Abstract
Chronic wounds represent a significant burden to health services and are associated with patient morbidity. Novel methods to diagnose and/or treat problematic wounds are needed. Interleukin (IL)-15 is a cytokine involved in a number of biological processes and disease states such as inflammation, healing and cancer progression. The current study explores the expression profile of IL-15 and IL-15 receptor α (IL-15Rα) in chronic wounds and its impact on keratinocytes. IL-15 and IL-15Rα expression were examined in healing and non-healing chronic wounds using qPCR and immunohistochemical analysis. The impact of recombinant IL-15 (rhIL-15) on human adult low calcium temperature (HaCaT) keratinocyte growth and migratory potential was further examined. IL-15 transcript expression was slightly, though non-significantly elevated in healing chronic wounds compared with non-healing chronic wounds. IL-15 protein staining was minimal in both subtypes of chronic wounds. By contrast, IL-15Rα transcript and protein expression were both observed to be enhanced in non-healing chronic wounds compared with healing chronic wounds. The treatment of HaCaT cells with rhIL-15 generally enhanced cell growth and promoted migration. Analysis with small molecule inhibitors suggested that the pro-migratory effect of rhIL-15 may be associated with ERK, AKT, PLCγ and FAK signalling. IL-15 may promote healing traits in keratinocytes and the differential expression of IL-15Rα is observed in chronic wounds. Together, this may imply a complex role for this interleukin in wound healing.
Keywords: interleukin-15, wound healing, migration, chronic wound
Introduction
The majority of wounds, such as a surgical incision or superficial injury will heal in an orderly manner within an acceptable period of time. In a chronic wound, there is often an underlying pathological process, either causing a prolonged injury to tissues or greatly impairing normal healing (1). Wounds that fail to heal within 3 months are considered chronic and 3.55/1,000 people in the UK and 3% of those over 60 years of age suffer from chronic wounds (2). The prevalence is likely to rise with an ageing population. Chronic wounds have a major financial impact. The cost to the NHS of frequent nurse and doctor appointments, dressings as well as outpatient and inpatient treatment complications is significant. The cost of chronic wound management in the UK has been estimated to be between £2.3 and £3.1 billion annually (3) or 2–3% of the NHS budget (4).
The process of repairing tissue after an injury is complex, involving overlapping steps. The phases are tightly regulated by a plethora of cytokines and growth factors controlling multiple cell types to proliferate, differentiate and migrate. Originally, 4 distinct phases were described. Namely: haemostasis, inflammation, proliferation and maturation or remodelling (5). In an acute wound, the phases progress in an orderly and timely fashion, with the prompt achievement of healing. Chronic wounds can become arrested in one of the above phases of healing or the phases progress more slowly than normal. In a chronic wound, the complex cascade of cytokines and cellular activity is impaired leading to prolonged inflammation, defective wound matrix and a failure to re-epithelialize. Several cytokines have been implicated as having a role in the process of wound healing. As inflammation is a key stage in wound healing, it is perhaps not surprising that many of these cytokines are known to be regulators of inflammation.
One such pro-inflammatory cytokine is interleukin (IL)-15. IL-15 was identified and characterised as a 14–18 kDa protein using the simian kidney epithelial cell line CV-1/EBNA (6). The data collected from this study allowed for the isolation of human IL-15 by specifically probing the human stromal cell line IMTLH cDNA library (6). The human IL-15 structure was defined as a four α-helical bundle cytokine and is located on the human chromosome 4q31 (6). IL-15 can exert its biological effects using two separate signalling pathways, the first of which involves conventional binding and signalling via the IL-15 receptor α (IL-15Rα), β and γ chains. IL-15 and its family member IL-2 are separate entities with different sequence homologies, but there is data to prove significant cross-over between IL-15 and the β and γ chains of the IL-2 receptor (6,7). This then results in the upregulation of natural killer (NK) cell and T-cell activity (6,8,9). Conversely, IL-15 in mast cells binds to its cognate IL-15RX receptor and does not require IL-2 receptor input (9,10). However, the mRNA expression of IL-15 in activated T cells is not a significant alternative to the large pools of IL-2 present (6). Previous findings confirm that IL-15Rα and its juxtracrine-signalling ligand IL-15 are present in human adult low calcium temperature (HaCaT) keratinocytes (11). Previous studies have explored IL-15 in wound biology, regeneration and inflammation and IL-15 has been demonstrated to promote the proliferation of the human keratinocyte HaCaT cell line, through ERK- and AKT-dependent pathways and to exert anti-apoptotic effects on epidermal keratinocytes (12,13). Other evidence has suggested that IL-15 promotes wound healing responses in the liver and may enhance regeneration following liver damage (14). A study by Kagimoto et al using transgenic IL-15 mouse models, has suggested a regulatory role for IL-15 in wound healing and mucosal infection, where transgenic IL-15 mice displayed accelerated wound healing but enhanced susceptibility to genital HSV-2 infection (15). IL-15 has also been implicated in a range of inflammatory diseases [reviewed in (16)].
As the clinical field of chronic wounds is such a significant burden, new diagnostic markers and potential therapeutic targets are needed. This study aims to assess whether IL-15 has a role in the healing of chronic wounds and to assess its effects in vitro on keratinocyte growth and migration.
