Abstract
Background
The effects of non-nutritive sweeteners (NNS) on glucose metabolism and appetite regulating hormones are not clear. There is an ongoing debate concerning NNS use and deleterious changes in metabolism.
Objectives
The aim of this review is to analyze the scientific available evidence regarding the effects of NNS on glucose metabolism and appetite regulating hormones.
Data Sources and Study Eligibility Criteria
We identified human observational studies evaluating the relation between NNS consumption and obesity, diabetes, and metabolic syndrome, in addition to clinical trials evaluating the effects of NNS in glucose metabolism and appetite regulating hormones.
Results
Fourteen observational studies evaluating the association between NNS consumption and the development of metabolic diseases and twenty-eight clinical trials studying the effects of NNS on metabolism were included. Finally, two meta-analyses evaluating the association between the consumption of NNS-containing beverages and the development of type 2 diabetes were identified.
Conclusions
Some observational studies suggest an association between NNS consumption and development of metabolic diseases; however, adiposity is a confounder frequently found in observational studies. The effects of the NNS on glucose metabolism are not clear. The results of the identified clinical trials are contradictory and are not comparable because of the major existing differences between them. Studies evaluating specific NNS, with an adequate sample size, including a homogeneous study group, identifying significant comorbidities, with an appropriate control group, with an appropriate exposure time, and considering adjustment for confounder variables such as adiposity are needed.
Introduction
The prevalence of obesity has more than doubled since 1980; in parallel in 2014, the estimated number of patients with diabetes in the world was 385 million with a projection to increase to 592 million by 2035. One of the contributing factors attributed to the increase in obesity, type 2 diabetes and other metabolic diseases is the consumption of a high sugar/high fat diet [1]. To avoid the negative health conditions associated with the excessive sugar intake, there has been an upsurge in the consumption of nonnutritive sweeteners (NNS) as an alternative [2]. At this time six NNS, sucralose, aspartame, saccharin, acesulfame-K, neotame, and advantame, are approved to be used as sweeteners in food, and two naturally derived NNS, steviol glycosides and Luo han guo extract, are generally recognized as safe and endorsed for use in food by the US Food Drug Administration (FDA) and the European Food Safety Authority (EFSA) [3, 4]. Nowadays, they are globally used and they are found in several products.
Recently, the EFSA conducted a re-evaluation of aspartame safety, and concluded that aspartame and its breakdown products are safe for the general population (including infants, children and pregnant women) [4]. Before the FDA approved NNS consumption, a series of toxicological and clinical studies in a number of species, including humans, were conducted to demonstrate that they are generally safe and well-tolerated [5]. There is an ongoing debate over whether NNS use may be associated to deleterious metabolic changes in humans [6]. This article aims to collect the information regarding the effects of NNS consumption on metabolic diseases, based on a systematic review of the scientific literature.
Study Search and Selection
We identified human studies evaluating the effects of NNS consumption in metabolic conditions through systematic searches and hand searches on April 8, 2015 (updated on March 25, 2016) in three electronic databases: PubMed, The Cochrane Library, and Trip Database. We conducted the search for observational studies to answer the following research question: Is there a relation between NNS consumption and the development of metabolic chronic diseases in adults? For clinical trials, we directed the search to answer the next research question: Is there an effect of NNS on glucose metabolism and appetite regulating hormones compared to water or other sweeteners in adults? The terms used in the systematic search were those related to NNS and artificially sweetened beverages including the next Medical Subject Headings (MeSH) terms: artificial sweeteners / non-nutritive sweeteners / carbonated beverages / sucralose / aspartame / stevia / saccharin / acesulfame potassium / diet soda / diabetes mellitus / obesity / metabolic syndrome. To complement the search, we also performed a hand-searching strategy through certain journals and references in other articles. Time and language of publication were not restricted. Inclusion criteria consisted in original studies of prospective design conducted in adult humans. For cohort studies, we considered those that evaluate the association between NNS consumption and the development of diabetes, metabolic syndrome or obesity, with a follow up of at least three years. For clinical trials we included those that evaluate the effects of any NNS on outcomes related to glucose metabolism and appetite regulating hormones (S1 File). One researcher (AR) screened the articles titles and abstracts to remove those that easily were detected to be not related to the objective of this review, and three researchers (AR, PA, and GB) read the articles that could be eligible in the systematic review and select those that finally are included. Articles evaluating the effects of NNS in other conditions or evaluating other outcomes not related were excluded.
