Abstract
A dominant appetite for protein drives increased energy intake in humans when the proportion of protein in the diet is reduced down to approximately 10% of total energy. Compensatory feeding for protein is apparent over a 1–2 d period but the mechanisms driving this regulation are not fully understood. Fibroblast growth factor-21 (FGF-21) has been identified as a candidate protein signal as levels increase in the circulation when dietary protein is low. The aim of this randomised controlled trial was to assess whether changes in percent dietary protein over a 4 d ad libitum experimental period in lean, healthy participants influenced energy intake, metabolic health, circulating FGF-21 and appetite regulating hormones including ghrelin, glucagon like peptide-1 and cholecystokinin. Twenty-two lean, healthy participants were fed ad libitum diets containing 10, 15 and 25% protein, over three, 4 d controlled, in-house experimental periods. Reduced dietary protein intake from 25% to 10% over a period of 4 d was associated with 14% increased energy intake (p = 0.02) as previously reported, and a 6-fold increase in fasting circulating plasma FGF-21 levels (p<0.0001), a 1.5-fold increase in serum triglycerides (p<0.0001), and a 0.9-fold decrease in serum total cholesterol (p = 0.02). Serum HDL cholesterol was reduced with a reduction in dietary protein from 15% to 10% (p = 0.01) over 4 d but not from 25% to 10% (p = 0.1) and the change from baseline was not different between diets. Plasma fasting insulin levels following the 4 d study period were significantly lower following the 25% ad libitum study period compared to the 15% protein period (p = 0.014) but not the 10% protein period (p = 0.2). Variability in interstitial glucose during each study period increased with a decrease in dietary protein from 25% to 15% and 10% (p = 0.001 and p = 0.04, respectively). Ghrelin, glucagon-like peptide-1 and cholecystokinin were unchanged. Increases in energy intake, plasma FGF-21 and serum triglycerides were associated with reductions in percent dietary protein from 25% to 10% energy over a 4 d ad libitum in-house feeding period and may be important in regulation of dietary protein intake.
Trial Registration
Australia New Zealand Clinical Trials Registry ACTRN12616000144415
Introduction
In a range of species, from locusts to rodents and humans, total energy intakes increase with a fall in percent dietary protein down to a point where excessive protein dilution fails to elicit increased intake. This response reflects an underlying nutrient-specific appetite for protein and has been termed the protein leverage effect [1–3]. A dominant appetite for protein driving energy intake has also been proposed to contribute to development of the human obesity epidemic [2, 3]. A recent analysis of 38 published experimental trials [4] and results from population studies and large dietary trials [5–7] shows that protein drives energy intake most strongly in humans when dietary protein is between 10 and 20% but not when percent dietary protein falls to lower levels of 5% [4, 8, 9] or is elevated to levels exceeding 30% [4]. Together these data suggest that protein leverage operates within a range of values for percent dietary protein that reflect the usual range seen in human populations with food sufficiency.
The mechanisms that control protein intake are not well understood. In humans protein leverage is expressed over a 1–2 d period [10, 11]. A shorter period of satiety follows a low protein meal when compared to a higher protein meal [11, 12] and may be important in driving increased food and energy intake in the longer term by increasing the frequency of feeding episodes. Dietary studies in humans suggest that taste cues associated with protein in food, such as umami, elicit elevated activity in the brain regions associated with hedonic responses when protein is depleted [13]. It has been hypothesised that there may be a systemic hormonal ‘protein signal’ that responds specifically to low levels of dietary protein, which can override nutritional feedbacks arising from high levels of carbohydrate and/or fat intake to permit continuing energy intake so that target protein intakes can be attained [2, 4]. Recently, Fibroblast Growth Factor-21 (FGF-21) was described as a potential candidate for such a protein signal. Laeger et al. [14] reported that FGF-21 was elevated, in mice and in humans, during fixed energy feeding of a low protein diet (5% dietary protein) [15]. The elevation in FGF-21 levels observed in humans following an overnight fast suggests that a sustained effect of a low intake of dietary protein triggers FGF-21 release. Appetite regulating hormones like cholecystokinin (CCK), glucagon like peptide -1 (GLP-1) and ghrelin respond to feeding [16–19] and in some studies, but not all, the response varies with dietary macronutrient composition (reviewed in [20, 21]). However, the relationships between the fasting levels of these hormones and sustained changes in percent dietary protein have not been studied in detail.
The aim of this study was to assess the plasma levels of FGF-21 and known appetite-regulating hormones including the pro-appetite hormone ghrelin and satiety hormones GLP-1 and CCK and various metabolic variables in lean, healthy individuals to a change in the effect of percent dietary protein under ad libitum conditions in which dietary macronutrient composition was disguised [11]. We hypothesised that a reduction in percent protein would increase the level of serum FGF-21 and ghrelin and lower the levels of the satiety hormone GLP-1 and CCK.
Methods
Ethics statement
The study protocol (S1 File) was approved in March 2007 by Sydney South West Area Health Service (Royal Prince Alfred Hospital) Human Research Ethics Committee (Protocol No. X07-0044) and the University of Sydney Human Research Ethics Committee (Ref No. 10153) and is registered with the Australian and New Zealand Clinical Trial Registry (ACTRN12616000144415). The trial was registered after participant recruitment began. The authors confirm that all ongoing and related trials for this intervention are registered. The study is an experimental study to test the response to a changed macronutrient composition of the diet rather than to test a clinical dietary intervention.