Materials and methods
Cells and materials
The HaCaT cell line was purchased from the German Cancer Research Institute (Heidelberg, Germany). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 with L-glutamine medium (Sigma-Aldrich, Gillingham, UK) supplemented with antibiotics (final concentration streptomycin 0.1 mg/ml, penicillin 100 U/ml, amphotericin 0.25 µg/ml) and 10% foetal calf serum (Sigma-Aldrich). Cells were routinely cultured in 25 or 75 cm2 tissue culture flasks (Greiner Bio-One Ltd., Stonehouse, UK) and incubated at 37°C, 5% CO2 and 95% humidity.
Recombinant IL-15 (rhIL-15) was purchased from Insight Biotechnology (Wembley, UK). ERK inhibitor II, AKT inhibitor and PLCγ inhibitor (U73122) were purchased from Calbiochem (Merck Chemicals Ltd., Nottingham, UK). FAK inhibitor was purchased from Tocris (Bristol, UK).
Clinical samples
Biopsies were taken from chronic venous leg ulcers in a cohort of 71 patients. The inclusion criteria were as follows: no evidence of other diagnosis, no evidence of infection and ulcer present for at least 3 months. The samples were collected at the wound healing clinic, University Hospital of Wales (UHW; Cardiff, UK), after obtaining ethics approval from the South East Wales Research Ethics Committee and informed written consent from the subjects. The patients were treated for 12 weeks with standard treatment. Specifically, this consisted of regular wound care with appropriate dressings and compression. Compression was produced with 3 layers of Tubigrip™ bandages (Mölnlycke Health Care, Dunstable, UK) at an appropriate size to deliver 40 mmHg of pressure. At the end of the 12 week treatment period, the wounds were assessed. The biopsies from those that had decreased in size or healed were termed 'healing'. The biopsies from those that had increased in size or had shown no change were termed 'non-healing'. The cohort has been described previously (17).
RNA extraction, quantification and reverse transcription
RNA was extracted using TRI reagent (Sigma-Aldrich) in accordance with the manufacturer's instructions. In brief, medium was aspirated from a flask containing a confluent monolayer of cells and the cells were lysed in TRI reagent. The cell lysate was placed in a 1.8 ml Eppendorf tube and incubated at 4°C for 5 min. Chloroform (0.2 ml) was subsequently added and the solution thoroughly shaken for 15 sec before standing for 15 min at room temperature. Samples were then centrifuged at 12,000 × g for 15 min at 4°C and the upper aqueous phase was removed with a pipette and added to an equal volume of isopropanol. This sample was incubated at 4°C for 10 min and then centrifuged at 12,000 × g for 10 min to precipitate RNA. The RNA pellet was washed twice in 75% ethanol in DEPC water before being dried in a drying oven (Techne Hybridiser HB-1D; Wolf Laboratories, York, UK) for 10 min at 55°C. The RNA pellet was dissolved in 50–100 µl of DEPC water prior to quantification using an Implen nanophotometer (Implen Gmbh, Munich, Germany) and standardisation.
Following quantification, standardised amounts of RNA were reverse transcribed to generate cDNA using a reverse transcription kit (iScript cDNA synthesis kit; Bio-Rad Laboratories, Hemel Hempstead, UK). Subsequently, cDNA was diluted appropriately and stored at −20°C until required.
Polymerase chain reaction (PCR)
RT-PCR was performed using the GoTaq Green master mix (Promega, Madison, WI, USA). In each reaction, GoTaq Green master mix, forward primer (10 pmol), reverse primer (10 pmol) and nuclease-free water were added to cDNA in a 200 µl PCR tube. The primers used in this study are detailed in Table I. Primer3 software was used to identify primer binding sites and predicted product size. The specific cycling conditions were 94°C for 5 min followed by 32–34 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 40 sec. Following this, a final extension was undertaken at 72°C for 10 min. Following PCR, samples were size separated using gel electrophoresis, stained in SYBR Safe (Life Technologies, Paisley, UK) and visualised under blue light using a Syngene U:Genius 3 system (Geneflow, Lichfield, UK).
Table I.
Primers used in the study.
| Primer | Sense | Antisense |
|---|---|---|
| GAPDH | GGCTGCTTTTAACTCTGGTA | GACTGTGGTCATGAGTCCTT |
| IL-15 | AACAGTCATTTTCTAACTGAAGC | ACTGAACCTGACCGTACATTCAAATCACTTATTACATTCACC |
| IL-15Rα | TCTCAGACAACAGCCAAGA | ACTGAACCTGACCGTACAGACAGTGGACGTGGAGATAG |
| GAPDH (qPCR) | AAGGTCATCCATGACAACTT | ACTGAACCTGACCGTACAGCCATCCACAGTCTTCTG |
ACTGAACCTGACCGTACA represents the z sequence. IL-15, interleukin 15; IL-15Rα, IL-15 receptor α.
Quantitative PCR (qPCR)
The Ampliflor UniPrimer Universal system (Intergen Co., Purchase, NY, USA) was used to quantify relative transcript copy numbers in each cDNA sample as previously described (18). Sample cDNA was combined with 2X iQ supermix (Bio-Rad Laboratories), forward primer (10 pmol), reverse primer containing the z sequence (1 pmol) and a UniPrimer probe (10 pmol). The specific primers are outlined in Table I, and the cycling conditions were initial denaturing at 94°C for 5 min followed by 60 cycles of 94°C for 10 sec, 55°C for 15 sec and 72°C for 20 sec. The incorporation and unfolding of the UniPrimer probe, due to its similarity to the z sequence, separates a fluorophore from a quencher moiety and allows detection of the fluorescent signal which is indicative of DNA amplification. Quantification of this signal against a standard set of samples allows the quantification of relative transcript copy numbers. Sample quantity was further normalised against the GAPDH housekeeping gene levels present in the samples.