Results
Literature search
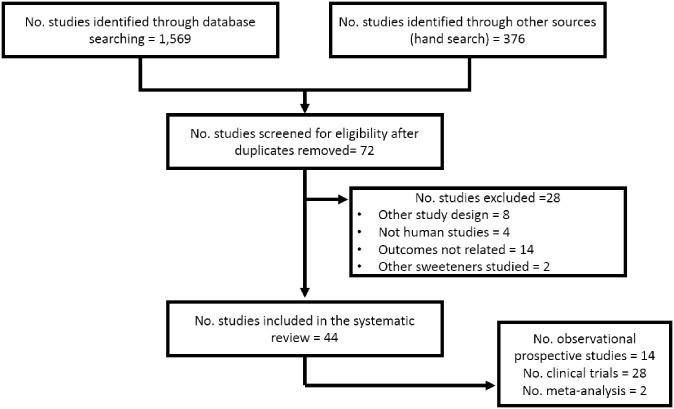
We identify 1569 studies through database searching; in addition, 376 were found by the hand searching strategy. After duplicates removal and initial screening, 72 studies were reviewed. Finally, 44 studies were included after the exclusion of 28 that did not fulfill the inclusion criteria. Fig 1 shows the flow chart describing the process of the systematic search.
Fig 1. Flow diagram of the systematic search.
Observational studies
We included fourteen observational studies evaluating the association between NNS consumption and the development of metabolic diseases including type 2 diabetes, obesity, and metabolic syndrome. All of these studies have considered NNS consumption in beverages and most of them in soft drinks.
Summarizing the results, the majority of these studies have found significant associations between the ingestion of NNS and the development of metabolic diseases. Among these studies there are two reports derived from the Nurses’ Health Study (NHS I and II) that included more than 70,000 and 90,000 women, with an average follow-up of 24 and 8 years for the first and the second studies, respectively. The first of these studies found a significant association between caffeinated artificially sweetened beverages consumption and development of type 2 diabetes (RR 1.35, 95% CI 1.24–1.47). However, this association was lost after the adjustment for body mass index (BMI) and energy intake (RR 1.01, 95% CI 0.93–1.10) [7]. In the NHS II no association was found [8].
Another large cohort study that evaluated the effect of artificially sweetened beverages consumption and the development of type 2 diabetes is the Health Professionals Follow-Up Study. This included approximately 40,000 male health professionals followed over 20 years. This study found a significant association between NNS consumption and type 2 diabetes development, even after multivariable adjustment (HR 1.40, 95% CI 1.26–1.56). However, this association was lost after the adjustment for BMI (HR 1.09, 95% CI 0.98–1.21) [9].
The European Prospective Investigation into Cancer and Nutrition (EPIC) Study, performed in eight European countries, included 340,234 men and women. This study reported a significant association between artificially sweetened soft drinks ingestion and type 2 diabetes development (HR 1.93, 95% CI 1.47–2.54). This association was attenuated after multivariable adjustment (HR 1.88, 95% CI 1.44–2.45), and lost statistical significance after further adjustment for BMI and energy intake (HR 1.13, 95% CI 0.85–1.52) [10].
Table 1 shows a summary of the results of the included cohort studies. On Table 2 the crude and adjusted risks reported in these studies are contrasted.
Table 1. Observational studies evaluating the association between artificially sweetened beverages consumption and the risk for development of metabolic diseases.
| Author, year, cohort, and country | Follow-up time | Population (Number and age) | Results |
|---|---|---|---|
|
8 years |
|
|
|
4 years |
|
|
|
9 years |
|
|
|
7–8 years |
|
|
|
4 years |
|
|
|
7 years |
|
|
|
20 years |
|
|
|
20 years |
|
|
|
24 years |
|
|
|
22 years |
|
|
|
16 years |
|
|
|
14 years |
|
|
|
7 years |
|
|
|
10.8 years |
|
|
T2D: type 2 diabetes, BMI: body mass index, RR: relative risk, CI: confidence interval, HR: hazard ratio, OR: odds ratio.
Table 2. Crude and adjusted associations between the consumption of artificially sweetened beverages and the development of metabolic diseases in observational prospective studies.