Study participants
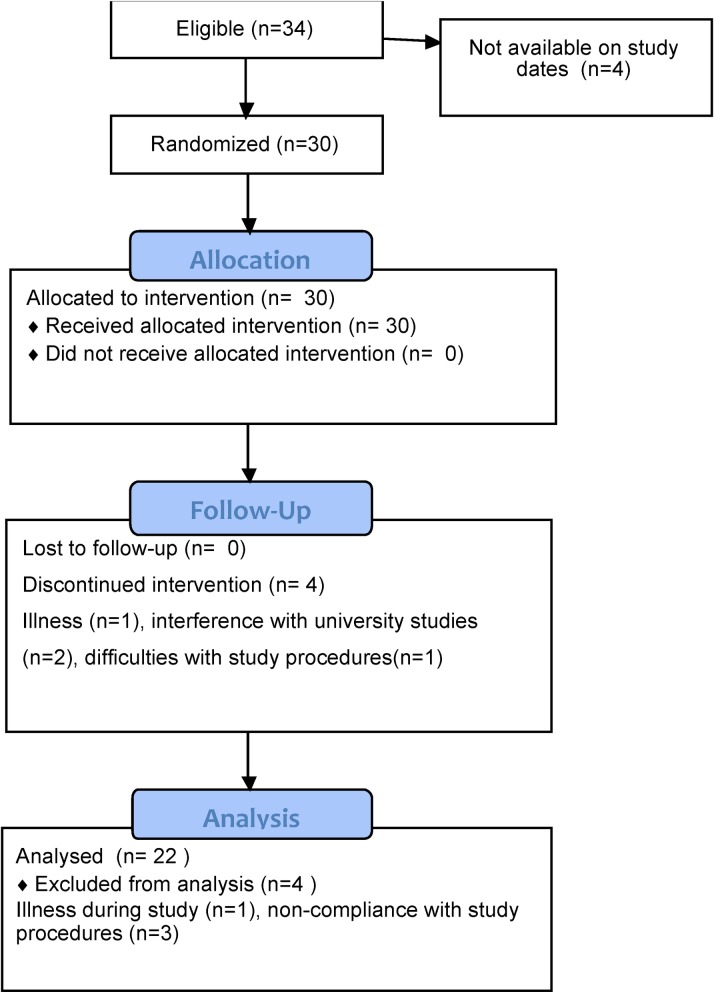
As previously described [11], lean, healthy (BMI:18–25 kg.m-2) male and female participants were recruited by advertising through casual employment sites at five universities within the Sydney region during October 2007—Dec 2009. All patient follow-up was completed by February 2011. Fig 1 presents the CONSORT 2010 flow diagram. 22 females and 12 males were eligible, indicated their willingness to undertake the trial and completed initial investigation day measures. From these, 20 females and 10 males commenced the trial. Subsequently, three females chose to discontinue due to interference with university studies, illness or difficulties with blood collection. One male was excluded after commencement following a diagnosis of hyperthyroidism. Overall, 17 lean female and 9 lean male participants completed the trial. On completion, four participants were excluded from the data analysis for reasons including gastrointestinal upset during one of the study weeks (n = 1 female) and failure of one sub-group to comply with study procedures (n = 3 males, consumed one another’s food). 16 lean female and 6 lean male participants were included in the final data analysis. Exclusion criteria were diabetes, high blood pressure, gastrointestinal problems, asthma, eczema or hay fever, chronic medical conditions, anaemia, allergies or strong dislikes to any study foods, smoking, following a weight reducing diet within the 3 months prior to the screening interview, pregnancy and breastfeeding. Participants completed the EAT-26 questionnaire and were excluded if they had a history of eating disorders or irregular eating habits. Vegetarians and vegans were excluded to aid in preparation of the treatment foods. All participants were given detailed verbal and written information regarding the purpose of the trial and the study procedures enabling written informed consent.
Fig 1. CONSORT 2010 flow diagram.
Dietary protocol
The full details of the dietary manipulation have been published previously [11, 22]. Briefly, recipes were modified to contain 10, 15 or 25% energy as protein. Carbohydrate was adjusted to be 60, 55 or 45% energy and dietary fat was kept constant at 30%. The final ad libitum menus for each of the three 4 d study periods were matched for energy density, palatability and variety [11]. The diets were ad libitum and participants were free to eat at anytime of the day. The final menus and the total nutrients available to each participant in this study have been published previously [11]. Habitual intakes and ad libitum intakes in response to the 10%, 15% and 25% protein diets consumed by each participant in this study are also reported elsewhere [11].
Study design
The details of the study design and participants are presented here (S1 File) and in Gosby et al. [11]. Following a screening interview each participant was allocated to a group of participants who were available to complete the trial on the same 3, 4 d periods of in-house dietary manipulation at the Woolcock Institute Sleep Study Centre, Glebe, NSW 2037, Australia. Once the subjects in each group were finalised the group was then randomly allocated an order of intervention by the study coordinator. Allocation of order was concealed by randomly selecting a sequence from an envelope. All possible sequences of interventions were printed the same number of times and placed in an envelope prior to the start of the study by the study coordinator. Participants were blinded to the sequence of interventions. It was estimated that 20 subjects would be required to detect a difference in ad libitum energy intake between dietary treatments. The sample size calculation was based on energy intakes from a previous ad libitum study where a 4.5MJ change in energy intake was measured in response to a reduction in percent dietary protein from 15% to 8% with a standard deviation of differences within pairs of approximately 1.2MJ. Using a paired, two-tailed design a difference of 0.8MJ, with a power of 80% and alpha of 0.05 could be detected with a sample size of 20.
Each study period was separated by at least 1 week. Participants arrived fasted on d 1 and were fasted from 10pm on d 4 of each 4 d ad libitum period. By the end of the experiment each group had undergone 4 continuous days on each of the 10, 15 and 25% dietary protein menus [11]. Participants were taken for a 1 hr walk on each of the study days.