In vitro growth assay
Cells were seeded into duplicate 96-well plates at a density of 3,000 cells/well. Subsequently, the same volume of either double concentrate rhIL-15 or normal medium was added to the wells to give final concentrations of 1, 10, 40 or 100 ng/ml as required. Each 96-well plate was incubated at 37°C with 5% CO2, either overnight or for 3 days. At the end of the incubation period, the medium was aspirated and the cells in each well were fixed in 4% formaldehyde (v/v) (Sigma-Aldrich) for 10 min. The formaldehyde was then removed and each well was stained with crystal violet (0.5% w/v) (Sigma-Aldrich) for 10 min. The crystal violet was then washed off with water and re-suspended in 10% acetic acid (Sigma-Aldrich) prior to reading absorbance on a plate reading spectrophotometer (ELx800; Bio-Tek, Swindon, UK). Percentage growth after a 3-day incubation period was calculated against the overnight plates.
Scratch assay
Cells were grown to confluence in a 24-well plate. Following this, the confluent monolayer was scratched using a 200 µl pipette tip to generate an artificial wound. Subsequently, the media was replaced with either normal media or media containing 100 ng/ml rhIL-15 and the wound tracked over a 120 min time period under an inverted microscope (Leica DMIL LED) on a heated plate at 37°C (both from Leica Microsystems GmbH, Wetzlar, Germany). Images were captured every 30 min using a digital camera (Leica DFC3000G; Leica Microsystems GmbH) and the distance between the wound fronts were calculated using ImageJ software. The distance migrated was calculated in comparison to the 0 h wound.
Electric cell-substrate impedance sensing (ECIS)
ECIS is a novel, real time method used to measure the rate of cells repopulating an area from which they have been eliminated using electrical wounding (19). This method can be used to screen large sample numbers and has been previously described (17). In brief, the ECIS Zθ system with 96WE1 array plates were used (Applied Biophysics Inc., Troy, NY, USA). Plates were stabilised prior to the addition of a standardised number of HaCaT cells and either rhIL-15 treatment or control media and incubated at 37°C. Resistance of the cells was monitored by the system and once confluence was achieved, the monolayer was wounded electrically (3,000 µA for 30 sec/well). Following wounding, the change in resistance across the array was monitored and recorded as cells migrated back into the wounded area.
Immunohistochemical (IHC) staining
A representative subset of tissues from chronic wounds that contained both healing (n=12) and non-healing (n=11) wounds was immunhistochemically analysed using a standard peroxidase technique. Briefly, 7 µm frozen sections were firstly fixed in dried acetone (Thermo Fisher Scientific, Loughborough, UK) for 15 min, air dried for a further 15 min, prior to rehydration in PBS. This was then followed by a permeabilisation step where the sections were washed with 0.1% saponin/PBS (Sigma-Aldrich) for 30 min. All subsequent washes contained 0.1% saponin/PBS since this reaction is reversible. The sections were then blocked for 1 h with a solution that contained 0.1% BSA/0.1% saponin/10% horse serum in PBS. All incubations were performed in a humidified box in order to prevent the sections from drying. Excess blocking solution was then removed and the sections incubated with the relevant primary antibody for 1 h. Two primary antibodies were used: anti-IL-15 (sc-8437;) and anti-IL-15Rα (sc-9172) (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at a concentration of 1:100 (final concentration 2 µg/ml). The sections were then washed with 0.1% saponin/PBS, prior to incubation for 30 min with biotinylated secondary antibody (Vector Laboratories, Nottingham, UK). Following washing, and a further 30 min incubation with the avidinbiotin complex (ABC) reagent, the final reaction product was developed with 3′3-diaminobenzidine solution (5 mg/ml). The sections were then washed in tap water, counterstained with Gill's hematoxylin (Vector Laboratories), dehydrated, cleared in xylene (Thermo Fisher Scientific) and mounted in DPX (Merck Chemicals Ltd.). Negative controls were performed by omitting the primary antibody and replacing it with PBS.
Statistical analysis
The SigmaPlot 11 statistical package was used for statistical analysis. Two-sample, two-tailed t-tests, Mann-Whitney U test and one or two way ANOVA analysis were undertaken depending on data normality. Experimental repeats were undertaken for all assays and p<0.05 was considered to indicate a statistically significant difference.