| Cohort | Pathology | Follow-up | n | Crude risk | Multivariable adjustment | Adiposity adjustment |
|---|---|---|---|---|---|---|
| NHS I [7] | T2D | 24 years | 74,749 | 1.59 (1.47–1.71) | 1.35 (1.24–1.47) | 1.01 (0.93–1.10) |
| NHS II [8] | T2D | 8 years | 91,249 | 1.21 (0.97–1.50) | ---- | ---- |
| Framingham Heart Study [11] | MS | 4 years | 6,039 | 1.42 (1.10–1.84) | 1.53 (1.10–2.15) | ---- |
| ARIC [12] | MS | 9 years | 9,514 | 1.20 (1.11–1.29) | 1.34 (1.24–1.44) | ---- |
| BWHS [14] | T2D | 4 years | 43,960 | 1.06 (0.83–1.36) | ---- | ---- |
| MESA [15] | MS | 7 years | 5,011 | 1.31 (1.07–1.60) | 1.36 (1.11–1.66) | 1.17 (0.96–1.44) |
| MESA [15] | T2D | 7 years | 5,011 | 1.63 (1.24–2.13) | 1.67 (1.27–2.20) | 1.38 (1.04–1.82) |
| HPFS [7] | T2D | 22 years | 39,059 | 1.87 (1.65–2.12) | 1.32 (1.15–1.51) | 1.06 (0.93–1.22) |
| HPFS– 2 [9] | T2D | 20 years | 40,389 | 1.91 (1.72–2.11) | 1.40 (1.26–1.56) | 1.09 (0.98–1.21) |
| CARDIAa [16] | MS | 20 years | 3,728 | 0.81 (0.69–0.95) | ---- | ---- |
| SAHS [13] | OB | 7–8 years | 3,682 | 2.03 (1.36–3.03) | ---- | ---- |
| EPIC [10] | T2D | 16 years | 340,234 | 1.93 (1.47–2.54) | 1.88 (1.44–2.45) | 1.13 (0.85–1.52) |
| EPIC-France [17] | T2D | 14 years | 66,118 | 3.50 (2.49–4.93) | 2.21 (1.56–3.14) | 1.68 (1.19–2.39) |
| EPIC-Norfolk [19] | T2D | 10.8 years | 24,653 | 1.70 (1.35–2.14) | 1.67 (1.33–2.11) | 1.17 (0.93–1.48) |
| Employee Factory Japan [18] | T2D | 7 years | 2,037 | 1.99 (1.33–2.98) | 1.82 (1.22–2.71) | 1.70 (1.13–2.55) |
NHS: Nurses’ Health Study, ARIC: the Atherosclerosis Risk in Communities study, BWHS: the Blacks Women’s Health Study, MESA: the Multi-Ethnic Study of Atherosclerosis, HPFS: the Health Professionals Follow-Up Study, CARDIA: the Coronary Artery Risk Development in Young Adults study, SAHS: the San Antonio Heart Study, EPIC: the European Prospective Investigation into Cancer and Nutrition study, T2D: type 2 diabetes, MS: metabolic syndrome, OB: obesity, n: individuals included in the studies. Associations between the highest range of artificially sweetened beverages consumption and the incidence of the specific metabolic disease studied, expressed in relative risks, odds ratios or hazard ratios with 95% confidence intervals (95% CI).
aThe CARDIA study evaluates the risk of the non-consumers of diet beverages to develop metabolic syndrome compared to the consumers.
Clinical trials
Twenty-eight clinical trials studying different effects of NNS were identified. Of these studies, 10 found significant effects on some or all the studied variables. All of these studies have analyzed glucose and most of them have measured insulin concentrations, 11 quantified GLP-1 concentrations. However, only one study has measured insulin sensitivity and pancreatic response, and another single study has evaluated the changes in the intestinal microbiome. The majority of the clinical trials have evaluated the effects of aspartame (14 trials), followed by sucralose (11 studies), and saccharin, acesulfame-K, and stevia (5 studies for saccharin, 5 for acesulfame-K, and 4 for stevia). Most of these studies have performed an acute single exposure to the NNS (n = 20) and the remaining (n = 8) have evaluated a longer exposure that varies between seven days to 18 weeks. Thirteen studies included individuals with diabetes.
The studies by Pepino [20] and Suez [21] demonstrate a deleterious effect increasing glucose concentrations after an acute and a 7-day exposure to sucralose and saccharin, respectively. Pepino, also reported a decrease in insulin sensitivity along with increased insulin and C-peptide concentrations. Remarkably, this study included subjects with a high degree of obesity (average BMI 42 kg/m2). In the study of Suez after a seven-day period of saccharin ingestion, in four of seven subjects glucose concentrations showed a significant increment. Subsequently, a feces transplant from some of the individuals with the glucose increase after saccharin exposure to mice was performed. After the transplant, glucose concentrations also increased in these mice, suggesting that NNS consumption modify intestinal microbiome in detriment of glucose tolerance. The microbiome showed a significant imbalance with an increase in the Bacteroides genus and Clostridiales order [21].
GLP-1 concentrations, measured in eleven studies, have been shown to be decreased in one report after aspartame ingestion [22] and increased in two studies after sucralose + acesulfame-K and sucralose exposure [23, 24]. Concentrations of appetite-regulating hormones, including cholecystokinin, ghrelin, and peptide YY, have only been studied in three studies. In none of them changes in the concentrations of these variables were found. In addition, no change in the subjective appetite ratings or on the quantity of food consumed after NNS exposure has been found.