Continuous glucose monitoring
The Continuous Glucose Monitoring System Gold (CGMS) (Medtronic, CA91325, USA) was used to collect continuous glucose measurements during each 4 d experimental period. The CGMS was fitted on d 2 of each experimental period. Participants were given instructions to calibrate the monitor before breakfast, lunch, dinner and prior to going to bed each day. The calibration was performed by entering the contemporaneous glucose reading obtained from a finger prick sample (Accu-Chek Performa, Roche, USA). If the monitor did not calibrate participants were instructed to inform and get assistance from the study coordinator. Participants were asked to enter meal, snack and activity events into the continuous glucose monitor. The continuous glucose monitor was worn until waking on d 5 at which point a last calibration was performed and the monitor removed from the participant. The continuous glucose monitor recorded readings every 5 minutes following initialisation.
Continuous glucose monitoring data analysis
To determine if there was an effect of percent dietary protein on the glucose profiles during d 3 and d 4 of ad libitum feeding of the 10%, 15% and 25% protein study periods the 24 h glucose data from the continuous glucose monitors on d 3 and 4 was used to calculate the average daily glucose, area under the curve for glucose and variance of glucose values during each 24-h period. The variance was determined by calculating the standard deviation of the blood glucose rate of change. The blood glucose rate of change (mg/dl/min) evaluates the dynamics of blood glucose fluctuations over 15-min periods [23]. A larger variation in the blood glucose rate of change indicates a more rapid and pronounced blood glucose fluctuation. Validation studies indicated that the Minimed CGMS is adequate for testing the glucose response to foods [24, 25].
Sample analysis
Serum and plasma samples were taken from fasting participants on the morning of d 1 and d 5 of each study period. Samples were stored on ice and transported to Sydney South West Pathology Service, Royal Prince Alfred Hospital, a NATA/RCPA accredited laboratory or stored at -80°C for subsequent analysis of glucose, serum triglycerides and total and HDL cholesterol. Plasma FGF-21 was measured using the Human FGF-21 Quantikine ELISA Kit (DF2100) by R&D systems. This assay achieves a sensitivity of 8.69 pg/mL and has a coefficient of variation of 2.9–3.9% (intra-assay) and 5.2–10.9% (inter-assay) over a range of 31.3–2000 pg/mL. The GHRT-89HK Total Ghrelin RIA kit (Millipore Corp, Billerica, MA, USA) was used to measure total ghrelin in stored plasma. This assay achieves a sensitivity of 27.6 pmol/L, the coefficient of variation was 3.3–10% (intra-assay) and 14.7–17.8% (inter-assay) for concentrations of total ghrelin over the range 296.7–889.9 pmol/L. Plasma GLP-1 was measured using the Millipore GLP-1A-35HK kit (Millipore Corp, Billerica, MA, USA). DPP-IV inhibitor was added to the collection tubes. The assay achieved a sensitivity of 3pmol/L, the intra-assay coefficient of variation was 27–30% (intra-assay) and 12–34% (inter-assay) for concentrations of 14–40 pmol/L. Insulin concentrations of 42–325 pmol/L, the intra-assay coefficient of variation was 2.2–4.4% and the inter-assay co-efficient of variation 2.9–6% with the measured recovery after the addition of known concentrations of insulin into serum from 93–100%.
Statistical analysis of experimental data
All data analysis and graphics were performed using R software (R version 3.2.3 [26]). The data are expressed as means ± SEM unless otherwise specified. All data were checked for normality using the Shapiro-Wilk test. Log transformation was used to normalise skewed data for body mass, diastolic blood pressure, triglycerides, total cholesterol, HDL cholesterol, ghrelin, GLP-1, CCK and for mean glucose, area under the curve and glucose variability data collected from CGMS. Square root transformation was used for FGF-21 data. Subjects with missing values for an analysis were removed from the analysis.
One-way within subject ANOVA was used to test for effect of percent dietary protein on d 5 circulating levels of insulin (n = 10, 54% missing), ghrelin (n = 19, 14% missing), CCK (n = 18, 18% missing) and GLP-1 (n = 19, 14% missing) following each 4 d study period. These data were checked for sphericity using Mauchlys sphericity test (Multcomp package [27]). If the sphericity assumption was violated, the Greenhouse-Geiser correction was applied to the F- and p-values for effect of percent dietary protein. Post-hoc analysis was performed with pair-wise comparisons using the Bonferroni correction for multiple comparisons. p < 0.05 was used to determine significance.
Mixed model linear regression was performed using the lme 4 [28] and lmerTest [29] packages in R to test for significant effects of percent dietary protein and for differences in the change from d 1 to d 5 between the dietary treatments. Percent dietary protein (10%, 15% and 25%) and time (d 1 and d 5) were used as fixed effects and participants as random effects. Day 1 and d 5 body mass (n = 22), systolic (n = 21, 5% missing) and diastolic (n = 21, 5% missing) blood pressure, fasting glucose (n = 18, 18% missing), FGF-21 (n = 11, 50% missing), triglycerides (n = 19, 14% missing), total cholesterol (n = 19, 5% missing) and HDL-cholesterol (n = 16, 27% missing) were collected prior to and after each 4 d dietary treatment. Mixed model linear regression was also used to assess the effect of percent dietary protein on d 3 and d 4 average daily glucose, area under the curve for glucose and standard deviation for blood glucose rate of change calculated from continuous glucose monitor data collected over each 4 d study period (n = 19, 14% missing). Each linear mixed model was checked to confirm that the errors had relatively constant variance, were independent and normally distributed. Mixed model linear regression analysis is presented as estimates, upper and lower confidence intervals (CI) and the p-value. p < 0.05 was used to determine significance.
Simple linear regression was used to test relationships between d 5 triglycerides with d 4 average daily glucose, d 4 area under the curve for glucose, d 4 glucose variability and d 4 energy intake.