Results
Expression of IL-15 in clinical samples
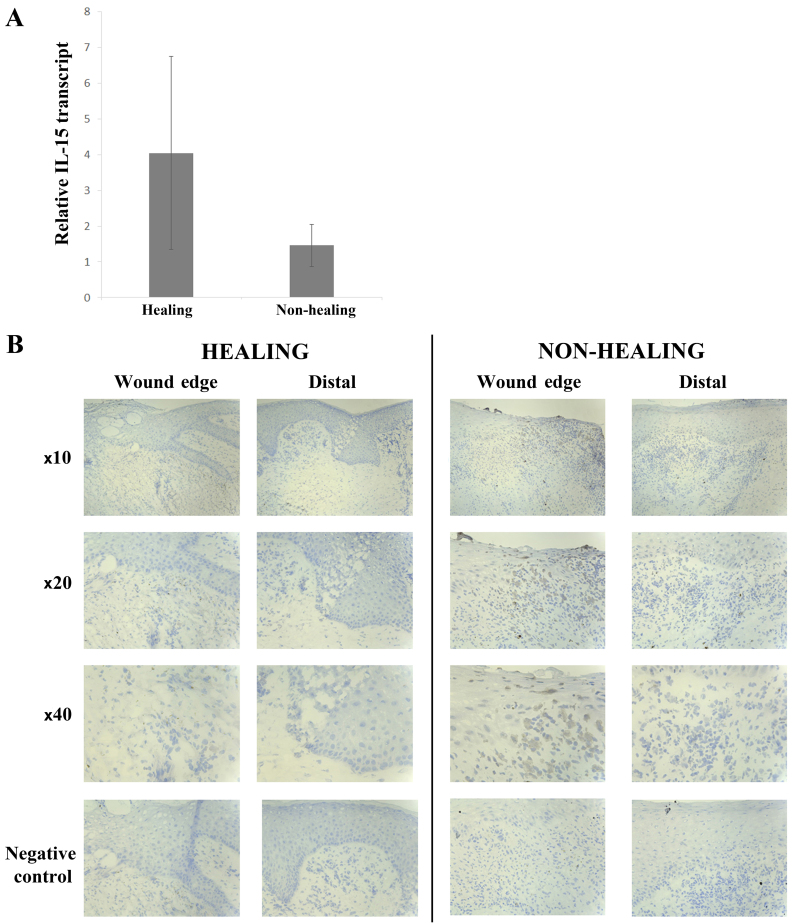
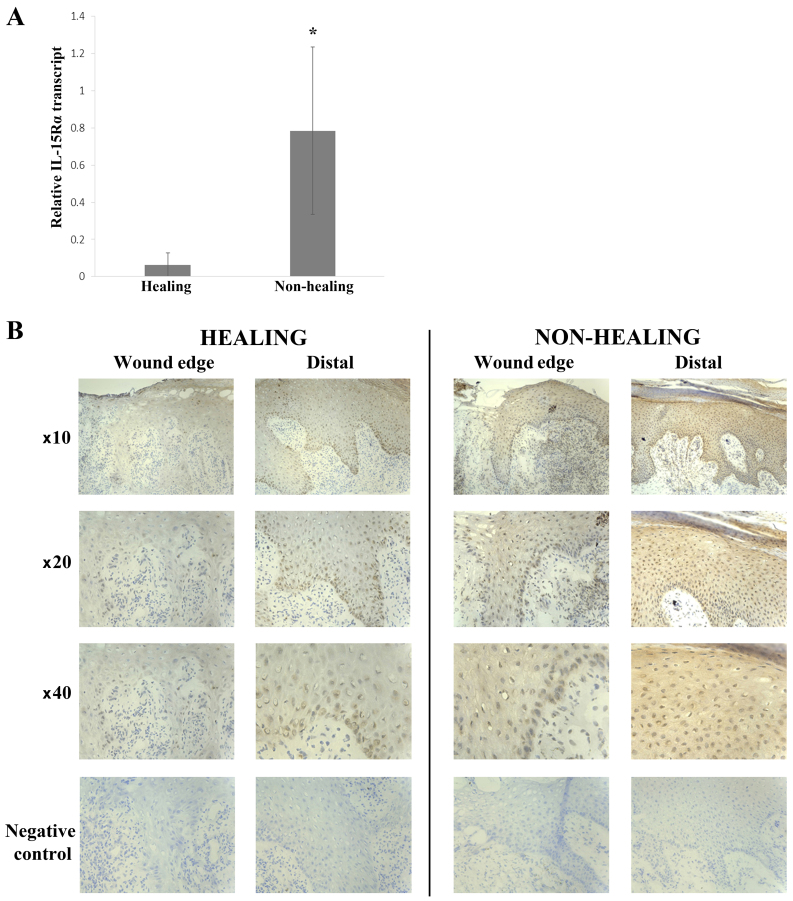
The expression profile of IL-15 was explored in clinical samples from chronic wounds using both qPCR transcript analysis and IHC staining. Quantitative transcript analysis of the clinical samples showed a greater expression of IL-15 mRNA in healing wounds (4.058±2.658) compared with non-healing wounds (1.458±0.587) (Fig. 1A), though this was not found to be statistically significant (p=0.17). Conversely, there was a significantly greater expression of IL-15Rα in non-healing wounds (0.785±0.45) compared with healing wounds (0.0638±0.0638) (p=0.031) (Fig. 2A).
Figure 1.
Expression profile of interleukin (IL)-15 in chronic wounds. (A) IL-15 transcript expression in healing (n=20) and non-healing (n=51) chronic wounds. (B) Immunohistochemical (IHC) analysis of IL-15 protein levels in healing (n=12) and non-healing (n=11) chronic wounds at both wound edge and distal locations. Minimal expression of IL-15 was observed in wound edge and distal locations of both healing and non-healing chronic wounds. Mean values shown, error bars represent SEM, representative images of wound sections shown.
Figure 2.
