;115(3):377–382. doi: 10.1289/ehp.9688. http://dx.doi.org/10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou SH, Tsai CSJ, Pelclova D, Schubauer-Berigan MK, Schulte PA. Assessing the first wave of epidemiological studies of nanomaterial workers. Journal of Nanoparticle Research. 2015;17(10):413. doi: 10.1007/s11051-015-3219-7. http://dx.doi.org/10.1007/s11051-015-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Hock L, Treumann S, Strauss V, Brill S, Luizi F, Mertler M, Wiench K, Gamer AO, van Ravenzwaay B, Landsiedel R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicological Sciences. 2009;112(2):468–481. doi: 10.1093/toxsci/kfp146. http://dx.doi.org/10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- Mangum JB, Turpin EA, Antao-Menezes A, Cesta MF, Bermudez E, Bonner JC. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part Fibre Toxicol. 2006;3:15. doi: 10.1186/1743-8977-3-15. http://dx.doi.org/10.1186/1743-8977-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, Andrew M, Castranova V, Porter DW. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Particle and Fibre Toxicologyl. 2013a;10:33. doi: 10.1186/1743-8977-10-33. http://dx.doi.org/10.1186/1743-8977-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Wang L, Battelli LA, McKinney W, Castranova V, Porter DW. Extrapulmonary transport of MWCNT following inhalation exposure. Particle and Fibre Toxicology. 2013b;10:38. doi: 10.1186/1743-8977-10-38. http://dx.doi.org/10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, McDonald JD. Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nature Nanotechnology. 2009;4(7):451–456. doi: 10.1038/nnano.2009.151. http://dx.doi.org/10.1038/nnano.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. Current intelligence bulletin 65: Occupational exposure to carbon nanotubes and nanofibers. Cincinnati, OH: US Department of Health and Human Services, Centers for Disease Control, National Institute for Occupational safety and Health; 2013. [accessed 2017 July 2013]. DHHS (NIOSH), Publication Number 2013-145, Available at: http://www.cdc.gov/niosh/docs/2013-2145/pdfs/2013-2145.pdf. [Google Scholar]
- NRC. Risk assessment in the federal government: Managing the process. Washington, DC: National Academy Press; 1983. [PubMed] [Google Scholar]
- NRC. Science and decisions: Advancing risk assessment. Washington, DC: National Academy Press; 2009. [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J. Subchronic 13-week inhalation exposure of rats to multiwalled carbon nanotubes: Toxic effects are determined by density of agglomerate structures, not fibrillar structures. Toxicological Sciences. 2010;113(1):226–242. doi: 10.1093/toxsci/kfp247. http://dx.doi.org/10.1093/toxsci/kfp247. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, Castranova V. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269(2–3):136–147. doi: 10.1016/j.tox.2009.10.017. http://dx.doi.org/10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Sager T, Wolfarth M, Keane M, Porter D, Castranova V, Holian A. Effects of nickel-oxide nanoparticle pre-exposure dispersion status on bioactivity in the mouse lung. Nanotoxicology. 2015:1–11. doi: 10.3109/17435390.2015.1025883. http://dx.doi.org/10.3109/17435390.2015.1025883. [DOI] [PubMed]
- Sargent LM, Porter DW, Staska LM, Hubbs AF, Lowry DT, Battelli L, Siegrist KJ, Kashon ML, Mercer RR, Bauer AK, Chen BT, Salisbury JL, Frazer D, McKinney W, Andrew M, Tsuruoka S, Endo M, Fluharty KL, Castranova V, Reynolds SH. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Particle and Fibre Toxicology. 2014;11:3. doi: 10.1186/1743-8977-11-3. http://dx.doi.org/10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubauer-Berigan MK, Dahm MM, Yencken MS. Engineered carbonaceous nanomaterials manufacturers in the United States: Workforce size, characteristics, and feasibility of epidemiologic studies. Journal of Occupational and Environmental Medicine. 2011;53(Suppl 6):S62–67. doi: 10.1097/JOM.0b013e31821b1e2c. http://dx.doi.org/10.1097/JOM.0b013e31821b1e2c. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2005;289(5):L698–708. doi: 10.1152/ajplung.00084.2005. http://dx.doi.org/10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Vermeulen R, Silverman DT, Garshick E, Vlaanderen J, Portengen L, Steenland K. Exposure-response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environmental Health Perspectives. 2014;122(2):172–177. doi: 10.1289/ehp.1306880. http://dx.doi.org/10.1289/ehp.1306880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicological Sciences. 2004;77(1):117–125. doi: 10.1093/toxsci/kfg228. http://dx.doi.org/10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]