Results
Subject characteristics
17 lean female and 9 lean male participants aged 24 ± 1 years (mean ± SEM; range 18–51) and BMI of 21.8 ± 0.4 (mean ± SEM; range 18–25.5) kg.m-2 completed the trial [11]. Participants’ habitual average intake was estimated to be 10.1 ± 0.6 MJ.d-1 and the macronutrient composition of the diet was 18.4 ± 0.7% protein, 47.2 ± 1.2%, carbohydrate and 34.4 ± 1.0% fat with an average protein intake of 78g.d-1[11]. The baseline measures and characteristics for males and females are presented in Table 1.
Table 1. Baseline characteristics of the participants.
| Female | Male | |
|---|---|---|
| N | 17 | 9 |
| age (years) | 25.2 ± 1.9 | 23.3 ± 0.8 |
| body mass (kg) | 55.5 ± 1.6 | 69.9 ± 3.0 |
| height (m) | 1.6 ± 0.02 | 1.8 ± 0.03 |
| BMI (kg.m-2) | 21.7 ± 0.5 | 22.1 ± 0.6 |
| energy intake (MJ.d-1) | 7.8 ± 0.2 | 10.1 ± 0.5 |
| protein (% of energy) | 17.6 ± 0.5 | 18.3 ± 0.9 |
| carbohydrate (% of energy) | 50.9 ± 0.7 | 49.8 ± 1.4 |
| fat (% of energy) | 31.4 ± 0.5 | 31.9 ± 0.9 |
Circulating levels of plasma FGF-21 responded to percent dietary protein
As previously reported [11], over the 4 d ad libitum periods of the study participants increased their total daily energy intake by 14% when percent dietary protein was reduced from 15% to 10% but did not change total energy intake when percent dietary protein increased from 15% to 25%. Mixed model linear regression analysis (Table 2) was used to determine differences in fasting levels of FGF-21 between the diet groups on the morning of d 5 following the 4 day ad libitum study period and the difference in the change between d 5 to d 1 between diet groups. On the morning of d 5 plasma FGF-21 levels were 1.6-fold greater after the period of the 10% protein diet than following 15% dietary protein (p = 0.17) and 6-fold greater than levels following 25% dietary protein (p<0.0001) (Table 2). The change between d 5 and d 1 values was greater in response to the 10% protein diet in comparison to the 25% protein diet (p = 0.007, respectively) but not the 15% protein diet (p = 0.2) (Table 2).
Table 2. Mixed model linear regression estimates for circulating fasting serum FGF-21 levels (pg/mL) prior to and following each experimental period.
| Model factor | estimate (CI lower, upper) | P-value* |
|---|---|---|
| Fixed effects | ||
| Intercept (10% protein, d 5) | 300 (206, 394) | |
| Slope (10% protein, d 1) | -153 (-260, -46) | 0.02 |
| Slope (15% protein, d 5) | -110 (-213, -6) | 0.2 |
| Slope (25% protein, d 5) | -251.64 (-355, -148) | <0.0001 |
| Slope (15% protein d 1 to d 5) | 131 (-15, 278) | 0.2 |
| Slope (25% protein d 1 to d 5) | 194 (47, 340) | 0.007 |
| Intercept (15% protein, d 5) | 190.0 (96, 284) | |
| Slope (15% protein, d 1) | -21.45 (-129, 86) | 0.4 |
| Slope (25% protein, d 5) | -142.0 (6, 213) | 0.0006 |
| Slope (25% protein d 1 to d 5) | 62.18 (-84, 209) | 0.1 |
| Intercept (25% protein, d 5) | 48.0 (-46, 142) | |
| Slope (25% protein, d 1) | 40.73 (-66, 148) | 0.2 |
*Square root transformation was used to normalise data for mixed model linear regression, untransformed data is presented.
Plasma levels of fasting ghrelin, GLP-1 and CCK did not change with percent dietary protein
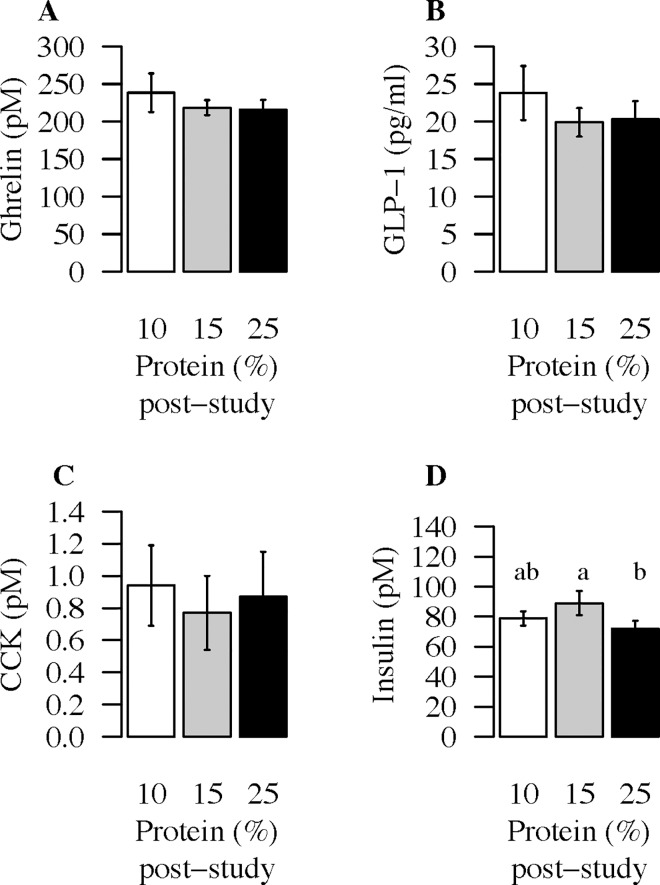
One way ANOVA did not show any significant effect of percent dietary protein on d 5 fasting plasma levels of ghrelin (F(2,36) = 0.5, p = 0.6), GLP-1 (F(2,36) = 0.3, p = 0.7), or CCK (F(2,34) = 1.9, p = 0.2) (Fig 2A–2C).