Expression profile of interleukin-15 receptor α (IL-15Rα) in chronic wounds. (A) IL-15Rα transcript expression in healing (n=20) and non-healing (n=51) chronic wounds. *p<0.05. (B) Immunohistochemical (IHC) analysis of IL-15Rα protein levels in healing (n=12) and non-healing (n=11) chronic wounds at both wound edge and distal locations. In both cases, IL-15Rα expression was enhanced in distal locations compared to wound edge tissue. Mean values shown, error bars represent SEM, representative images of wound sections shown.
IHC staining for IL-15 was generally negative in keratinocytes of all chronic wounds, both healing and non-healing (Fig. 1B). Some cells of the inflammatory infiltrate directly below the leading migratory wound edge showed positive cytoplasmic staining for IL-15. These cells had the appearance of macrophages. This was found mostly in non-healing wounds (8/11 or 73%) compared with healing wounds (2/12 or 17%). IL-15Rα expression was seen in the majority of chronic wounds (14/23) (Fig. 2B) with a slightly higher number of non-healing wounds expressing IL-15Rα compared with healing wounds [8/11 (73%) and 6/12 (50%), respectively]. Generally, IL-15Rα expression in the healing wounds was nuclear in the basal and lower layers of the epidermis, increasing with intensity towards the distal/normal tissue (moving away from the wound edge). This staining pattern was also seen in the non-healing chronic wounds accompanied by cytoplasmic expression in the mature keratinocytes distal to the wound edge.
Impact of IL-15 on HaCaT proliferation
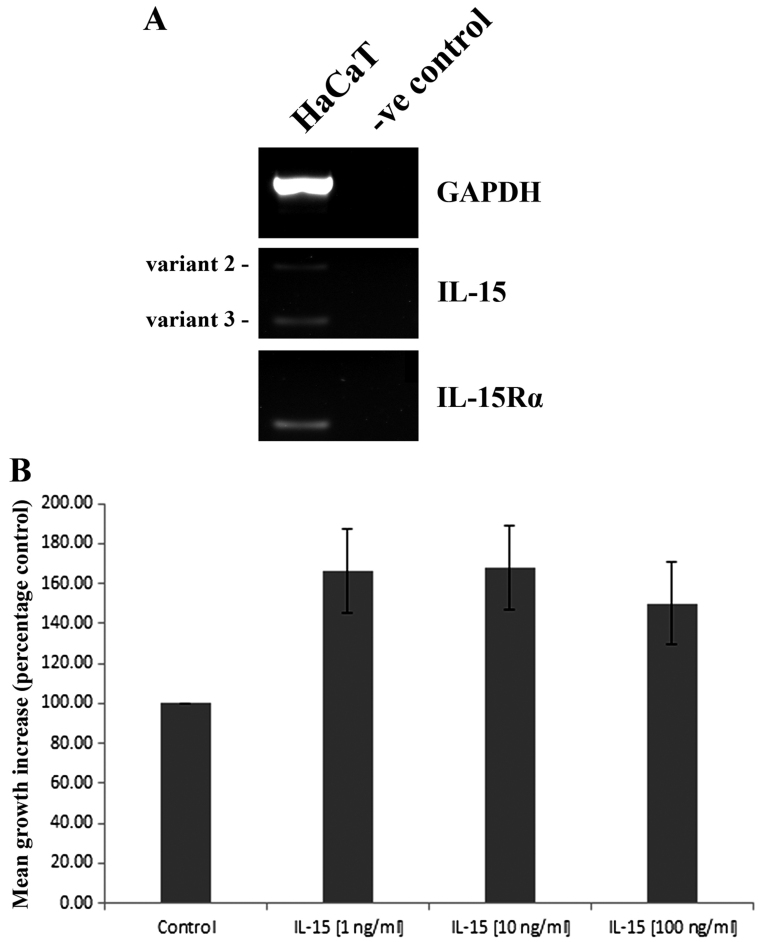
RT-PCR analysis indicated that HaCaT cells express both IL-15 and IL-15Rα transcripts (Fig. 3A). Expression of the receptor appears to be stronger than IL-15, of which two transcript variants were detected. A recombinant form of IL-15 (rhIL15) was subsequently used to explore the effects of this molecule on HaCaT cell function. The treatment of HaCaT cells with either 1, 10 or 100 ng/ml appeared to enhance HaCaT cell growth over a 3 day incubation, though ANOVA analysis was just outside of statistical significance (p=0.07) (Fig. 3B).
Figure 3.
Expression of interleukin (IL)-15 in human adult low calcium temperature (HaCaT) keratinocytes and impact on HaCaT growth. (A) RT-PCR analysis of IL-15 and IL-15 receptor α (IL-15Rα) expression in HaCaT human keratinocytes, -ve control represents no template control. (B) Impact of recombinant IL-15 (rhIL-15) on HaCaT cell growth at a range of concentrations. Representative images shown. Values represent mean percentage control data. Error bars represent SEM.