On Table 3 the description and results of these studies are shown. As a reference, one 12 oz diet-coke contains approximately 140 mg of aspartame and acesulfame K mix, one 12 oz diet-Dr. Pepper can contains approximately 65 and 22 mg of sucralose and acesulfame, respectively, and one 12-oz Coca-Cola Life can contains 27 mg of stevia. Some of the NNS available as individual packets include Sweet and Low, containing 34 mg of saccharin, and Splenda containing 12 mg of sucralose. On Table 4 a summary of the studies indicating the methodology used, studied variables, and the NNS evaluated are presented.
Table 3. Clinical trials evaluating the effect of non-nutritive sweeteners consumption on glucose metabolism and appetite regulating hormones.
| No. | Author and year | Population | Methodology | Variables | Results |
|---|---|---|---|---|---|
| 1 |
|
62 subjects with diabetes (31 insulin-dependent and 31 non-insulin-dependent) aged 18–65 years |
|
|
No changes in plasma glucose or HbA1c levels during the treatment. |
| 2 |
|
|
|
|
|
| 3 |
|
12 normal subjects and 10 subjects with non-insulin-dependent diabetes aged 18–65 years |
|
|
|
| 4 |
|
17 subjects with non-insulin-dependent diabetes, aged 62.2±14.0 years, and BMI 26.0±3.0 kg/m2 |
|
|
No changes on glucose, insulin or triglycerides were found with the saccharin ingestion |
| 5 |
|
9 subjects with non-insulin-dependent diabetes, aged 66±5 years, and BMI 26.4±2.1 kg/m2 |
|
|
Aspartame ingestion did not generate changes on any of the variables measured |
| 6 |
|
12 overweight and 12 normal-weight subjects, aged 22–50 years |
|
|
|
| 7 |
|
14 healthy subjects aged 19–52 years with normal glucose tolerance |
|
|
|
| 8 |
|
13 subjects with T1D and 13 subjects with T2D (HbA1c <10%) |
|
|
|
| 9 |
|
10 healthy non-smorkers men, aged 19–31 years, BMI 23.4±1.9 kg/m2 |
|
|
|
| 10 |
|
128 subjects with T2D, aged 31–70 years, and HbA1c levels ≤10% |
|
|
No effects were found on glucose, C-peptide or changes in HbA1c after sucralose consumption |
| 11 |
|
6 subjects aged 24–31 years and BMI <25 kg/m2 |
|
|
|
| 12 |
|
12 subjects with T2D, BMI 25–32 kg/m2, and HbA1c levels <10% |
|
|
|
| 13 |
|
|
|
|
Steviol glycosides did not generate changes on any of the studied variables |
| 14 |
|
122 subjects with diabetes aged 33–75 years |
|
|
The consumption of rebaudioside A over 16 weeks did not shown effects in any variable |
| 15 |
|
7 healthy subjects with BMI 21.6±1.2 kg/m2, age 24±2 years, non-smokers, and alcohol consumption <20 g per day |
|
|
Sucralose did not showed effects at any dose on glucose, insulin, GLP-1, GIP, and gastric emptying compared to saline |
| 16 |
|
|
|
|
|
| 17 |
|
10 healthy subjects, with BMI 23.4±0.8 kg/m2, and age 27±2 years |
|
|
No effects on glucose intestinal absorption or GLP-1 secretion were observed with sucralose consumption |
| 18 |
|
8 healthy subjects aged 22–27 years, with BMI 18.8 kg/m2, and non-smokers |
|
|
|
| 19 |
|
8 female volunteers with BMI 22.16±1.71 kg/m2, aged 21.75±2.25 years, non-smokers, without diabetes or alcohol consumption |
|
|
No significant differences were observed in any of the variables with the consumption of sucralose compared to water |
| 20 |
|
12 healthy subjects aged 23.3±0.7 years, BMI 23.0±0.5 kg/m2, non-smokers and without chronic diseases |
|
|
|
| 21 |
|
|
|
|
Aspartame-containing beverage did not showed effects on any of the variables |
| 22 |
|
10 healthy subjects aged 28.8±4.0 years, and BMI 25.5±1.5 kg/m2 |
|
|
|
| 23 |
|
|
|
|
|
| 24 |
|
80 subjects with T2D aged 49.3±9.06 years, BMI 30.5±4.30 kg/m2, and less than 10 years of diabetes evolution |
|
Capillary glucose | No effects of diet soda on capillary glucose levels |
| 25 |
|
17 subjects with BMI 42.3±1.6 kg/m2 with low previous NNS consumption (less than one can of diet beverage or one spoonful of NNS per week) |
|
|
|
| 26 |
|
|
|
|
|
| 27 |
|
|
|
|
|
| 28 |
|
8 newly diagnosed T2D subjects without pharmacological treatment, aged 51.5±9.2 years and 8 apparently healthy subjects aged 45.0±4.1 years |
|
|
|
BMI: body mass index, HbA1c: glycated hemoglobin, HDL: high density lipoproteins, LDL: low density lipoproteins, T1D: type 1 diabetes, T2D: type 2 diabetes, VAS: visual analogue scales, GLP-1: glucagon like peptide type 1, GIP: glucose-dependent insulinotropic peptide, CCK: cholecystokinin, PYY: tyrosine tyrosine peptide, NNS: non-nutritive sweeteners, OGTT: oral glucose tolerance test, AUC: area under the curve.