Fig 2. Plasma levels of fasting ghrelin, GLP-1, CCK and insulin in response to dietary treatments.
Plasma (A) ghrelin (pM), (B) GLP-1 (pM) (C) CCK (pM) and insulin (pM) (D) on d 5 following each 4 d ad libitum 10% (white), 15% (grey) and 25% (black) protein study periods. A one-way within subject ANOVA was used to test for differences between dietary interventions for ghrelin, GLP-1, CCK and insulin. D 5 ghrelin, GLP-1 and CCK did not differ between diets, p>0.05. D 5 insulin concentrations were different between the diets (F(2,38) = 6.0, p = 0.006). Different letters indicate significant differences between the groups, (d5 insulin: 10% vs 25% protein p = 0.2, 15% vs 25% p = 0.014).
Effect of percent dietary protein on body mass and blood pressure after 4 d of ad libitum feeding
Mixed model linear regression analysis (Table 3) was used to determine differences in body mass, and blood pressure between the different diet groups on d 5 and to assess whether there were any differences in the changes between d 1 and d 5 between diet groups. Day 5 body mass and systolic blood pressure was not significantly different following the 10, 15 and 25% protein diets (Table 3). However, d 5 diastolic blood pressure was reduced in comparison to 10% and 25% dietary protein (Table 3).
Table 3. Fixed effects estimates from mixed model linear regression analysis for body mass, systolic and diastolic BP measured on d 1 and d 5 of each 10, 15 and 25% protein study period.
| Model factor | Body mass (kg) | Systolic BP (mmHg) | Diastolic BP (mmHg) | |||
|---|---|---|---|---|---|---|
| estimate | p-value* | estimate | p-value | estimate | p-value* | |
| Intercept (10% protein, d 5) | 59.4 (55.5, 63.3) | 102.5 (99.3,105.8) | 66.3 (63.9, 68.8) | |||
| Slope (10% protein, d 1) | 0.6 (0.1, 1.0) | 0.016 | -0.95 (-4.16,2.27) | 0.56 | -1.1 (-3.4, 1.1) | 0.3 |
| Slope (15% protein, d 5) | -0.25 (-0.7, 0.2) | 0.3 | -1.48 (-4.59,1.64) | 0.4 | -2.7 (-4.8, -0.6) | 0.01 |
| Slope (25% protein, d 5) | -0.2 (-0.6, 0.3) | 0.5 | -0.3 (-3.45,2.79) | 0.8 | -0.3 (-2.4, 1.8) | 0.7 |
| Slope (15% protein d 1 to d 5) | -0.5 (-1.1, 0.1) | 0.08 | -0.14 (-4.55,4.27) | 0.95 | 3.6 (0.7, 6.6) | 0.02 |
| Slope (25% protein d 1 to d 5) | -0.4 (-1.0, 0.2) | 0.2 | -2.67 (7.08,1.74) | 0.2 | -1.4 (-4.4, 1.5) | 0.3 |
| Intercept (15% protein, d 5) | 59.2 (55.3, 63.1) | 101.1 (97.9,104.3) | 63.6 (61.2, 66.1) | |||
| Slope (15% protein, d 1) | 0.02 (-0.4, 0.5) | 0.92 | -1.1 (-4.3,2.1) | 0.5 | 2.5 (0.3, 4.7) | 0.04 |
| Slope (25% protein, d 5) | 0.1 (-0.3, 0.5) | 0.73 | 1.14 (-1.98,4.26) | 0.5 | 2.4 (0.3, 4.5) | 0.026 |
| Slope (25% protein d 1 to d 5) | 0.2 (-0.4, 0.8) | 0.6 | -2.5 (-6.93,1.89) | 0.3 | -5.0 (-8.0, 2.1) | 0.001 |
| Intercept (25% protein, d 5) | 59.2 (55.4, 63.2) | 102.2 (99.0,104.5) | 66.0 (63.5, 68.5) | |||
| Slope (25% protein, d 1) | 0.2 (-0.3, 0.6) | 0.4 | -3.6 (-6.82,-0.41) | 0.03 | -2.6 (-4.8, -0.3) | 0.019 |
*Log transformation was used to normalise data for mixed model linear regression, untransformed data is presented.
Glucose control
Linear mixed model regression analysis showed that fasting glucose did not differ between the 10%, 15% or 25% protein diets (F(2,34) = 1.29, p = 0.3) (Table 4). Using a within subject one-way ANOVA, however, d 5 fasting insulin concentrations were different between the diets (F(2,38) = 6.0, p = 0.006). On the morning of d 5 following the 4 d study period fasting insulin levels were significantly lower following the 25% ad libitum study period compared to the 15% protein period (p = 0.014) but not the 10% protein period (p = 0.2) (Fig 2D).