IL-15 impacts on HaCaT cell migration
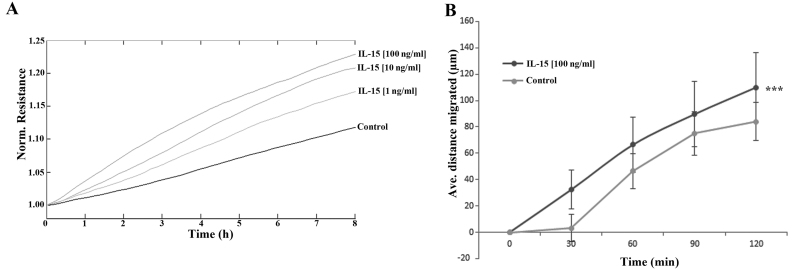
The impact of rhIL-15 was explored using both ECIS- (Fig. 4A) and conventional scratch- (Fig. 4B) based assays. ECIS-based analysis indicated a dose-dependent enhancement of HaCaT cell migration following treatment with rhIL-15, with the greatest effects observed following treatment with 100 ng/ml rhIL-15. The effect of this concentration was similarly confirmed using a scratch assay methodology. Similar to the findings of the ECIS assay, 100 ng/ml rhIL-15 enhanced HaCaT cell migration in comparison with the untreated control cells and this effect was found to be statistically significant (p<0.001).
Figure 4.
Impact of recombinant IL-15 (rhIL-15) on human adult low calcium temperature (HaCaT) cell migration. (A) Electric cell-substrate impedance sensing (ECIS)-based analysis demonstrating effects of rhIL-15 on HaCaT cell migration at a range of concentrations. (B) Scratch wound healing assay demonstrating the impact of 100 ng/ml rhIL-15 on HaCaT cell migration. Representative data shown. Error bars represent standard deviation, ***p<0.001.
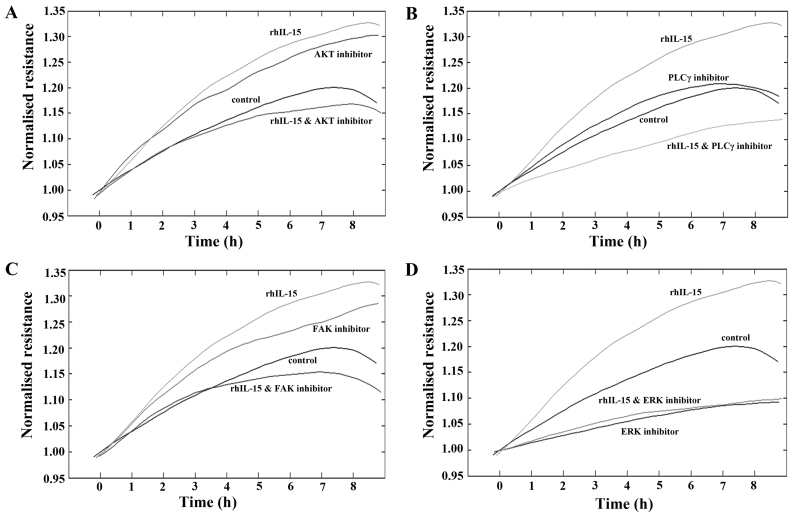
To further explore the potential mechanisms responsible for the pro-migratory effects of rhIL-15 on HaCaT cells, ECIS assays were undertaken using a number of small molecule inhibitors of key migratory pathways (Fig. 5). Similar to previous findings, treatment with rhIL-15 (40 ng/ml) continued to display a pro-migratory response in comparison with the untreated cells.
Figure 5.
Impact of pathway inhibitors on recombinant IL-15 (rhIL-15) related migration. Small molecule pathway inhibitors of (A) AKT, (B) PLCγ, (C) FAK and (D) ERK were added independently and in conjunction with rhIL-15 to explore potential signalling interactions. Electric cell-substrate impedance sensing (ECIS) migration analysis was used, representative data shown.
The treatment of HaCaT cells with AKT inhibitor appeared to enhance cell migration. Notably, the pro-migratory effects observed due to both rhIL-15 or AKT inhibitor alone were negated when these compounds were combined (Fig. 5A). The addition of a PLCγ inhibitor did not substantially impact on the migration rates of HaCaT cells in comparison with the untreated controls. However, in combination with rhIL-15, PLCγ inhibition negated the pro-migratory effect of rhIL-15 alone, and also substantially inhibited HaCaT cell migration rates in comparison with the untreated control cells (Fig. 5B). Similar trends to the AKT inhibitor were observed with the addition of FAK inhibitor, which appeared to exert pro-migratory effects independently but when added in combination with rhIL-15, negated the pro-migratory effect of both compounds (Fig. 5C). Finally, the addition of ERK inhibitor substantially reduced HaCaT cell migration in comparison with the untreated control cells and when added in combination with rhIL-15, completely abolished the rhIL-15 pro-migratory effect, demonstrating migratory rates similar to those seen with ERK inhibition alone.
Discussion
IL-15 is a cytokine involved in a wide range of biological processes and disease states. Given its involvement in inflammatory diseases, keratinocyte function and potential to affect wound healing, the present study aimed to explore the role of this interleukin in chronic wounds.