Table 4. Summary of the studied variables, non-nutritive sweetener used, study methodology and findings of the clinical trials evaluated in Table 3.
| Study number (according to Table 3) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | |
| Glucose | ||||||||||||||||||||||||||||
| Insulin | ||||||||||||||||||||||||||||
| GLP-1 | ||||||||||||||||||||||||||||
| GIP | ||||||||||||||||||||||||||||
| HbA1c | ||||||||||||||||||||||||||||
| C-peptide | ||||||||||||||||||||||||||||
| Glucagon | ||||||||||||||||||||||||||||
| PYY | ||||||||||||||||||||||||||||
| Ghrelin | ||||||||||||||||||||||||||||
| CCK | ||||||||||||||||||||||||||||
| Triglycerides | ||||||||||||||||||||||||||||
| VAS to measure appetite | ||||||||||||||||||||||||||||
| Caloric intake | ||||||||||||||||||||||||||||
| Insulin sensitivity | ||||||||||||||||||||||||||||
| β-Cell function | ||||||||||||||||||||||||||||
| Insulin clearance | ||||||||||||||||||||||||||||
| Insulinogenic index | ||||||||||||||||||||||||||||
| Gut microbiota | ||||||||||||||||||||||||||||
| Gastric emptying | ||||||||||||||||||||||||||||
| Saccharin | ||||||||||||||||||||||||||||
| Aspartame | ||||||||||||||||||||||||||||
| Acesulfame-K | ||||||||||||||||||||||||||||
| Sucralose | ||||||||||||||||||||||||||||
| Stevia | ||||||||||||||||||||||||||||
| Short-term exposition | ||||||||||||||||||||||||||||
| Crossover design | ||||||||||||||||||||||||||||
| Had found effects* | ||||||||||||||||||||||||||||
GLP-1: glucagon like peptide type 1, GIP: glucose-dependent insulinotropic peptide, HbA1c: glycated hemoglobin, PYY: tyrosine tyrosine peptide, CCK: cholecystokinin, VAS: visual analogue scales
*This refers to studies that have found significant changes in some or all the studied variables without signifying positive or negative effects. For review of these effects, please refer to Table 3.
Meta-analysis
Two meta-analyses have been published to evaluate the association between the consumption of NNS-containing beverages and the development of type 2 diabetes to clarify if this relation is clearly linked to the consumption of these products or related to other lifestyle factors. Both meta-analyses evaluated the association between NNS consumption, without specifying of stratifying for the specific NNS ingested. While both studies excluded cohorts including individuals with a known diagnosis of diabetes, the article by Grenwood only included four studies. This may be due to the selection criteria that specify that only studies including individuals “from a generally healthy population” were considered [48]. In contrast, the study by Imamura evaluated ten studies estimating the risk of type 2 diabetes associated to consumption of NNS-containing beverages [49]. None of the studies disclosed significant competing interests.
In the first meta-analysis that included 4 observational prospective studies, the pooled estimated relative risk (RR) was 1.13 (95% CI: 1.02–1.25; P = 0.02) for the consumption of 330 ml per day of artificially sweetened beverages and the development of type 2 diabetes. There was high heterogeneity between studies, and the positive association was less consistent for this type of beverages compared to the sugar-sweetened drinks [48].
In the second meta-analysis with 10 studies included, the crude RR was 1.48 (95% CI: 1.35–1.62; P<0.05). However, after adjustment for BMI and the calibration for information and publication bias, the association was no longer statistically significant (RR: 1.22; 95% IC: 0.98–1.52; P = 0.07) [49].
Discussion
The aim of this systematic review is to evaluate the scientific available evidence regarding the association between NNS consumption and metabolic diseases as well as the effects of NNS on glucose metabolism and appetite regulating hormones. The results indicate that the association between NNS intake and the development of metabolic diseases, mainly type 2 diabetes, is not clear. A common identified confounding factor in the observational prospective studies is adiposity. In addition, it is unknown if the NNS are associated with deleterious effects on glucose metabolism or appetite regulation. Based on the available evidence, an effect of NNS on glucose metabolism cannot be established. The study of appetite and its regulation is complex, the evidence presented concerning this issue is scarce and an effect of NNS in appetite cannot be demonstrated either. The studies found are varied regarding the NNS studied; therefore, a class effect cannot be determined and no solid conclusions regarding a specific NNS can be stated.