Table 4. Fixed effects estimates from mixed model linear regression analysis for glucose control in response to 10, 15 and 25% protein diets.
| Model factor | Fasting glucose (mM) | Mean glucose* (mM) | Glucose AUC* | SDROC | |
|---|---|---|---|---|---|
| estimate | estimate | estimate | estimate | p-value* | |
| Intercept (10% protein, d 4) | 4.6 (4.5, 4.8) | 5.57 (5.32, 5.82) | 8009 (7622, 8397) | 0.0215 (0.018,0.025) | |
| Slope (10% protein, d 3) | -0.02 (-0.22, 0.18) | -0.04 (-0.24, 0.17) | -119 (-437, 198) | -0.001 (-0.005,0.002) | 0.4 |
| Slope (15% protein, d 4) | 0.06 (-0.11, 0.23) | -0.22 (-0.43, -0.02) | -224 (-546, 98) | -0.002 (-0.0016, 0.006) | 0.2 |
| Slope (25% protein, d 4) | 0.08 (-0.09, 0.25) | -0.12 (-0.32, 0.09) | -172 (-494, 150) | -0.004 (-0.007, -0.0004) | 0.04 |
| Slope (15% protein d 4 to d 3) | 0.07 (-0.17, 0.31) | 0.04 (-0.25, 0.33) | 97 (-339, 533) | -0.001(-0.006,0.004) | 0.8 |
| Slope (25% protein d 4 to d 3) | 0.01 (-0.23, 0.25) | -0.08 (-0.37, 0.21) | -15 (-451, 421) | 0.001 (-0.004, 0.006) | 0.8 |
| Intercept (15% protein, d 4) | 4.70 (4.51, 4.89) | 5.35 (5.10, 5.60) | 7786 (-7398, 8173) | 0.023 (0.020,0.026) | |
| Slope (15% protein, d 3) | 0.06 (-0.14, 0.26) | 0.01 (-0.20, 0.21) | -22 (-340, 295) | -0.002 (-0.006, 0.001) | 0.3 |
| Slope (25% protein, d 4) | 0.02 (-0.15, 0.19) | 0.11 (-0.1, 0.31) | 51 (-271, 373) | -0.006 (-0.010, -0.002) | 0.001 |
| Slope (25% protein d 4 to d 3) | -0.06 (-0.30, 0.18) | -0.12 (-0.41, 0.17) | -112 (-548, 324) | 0.002 (-0.003, 0.007) | 0.6 |
| Intercept (25% protein, d 4) | 4.72 (4.54, 4.91) | 5.45 (5.20, 5.70) | 7837 (7450, 8224) | 0.017 (0.014, 0.02) | |
| Slope (25% protein, d 3) | -0.01(-0.18, 0.30) | -0.12 (-0.32, 0.09) | -134 (-451, 183) | -0.0004 (-0.004, 0.003) | 0.7 |
*Log transformation was used to normalise data for mixed model linear regression, untransformed data is presented. P-value is for analysis of Std deviation rate of change (SDROC).
Linear mixed model regression analysis was used to analyse the effects of percent dietary protein on continuous glucose monitoring results collected over d 3 and d 4 of each experimental period. The analysis suggested that average glucose and area under the curve (AUC) for glucose measured over d 3 and d 4 of each 4 d ad libitum study period did not change in response to percent dietary protein (Table 4). However, by study d 4, the standard deviation for blood glucose rate of change (SDROC), a measure of variability in interstitial glucose level, decreased as percent dietary protein increased from 10% to 25%. Then, glucose variability was reduced for the 25% protein diet in comparison to both the 10% (p = 0.04) and the 15% protein diets (p = 0.001) (Table 4).
Triglycerides increased and total cholesterol decreased with a reduction in the proportion of protein in the diet
Table 5 presents a summary of the linear mixed model regression analysis of the effect of percent dietary protein on fasting serum levels of triglycerides, total cholesterol and HDL cholesterol measured on the morning of d 1 and d 5 prior to and following each 4 d ad libitum period. Day 5 triglyceride levels were 1.2-fold and 1.5-fold greater on the 10% protein diet than 15% protein diet (p = 0.04) and 25% protein diet (p<0.0001) (Table 5). Consistent with this, the change in the fasting serum triglycerides from d 1 to d 5 following 4 d of 10% dietary protein was greater than the change following 4 d of 15% (p = 0.08) and 25% dietary protein (p = 0.0005). Simple linear regression showed that an increased serum triglyceride level was associated with the dilution of percent dietary protein (p = 0.03), increased d 4 mean daily glucose level (p = 0.002) and increased d 4 area under the curve for glucose (p = 0.0009) but not with increased d 4 energy intake (11) (p = 0.3) or increased d 4 glucose variability (p = 0.9). D 5 total cholesterol level was 0.9-fold for 10% protein when compared to either 15% (p = 0.02) or 25% dietary protein (p = 0.03) (Table 5). The change from d 1 to d 5 following 10% dietary protein was significantly different from the change from d 1 following 15% and 25% dietary protein (p = 0.02 and p = 0.05, respectively). D 5 HDL-cholesterol levels were lower following 10% dietary protein in comparison to 15% (p < 0.01) but not 25% (p = 0.1) protein diets. The changes from d 1 to d 5 were not different between diets (Table 5). Despite the changes measured all serum lipid levels remained within their normal reference ranges during the course of these experiments.
Table 5. Fixed effects estimates from mixed model linear regression analysis for fasting serum lipids in response to percent dietary protein.