Quantitative transcript analysis of the clinical samples from the chronic wound cohort indicated a greater expression of IL-15 mRNA in chronic wounds that go on to heal when compared with those that do not heal, though this difference was not found to be statistically significant. To the best of our knowledge, this observation has not been previously documented as most studies on IL-15 to date have focused on its role in tumourigenesis, particularly in blood-borne tumours including multiple myeloma (20), cutaneous T-cell lymphoma (21) and T-cell leukaemia (22). IHC staining indicated little IL-15 protein expression in the keratinocytes of healing or non-healing wounds, with little difference in expression levels observed between the healing and non-healing chronic wound samples. This discrepancy may be attributed to previous observations that IL-15 transcripts are abundantly expressed whereas IL-15 protein is tightly controlled with expression generally limited to monocytes, macrophages and dendritic cells (6,23). This is consistent with the IHC observation that IL-15 protein was expressed in cells in the inflammatory infiltrate directly below the migratory wound edge. The cells in this infiltrate have been reported as being predominantly leukocytes and macrophages (24). However, this was mostly found in non-healing wounds compared with healing wounds, conflicting with the qPCR results. Several translational and intracellular protein trafficking mechanisms serve to limit the production of IL-15 protein from the far larger stores of transcript (25). This could explain the apparent discrepancy between the mRNA and protein expression of IL-15. Somewhat in contrast to this, the IL-15Rα receptor was found to be upregulated in non-healing chronic wounds compared with healing chronic wounds at both transcript and protein levels.
Following on from the clinical data, we explored the impact of rhIL-15 on HaCaT keratinocytes and its impact on cell growth and migration. The PCR results confirmed that IL-15 mRNA and that of IL-15Rα are expressed in HaCaT keratinocytes. This is consistent with previous studies of IL-15 expression in keratinocytes (11,26). In vitro treatment of HaCaT cells with rhIL-15 did not result in statistically significant changes to 3 day growth rates, though all treatment concentrations displayed a trend of enhanced growth. Previous studies have demonstrated a pro-proliferative role for IL-15 in HaCaT cells (12) and this interleukin is associated with the enhancement of cell proliferation and the inhibition of apoptosis in numerous cell lines (11,27–29). One key effect outlined in this study was the impact of rhIL-15 on HaCaT cell migration. Using both ECIS- and scratch assay-based methods, the addition of rhIL-15 resulted in an increased rate of HaCaT cell migration. Our data indicates that IL-15 is capable of enhancing the migratory capacity of HaCaT keratinocytes. Similar trends have been seen in other cell types and IL-15 has recently been reported to act in a pro-migratory manner on the 5637 bladder carcinoma cell line in a wound healing-based assay (30). Subsequently, we explored the ability of a number of small molecule inhibitors of key pathways to alter the effect of rhIL-15 on HaCaT cell migration. A combination of rhIL-15 with either AKT, FAK, PLCγ or ERK small molecule pathway inhibitors was capable of negating the pro-migratory effect of rhIL-15, reducing migrational rates to control or below control levels. Notably, the combination of rhIL-15 with either AKT, FAK or PLCγ inhibitor all appeared to bring about migrational rates below that of the individual inhibitor alone, suggesting potential links between these pathways and the pro-migratory effect of IL-15. IL-15 has previously been shown to activate/signal via AKT and ERK1/2 pathways (12,30) and the subsequent inhibition of these pathways could negate the proliferative role of IL-15 in HaCaT keratinocytes (12) and the pro-migratory role of IL-15 in 5637 bladder cancer cells (30). The present data implies a possible association between these tested pathways and HaCaT cell migration, though further research is warranted in order to fully explore this association.
The present study suggests some complex roles for IL-15 in chronic wound healing. Enhanced IL-15Rα levels are observed in non-healing chronic wounds in comparison with healing chronic wounds, though treatment of keratinocytes with rhIL-15 enhanced pro-healing traits such as growth, albeit non-significantly, and migration. Further studies are necessary to establish expression profiles in larger wound healing cohorts containing normal skin as well as acute and chronic wound subtypes.
Acknowledgments
The authors wish to thank the A4B Scheme of the Welsh Government Ser Cymru, NRN Life Sciences Research Network Wales and Cancer Research Wales for supporting this study.
References
- 1.Wright K. Acute and chronic wounds: current managment concepts. Clin Nurse Spec. 2007;21:172–173. doi: 10.1097/01.NUR.0000270010.74587.3b. [DOI] [Google Scholar]
- 2.Vowden K, Vowden P, Posnett J. The resource costs of wound care in Bradford and Airedale primary care trust in the UK. J Wound Care. 2009;18:93–94. 96–98. doi: 10.12968/jowc.2009.18.3.39814. 100 passim. [DOI] [PubMed] [Google Scholar]
- 3.Posnett J, Franks PJ. The burden of chronic wounds in the UK. Nurs Times. 2008;104:44–45. [PubMed] [Google Scholar]