A possible explanation for the associations found in some of the observational studies among NNS consumption and the development of metabolic diseases might be that these cohorts included participants prone to develop these outcomes, for example with family members with diabetes or with a predisposition for weight gain, that are likely to consume these products. For example, people with higher BMI, already at risk to develop diabetes, consume NNS-containing beverages as a strategy to minimize calorie intake.
An additional limitation of observational studies is that the majority has evaluated the consumption of NNS containing-beverages and the non-consuming population may actually have consumed these substances from other non-acknowledged products. Finally, the evidence level of observational studies cannot establish causality.
Most of the clinical trials included have small sample sizes and the majority does not provide a justification for these calculations. Many of the clinical trials are crossover studies and a main limitation of this design is the residual effect between treatments. In most cases there is no information regarding the washout period. Another variable that needs to be considered is the amount of NNS used and the exposition type, for example acute or long-term exposition. Moreover, there is no uniformity in the exposition time between studies evaluating a long-term exposure. Finally, a number of confounding variables are not mentioned or adjusted in these trials, including BMI, previous NNS intake, and presence of metabolic alterations such as glucose intolerance or diabetes, among others. These drawbacks may confuse the results presented.
We can conclude that some clinical trials have found effects of NNS on glucose metabolism. However the results are contradictory and there is no possible comparison between the trials due to the heterogeneity in the population included, NNS studied, placebo use, exposure time, outcomes evaluated, among many other. For example, after sucralose consumption, one study reported higher concentrations of glucose, however, another study report lower concentrations and nine studies did not observed changes in glucose. In addition, two studies found that sucralose increase GLP-1 levels compared to water, an effect that other six studies could not confirm. One study found that sucralose decreases insulin sensitivity and insulin clearance in morbid obese population, nevertheless, this is the only one trial that has evaluated these outcomes.
The consumption of aspartame showed lower concentrations of glucose in two of fourteen studies, one compared to water and the other one to glucose. One study observed lower concentrations of insulin after aspartame vs. sucrose and another study found higher concentrations of insulin after aspartame vs. water. Finally, one trial reported that aspartame decreases GLP-1 concentrations compared to placebo.
For stevia, one trial observed lower glucose and insulin concentrations compared to sucrose, and another study found lower glucose concentrations and an increment in the insulinogenic index compared to placebo.
One trial reflected an important impact of saccharin consumption for seven days promoting glucose intolerance in four of seven subjects studied; this trial suggest that this effect is caused by altering the gut microbiome performing a fecal transplantation to mice showing a similar increase in glucose levels. In contrast, one study showed lower glucose and insulin concentrations after saccharin ingestion compared to water or sucrose, respectively.
The findings of the two meta-analyses should be interpreted cautiously. In the first report few studies were included, without considering other variables that may be involved in the development of diabetes, and in the second the association between NNS-containing beverages and development of diabetes was lost after the adjustment for body mass index, indicating that adiposity may be influencing the findings.
Randomized clinical trials testing each of the NNS, including a homogeneous group of participants, without metabolic conditions that may confound the results, including an adequate sample size, with an appropriate control group, during an appropriate exposure time, and considering adjustment or control for significant variables such as adiposity are needed. In addition, the mechanisms involved in the glucose metabolism changes after a long-term exposition to NNS should explored in human studies.
Based on the scientific evidence presented, the consumption of NNS is not encouraged, but they could be considered a useful tool in the nutritional treatment of certain metabolic diseases as sugar substitutes as long as the quantity consumed is within the acceptable daily intake (ADI) and without compensation by ingesting other energy-rich foods. Lastly, health professionals should not promote the consumption of sweet tasting foods regardless its source.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The authors have no support or funding to report.
References
- 1.Barquera S, Hernandez-Barrera L, Tolentino ML, Espinosa J, Ng SW, Rivera JA, et al. Energy intake from beverages is increasing among Mexican adolescents and adults. The Journal of nutrition. 2008;138(12):2454–61. 10.3945/jn.108.092163 . [DOI] [PubMed] [Google Scholar]
- 2.Shankar P, Ahuja S, Sriram K. Non-nutritive sweeteners: review and update. Nutrition. 2013;29(11–12):1293–9. 10.1016/j.nut.2013.03.024 . [DOI] [PubMed] [Google Scholar]
- 3.Food U.S. and Drug Administration. Additional Information about High-Intensity Sweeteners Permitted for use in Food in the United States 2015 [updated 05/25/2015; cited 2016 02/01/2016]. Available: http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm.