| Model factor | Triglycerides (mM) | Cholesterol (mM) | HDL (mM) | |||
|---|---|---|---|---|---|---|
| Estimate | p-value* | estimate | p-value* | estimate | p-value* | |
| Intercept (10% protein, d 5) | 1.79 (1.42, 2.16) | 4.26 (3.79, 4.73) | 1.27 (1.15, 1.38) | |||
| Slope (10% protein, d 1) | -1.02 (-1.35, -0.68) | <0.0001 | 0.06 (-0.16, 0.30) | 0.46 | 0.19 (0.10, 0.28) | <0.0001 |
| Slope (15% protein, d 5) | -0.34 (-0.59, -0.08) | 0.04 | 0.27 (0.05, 0.50) | 0.017 | 0.11 (0.02, 0.19) | 0.0099 |
| Slope (25% protein, d 5) | -0.64 (-0.90, -0.38) | <0.0001 | 0.24 (0.02, 0.47) | 0.03 | 0.06 (-0.03, 0.15) | 0.151 |
| Slope (15% protein d 5 to d 1) | 0.37 (0.01, 0.74) | 0.078 | -0.35 (-0.67, -0.03) | 0.02 | -0.09 (-0.22, 0.03) | 0.091 |
| Slope (25% protein d 1 to d 5) | 0.68 (0.32, 1.05) | 0.0005 | -0.27 (-0.59, 0.05) | 0.05 | 0.0006 (-0.12, 0.12) | 0.94 |
| Intercept (15% protein, d 5) | 1.45 (1.08, 1.82) | 4.54 (4.07, 5.0) | 1.37 (1.26, 1.49) | |||
| Slope (15% protein, d 1) | -0.64 (-0.98, -0.31) | <0.0001 | -0.28 (-0.52, -0.05) | 0.01 | 0.1 (0.01, 0.18) | 0.04 |
| Slope (25% protein, d 5) | -0.34 (-0.56, -0.05) | 0.02 | -0.03 (-0.26, 0.19) | 0.88 | -0.05 (-0.14, 0.04) | 0.23 |
| Slope (25% protein d 1 to d 5) | 0.31 (-0.05, 0.67) | 0.07 | 0.08 (-0.23, 0.40) | 0.68 | 0.09 (-0.03, 0.22) | 0.1 |
| Intercept (25% protein, d 5) | 1.15 (0.76, 1.52) | 4.51 (4.04, 4.97) | <0.0001 | 1.32 (1.21, 1.44) | ||
| Slope (25% protein, d 1) | -0.33 (-0.67, 0.05) | 0.0004 | -0.2 (-0.43, 0.03) | 0.046 | 0.19 (0.10, 0.28) | <0.0001 |
*Log transformation was used to normalise for mixed model linear regression, untransformed data is presented.
Discussion
In this study, the fasting plasma level of FGF-21 was higher following 4 d ad libitum intake of a reduced percent protein diet (10%), consistent with published work in mice and in humans [14]. Furthermore, studies in FGF-21 knockout mice indicate that FGF-21 is required for the increased energy intake that occurs on low percent protein diets [14]. In the present study, protein concentration in the diet was varied by substitution with carbohydrate with fat kept constant. Therefore the elevated serum FGF-21 levels and energy intakes observed in subjects on the 10% protein diets also occurred in the context of higher carbohydrate intakes (see also [14,30]).
Rodents have separate appetites for both protein and carbohydrate with protein dominating (to a somewhat lesser extent in mice than rats on the basis of the literature) [31, 32]. If the regulations of protein and carbohydrate intakes are comparable in humans, as indicated by other clinical trial and population data [5–7], the increase in energy intake on the 10% protein diet is predominantly due to limited protein availability rather than high levels of carbohydrate [11]. However, systematically testing the interactions between protein, fat and carbohydrate with other dietary factors such as carbohydrate quality, fiber content, amino acid and fatty acid composition, and dietary energy density on energy intake is required to unravel relative significance of each of these nutritional factors on energy intake and satiety.
The plasma level of FGF-21 was increased in mice fed a protein restricted-normal energy diet (10% dietary protein) in comparison to mice fed a protein restricted-low energy diet (20% protein) [14]. In healthy men and women plasma FGF-21 levels increased 1.7-fold above baseline [14] in response to a 28 d overfeeding protocol using a 5% protein diet [15] but did not change from baseline in response to overfeeding using a 15% protein dietary protocol [15]. In the current study plasma FGF-21 levels and energy intakes were increased following 4 d reduction in dietary protein from 25% to 10% using an approach in which dietary fat was held constant. Vienberg et al. also observed substantial increases in serum FGF-21 levels when dietary protein fell from 15 to 7.5% by increasing dietary fat content [33]. In contrast, a delayed increase in serum FGF-21 level was observed after a 9 d fast in humans [34]. In a recent study by Dushay et al. serum levels of FGF-21 were increased at 60 min in response to a dietary fructose load [35] indicating that increased carbohydrate intake on the 10% protein diet may also contribute to increased secretions of FGF-21. Coincident increases in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels suggest that liver stress occurred under the fasting conditions [34]. ALT also increases on low protein, high energy diets used to determine protein requirements in nitrogen balance studies [36]. These findings raise the possibility that hepatic stress and catabolism indicative of liver inflammation or damage may be related to elevated serum FGF-21 levels.
Once stimulated, FGF-21 secretion from the liver may act on the hypothalamus to drive increased energy intake [37]. It has been suggested that essential amino acid deficiency may be detected by an alternative pathway involving the protein kinase General Control Nonderepressible 2 (GCN2) in the anterior piriform cortex (APC) and consequent phosphorylation of eukaryotic initiation factor 2α (eIF2α) which stimulates FGF-21 but also prevents further consumption of foods deficient in essential amino acids [37] and this can be reversed by the addition of methionine to a low carbohydrate ketogenic diet which may indicate an amino acid specific response [38]. Functional magnetic resonance imaging has been used to show an association between protein status and brain responses in reward regions to protein cues such as umami taste, protein depleted participants showed increased responses in comparison to protein replete participants [13], this may enable aversion to low protein foods in a protein-depleted state. In the current study, where diet derived amino acids were reduced the 14% increase in energy intake during the 10% protein period was largely achieved by preferential selection of savoury-flavoured scones and muffins between meals [11]. The differential response to low total levels of amino acids versus selective amino acid deficiency may partly explain why protein intakes are not fully stabilised across a range of values for percent dietary protein [4, 11]. For example, Martens et al. [8, 9, 39] found that food and energy intakes did not continue to increase when dietary protein was reduced to 5% protein, and energy intake did not fall in the current study at 25% protein [11]. A recent clinical trial in which disguised diets were offered to subjects for a period of 5 d found a monotonic decline in food intake as percent protein rose from 10, 15 to 25%, but even then total intakes of protein fell as percent dietary protein fell indicating that protein leverage was incomplete [40]. Such incomplete protein leverage is a widespread feature among animals, including mice. Among mammals, spider monkeys come the closest to complete expression of protein leverage by maintaining protein intakes almost constant as percent dietary protein of the diet changes in response to season from approximately 4% to 20% protein [41]. Food choice by taste preference may be disrupted when processed foods that are savoury in taste (eg. by addition of sodium glutamate) have low protein and high fat or carbohydrate content [42].