- 4.Drew P, Posnett J, Rusling L, Wound Care Audit T. Wound Care Audit Team The cost of wound care for a local population in England. Int Wound J. 2007;4:149–155. doi: 10.1111/j.1742-481X.2007.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schilling JA. Wound healing. Surg Clin North Am. 1976;56:859–874. doi: 10.1016/S0039-6109(16)40983-7. [DOI] [PubMed] [Google Scholar]
- 6.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 7.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–766. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 8.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Tagaya Y, Burton JD, Miyamoto Y, Waldmann TA. Identification of a novel receptor/signal transduction pathway for IL-15/T in mast cells. EMBO J. 1996;15:4928–4939. [PMC free article] [PubMed] [Google Scholar]
- 11.Rückert R, Asadullah K, Seifert M, Budagian VM, Arnold R, Trombotto C, Paus R, Bulfone-Paus S. Inhibition of keratinocyte apoptosis by IL-15: a new parameter in the pathogenesis of psoriasis? J Immunol. 2000;165:2240–2250. doi: 10.4049/jimmunol.165.4.2240. [DOI] [PubMed] [Google Scholar]
- 12.Yano S, Komine M, Fujimoto M, Okochi H, Tamaki K. Interleukin 15 induces the signals of epidermal proliferation through ERK and PI 3-kinase in a human epidermal keratinocyte cell line, HaCaT. Biochem Biophys Res Commun. 2003;301:841–847. doi: 10.1016/S0006-291X(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 13.Lindner G, Rückert R, Bulfone-Paus S, Paus R. Inhibition of chemotherapy-induced keratinocyte apoptosis in vivo by an interleukin-15-IgG fusion protein. J Invest Dermatol. 1998;110:457–458. doi: 10.1046/j.1523-1747.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, McCall S, Choi SS, Sicklick JK, Huang J, Qi Y, Zdanowicz M, Camp T, Li YX, Diehl AM. Interleukin-15 increases hepatic regenerative activity. J Hepatol. 2006;45:410–418. doi: 10.1016/j.jhep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Kagimoto Y, Yamada H, Ishikawa T, Maeda N, Goshima F, Nishiyama Y, Furue M, Yoshikai Y. A regulatory role of interleukin 15 in wound healing and mucosal infection in mice. J Leukoc Biol. 2008;83:165–172. doi: 10.1189/jlb.0307137. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB, Gracie JA. Interleukin-15: A new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Bosanquet DC, Harding KG, Ruge F, Sanders AJ, Jiang WG. Expression of IL-24 and IL-24 receptors in human wound tissues and the biological implications of IL-24 on keratinocytes. Wound Repair Regen. 2012;20:896–903. doi: 10.1111/j.1524-475X.2012.00840.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang WG, Martin TA, Lewis-Russell JM, Douglas-Jones A, Ye L, Mansel RE. Eplin-alpha expression in human breast cancer, the impact on cellular migration and clinical outcome. Mol Cancer. 2008;7:71. doi: 10.1186/1476-4598-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keese CR, Wegener J, Walker SR, Giaever I. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci USA. 2004;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinhofer I, Marschitz I, Henn T, Egle A, Greil R. Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. Blood. 2000;95:610–618. [PubMed] [Google Scholar]
- 21.Leroy S, Dubois S, Tenaud I, Chebassier N, Godard A, Jacques Y, Dréno B. Interleukin-15 expression in cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome) Br J Dermatol. 2001;144:1016–1023. doi: 10.1046/j.1365-2133.2001.04192.x. [DOI] [PubMed] [Google Scholar]
- 22.Kukita T, Arima N, Matsushita K, Arimura K, Ohtsubo H, Sakaki Y, Fujiwara H, Ozaki A, Matsumoto T, Tei C. Autocrine and/or paracrine growth of adult T-cell leukaemia tumour cells by interleukin 15. Br J Haematol. 2002;119:467–474. doi: 10.1046/j.1365-2141.2002.03813.x. [DOI] [PubMed] [Google Scholar]
- 23.Bamford RN, Battiata AP, Waldmann TA. IL-15: the role of translational regulation in their expression. J Leukoc Biol. 1996;59:476–480. doi: 10.1002/jlb.59.4.476. [DOI] [PubMed] [Google Scholar]
- 24.Moore K, Ruge F, Harding KG. T lymphocytes and the lack of activated macrophages in wound margin biopsies from chronic leg ulcers. Br J Dermatol. 1997;137:188–194. doi: 10.1046/j.1365-2133.1997.18041895.x. [DOI] [PubMed] [Google Scholar]
- 25.Kurys G, Tagaya Y, Bamford R, Hanover JA, Waldmann TA. The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15. J Biol Chem. 2000;275:30653–30659. doi: 10.1074/jbc.M002373200. [DOI] [PubMed] [Google Scholar]
- 26.Blauvelt A, Asada H, Klaus-Kovtun V, Altman DJ, Lucey DR, Katz SI. Interleukin-15 mRNA is expressed by human keratinocytes Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J Invest Dermatol. 1996;106:1047–1052. doi: 10.1111/1523-1747.ep12338641. [DOI] [PubMed] [Google Scholar]
- 27.Kuniyasu H, Ohmori H, Sasaki T, Sasahira T, Yoshida K, Kitadai Y, Fidler IJ. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res. 2003;9:4802–4810. [PubMed] [Google Scholar]
- 28.Shinozaki M, Hirahashi J, Lebedeva T, Liew FY, Salant DJ, Maron R, Kelley VR. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J Clin Invest. 2002;109:951–960. doi: 10.1172/JCI0214574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hjorth-Hansen H, Waage A, Börset M. Interleukin-15 blocks apoptosis and induces proliferation of the human myeloma cell line OH-2 and freshly isolated myeloma cells. Br J Haematol. 1999;106:28–34. doi: 10.1046/j.1365-2141.1999.01510.x. [DOI] [PubMed] [Google Scholar]
- 30.Park SL, Kim WJ, Moon SK. p21WAF1 mediates the IL-15-induced migration and invasion of human bladder cancer 5637 cells via the ERK1/2/NF-κB/MMP-9 pathway. Int Immunopharmacol. 2014;22:59–65. doi: 10.1016/j.intimp.2014.06.008. [DOI] [PubMed] [Google Scholar]