- 4.European Food Safety Authority [Internet]. Sweetners [cited 2016 Feb 01]. Available: http://www.efsa.europa.eu/en/topics/topic/sweeteners.
- 5.Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiology & behavior. 2015;152(Pt B):450–5. 10.1016/j.physbeh.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qurrat ul A, Khan SA. Artificial sweeteners: safe or unsafe? JPMA The Journal of the Pakistan Medical Association. 2015;65(2):225–7. . [PubMed] [Google Scholar]
- 7.Bhupathiraju SN, Pan A, Malik VS, Manson JE, Willett WC, van Dam RM, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. The American journal of clinical nutrition. 2013;97(1):155–66. 10.3945/ajcn.112.048603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004;292(8):927–34. 10.1001/jama.292.8.927 . [DOI] [PubMed] [Google Scholar]
- 9.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. The American journal of clinical nutrition. 2011;93(6):1321–7. 10.3945/ajcn.110.007922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.InterAct C, Romaguera D, Norat T, Wark PA, Vergnaud AC, Schulze MB, et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56(7):1520–30. 10.1007/s00125-013-2899-8 . [DOI] [PubMed] [Google Scholar]
- 11.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480–8. 10.1161/CIRCULATIONAHA.107.689935 . [DOI] [PubMed] [Google Scholar]
- 12.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–61. 10.1161/CIRCULATIONAHA.107.716159 . [DOI] [PubMed] [Google Scholar]
- 13.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16(8):1894–900. 10.1038/oby.2008.284 . [DOI] [PubMed] [Google Scholar]
- 14.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Archives of internal medicine. 2008;168(14):1487–92. 10.1001/archinte.168.14.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes care. 2009;32(4):688–94. 10.2337/dc08-1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffey KJ, Steffen LM, Van Horn L, Jacobs DR Jr., Popkin BM. Dietary patterns matter: diet beverages and cardiometabolic risks in the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) Study. The American journal of clinical nutrition. 2012;95(4):909–15. 10.3945/ajcn.111.026682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l'Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. The American journal of clinical nutrition. 2013;97(3):517–23. 10.3945/ajcn.112.050997 . [DOI] [PubMed] [Google Scholar]
- 18.Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Nagasawa SY, et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. European journal of nutrition. 2014;53(4):1137–8. 10.1007/s00394-014-0681-4 . [DOI] [PubMed] [Google Scholar]
- 19.O'Connor L, Imamura F, Lentjes MA, Khaw KT, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia. 2015;58(7):1474–83. 10.1007/s00125-015-3572-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepino MY, Tiemann CD, Patterson BW, Wice BM, Klein S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes care. 2013;36(9):2530–5. 10.2337/dc12-2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. 10.1038/nature13793 . [DOI] [PubMed] [Google Scholar]
- 22.Hall WL, Millward DJ, Rogers PJ, Morgan LM. Physiological mechanisms mediating aspartame-induced satiety. Physiology & behavior. 2003;78(4–5):557–62. . [DOI] [PubMed] [Google Scholar]
- 23.Brown AW, Bohan Brown MM, Onken KL, Beitz DC. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutrition research. 2011;31(12):882–8. 10.1016/j.nutres.2011.10.004 . [DOI] [PubMed] [Google Scholar]
- 24.Temizkan S, Deyneli O, Yasar M, Arpa M, Gunes M, Yazici D, et al. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. European journal of clinical nutrition. 2015;69(2):162–6. 10.1038/ejcn.2014.208 . [DOI] [PubMed] [Google Scholar]
- 25.Nehrling JK, Kobe P, McLane MP, Olson RE, Kamath S, Horwitz DL. Aspartame use by persons with diabetes. Diabetes care. 1985;8(5):415–7. . [DOI] [PubMed] [Google Scholar]
- 26.Okuno G, Kawakami F, Tako H, Kashihara T, Shibamoto S, Yamazaki T, et al. Glucose tolerance, blood lipid, insulin and glucagon concentration after single or continuous administration of aspartame in diabetics. Diabetes research and clinical practice. 1986;2(1):23–7. . [DOI] [PubMed] [Google Scholar]
- 27.Horwitz DL, McLane M, Kobe P. Response to single dose of aspartame or saccharin by NIDDM patients. Diabetes care. 1988;11(3):230–4. . [DOI] [PubMed] [Google Scholar]
- 28.Cooper PL, Wahlqvist ML, Simpson RW. Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: short- and medium-term metabolic effects. Diabetic medicine: a journal of the British Diabetic Association. 1988;5(7):676–80. . [DOI] [PubMed] [Google Scholar]
- 29.Colagiuri S, Miller JJ, Edwards RA. Metabolic effects of adding sucrose and aspartame to the diet of subjects with noninsulin-dependent diabetes mellitus. The American journal of clinical nutrition. 1989;50(3):474–8. . [DOI] [PubMed] [Google Scholar]
- 30.Rodin J. Comparative effects of fructose, aspartame, glucose, and water preloads on calorie and macronutrient intake. The American journal of clinical nutrition. 1990;51(3):428–35. . [DOI] [PubMed] [Google Scholar]
- 31.Härtel B G H. Schneider B. Bier A.. The influence of sweetener solutions on the secretion of insulin and blood glucose level. Ernährungsunschau. 1993;40(4):152–5. [Google Scholar]
- 32.Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes care. 1996;19(9):1004–5. . [DOI] [PubMed] [Google Scholar]
- 33.Melanson KJ, Westerterp-Plantenga MS, Campfield LA, Saris WH. Blood glucose and meal patterns in time-blinded males, after aspartame, carbohydrate, and fat consumption, in relation to sweetness perception. The British journal of nutrition. 1999;82(6):437–46. . [PubMed] [Google Scholar]
- 34.Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. Journal of the American Dietetic Association. 2003;103(12):1607–12. 10.1016/j.jada.2003.09.021 . [DOI] [PubMed] [Google Scholar]
- 35.Gregersen S, Jeppesen PB, Holst JJ, Hermansen K. Antihyperglycemic effects of stevioside in type 2 diabetic subjects. Metabolism: clinical and experimental. 2004;53(1):73–6. . [DOI] [PubMed] [Google Scholar]
- 36.Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N, et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regulatory toxicology and pharmacology: RTP. 2008;51(1):37–41. 10.1016/j.yrtph.2008.02.006 . [DOI] [PubMed] [Google Scholar]
- 37.Maki KC, Curry LL, Reeves MS, Toth PD, McKenney JM, Farmer MV, et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2008;46 Suppl 7:S47–53. 10.1016/j.fct.2008.05.007 . [DOI] [PubMed] [Google Scholar]
- 38.Ma J, Bellon M, Wishart JM, Young R, Blackshaw LA, Jones KL, et al. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. American journal of physiology Gastrointestinal and liver physiology. 2009;296(4):G735–9. 10.1152/ajpgi.90708.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anton SD, Martin CK, Han H, Coulon S, Cefalu WT, Geiselman P, et al. Effects of stevia, aspartame, and sucrose on food intake, satiety, and postprandial glucose and insulin levels. Appetite. 2010;55(1):37–43. 10.1016/j.appet.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, Chang J, Checklin HL, Young RL, Jones KL, Horowitz M, et al. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. The British journal of nutrition. 2010;104(6):803–6. 10.1017/S0007114510001327 . [DOI] [PubMed] [Google Scholar]
- 41.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, et al. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. European journal of clinical nutrition. 2011;65(4):508–13. 10.1038/ejcn.2010.291 . [DOI] [PubMed] [Google Scholar]
- 42.Steinert RE, Frey F, Topfer A, Drewe J, Beglinger C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. The British journal of nutrition. 2011;105(9):1320–8. 10.1017/S000711451000512X . [DOI] [PubMed] [Google Scholar]
- 43.Maersk M, Belza A, Holst JJ, Fenger-Gron M, Pedersen SB, Astrup A, et al. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: a controlled trial. European journal of clinical nutrition. 2012;66(4):523–9. 10.1038/ejcn.2011.223 . [DOI] [PubMed] [Google Scholar]
- 44.Wu T, Zhao BR, Bound MJ, Checklin HL, Bellon M, Little TJ, et al. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. The American journal of clinical nutrition. 2012;95(1):78–83. 10.3945/ajcn.111.021543 . [DOI] [PubMed] [Google Scholar]
- 45.Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes care. 2012;35(5):959–64. 10.2337/dc11-2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olalde-Mendoza L, Moreno-Gonzalez YE. [Modification of fasting blood glucose in adults with diabetes mellitus type 2 after regular soda and diet soda intake in the State of Queretaro, Mexico]. Archivos latinoamericanos de nutricion. 2013;63(2):142–7. . [PubMed] [Google Scholar]
- 47.Bryant CE, Wasse LK, Astbury N, Nandra G, McLaughlin JT. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. European journal of clinical nutrition. 2014;68(5):629–31. 10.1038/ejcn.2014.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenwood DC, Threapleton DE, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. The British journal of nutrition. 2014;112(5):725–34. 10.1017/S0007114514001329 . [DOI] [PubMed] [Google Scholar]
- 49.Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Bmj. 2015;351:h3576 10.1136/bmj.h3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.