Elevated plasma levels of FGF-21 have been linked with improvements in metabolic health indicators [43–45]. The present study was relatively short in duration and cannot be used to comment on longer term effects of percent dietary protein and FGF-21 on metabolic health. Also, in contrast to habitual diets all foods in each dietary treatment were of a fixed macronutrient composition and it is not known whether this difference influences metabolic health. Deleterious changes in lipids such as increased serum triglyceride levels followed reductions in percent dietary protein whereas total cholesterol was reduced on 10% relative to 15% and 25% dietary protein. Indicators of glucose control (glucose variability and insulin) improved on the 25% relative to 15% and 10% dietary protein however the change in glucose variability was relatively small and may not be physiologically significant in this lean healthy population. The increase in serum triglycerides in response to the 10% protein diet could be detrimental to health if continued over longer periods. Dietary recommendations to increase carbohydrate by increasing complex carbohydrates rather than refined sources may reduce these detrimental effects on triglycerides and HDL-cholesterol [46] observed in the case of a high carbohydrate, low fat diet [47]. Longer-term dietary studies in mice and humans show increased longevity and better metabolic health on low protein, high carbohydrate diets [48–50]. The work by Solon-Biet et al. measures the metabolic response over a much larger range of dietary macronutrient compositions highlighting the importance of higher carbohydrate and lower levels of fat in improvement of cardio-metabolic disease and lifespan measures [48].
The current study did not provide any evidence for involvement of ghrelin, GLP-1 and CCK in percent dietary protein dependent changes in energy intake. A single, very high percent dietary protein meal (58.1% vs a control meal of 19.3% and 71% vs a control meal of 1% protein) enhanced post-meal suppression of ghrelin [51, 52] but had no effect when changes in percent dietary protein more closely resembled those of a normal balanced diet (ie. 35% vs 20% and 25% vs 10% dietary protein) [53, 54]. Secretion of GLP-1 may be stimulated by increased production of the short chain fatty acid, propionate, in the lumen of the gut to act on fatty acid receptor 2 [55]. Results from the large randomized controlled trial, DIOGENES, support an association between GLP-1, increased dietary fiber and better maintenance of weight loss [56]. Fasting CCK has been shown to respond to low compared to high fat diets [57] as well as other nutrients. The absence of a CCK response in this study may be because fat was held constant at 30% in each of the 10%, 15% and 25% protein diets.
This study suggests there may be a potential role for FGF-21 in driving increases in energy intake when the proportion of protein in the diet is reduced under controlled ad libitum study conditions. Changes in FGF-21 as a result of protein restriction may have consequences not only for total energy intake but also for body weight and metabolic health.
Supporting Information
(PDF)
(DOC)
Acknowledgments
We thank the Woolcock Institute of Medical Research (Sydney, Australia) for the use of their facilities. We would also like to thank all the study volunteers for their participation.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by a National Health and Medical Research Council Project grant (#457522). CST was supported by a National Health and Medical Research Council Early Career Fellowship (#1037275). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6(2):133–42. [DOI] [PubMed] [Google Scholar]
- 2.Simpson SJ, Raubenheimer D. The Nature of Nutrition: A Unifying Framework from Animal adaptation to Human Obesity: Princeton University Press; 2012. [Google Scholar]
- 3.Raubenheimer D, Machovsky-Capuska GE, Gosby AK, Simpson S. Nutritional ecology of obesity: from humans to companion animals. Br J Nutr. 2015;113 Suppl:S26–39. 10.1017/S0007114514002323 [DOI] [PubMed] [Google Scholar]
- 4.Gosby AK, Conigrave AD, Raubenheimer D, Simpson SJ. Protein leverage and energy intake. Obesity Reviews. 2014;15(3). [DOI] [PubMed] [Google Scholar]
- 5.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363(22):2102–13. 10.1056/NEJMoa1007137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Cordero C, Kuzawa CW, Sloboda DM, Stewart J, Simpson SJ, Raubenheimer D. Testing the Protein Leverage Hypothesis in a free-living human population. Appetite. 2012;59(2):312–5. 10.1016/j.appet.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 7.Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. American Journal of Clinical Nutrition. 2011;93(4):836–43. 10.3945/ajcn.110.000141 [DOI] [PubMed] [Google Scholar]
- 8.Martens EA, Lemmens SG, Westerterp-Plantenga MS. Protein leverage affects energy intake of high-protein diets in humans. Am J Clin Nutr. 2013;97(1):86–93. 10.3945/ajcn.112.046540 [DOI] [PubMed] [Google Scholar]
- 9.Martens EA, Tan SY, Dunlop MV, Mattes RD, Westerterp-Plantenga MS. Protein leverage effects of beef protein on energy intake in humans. Am J Clin Nutr. 2014;99(6):1397–406. 10.3945/ajcn.113.078774 [DOI] [PubMed] [Google Scholar]
- 10.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82(1):41–8. [DOI] [PubMed] [Google Scholar]
- 11.Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, Jebb SA, et al. Testing Protein Leverage in Lean Humans: A Randomised Controlled Experimental Study. PLoS One. 2011;6(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmonier C, Chapelot D, Louis-Sylvestre J. Effects of macronutrient content and energy density of snacks consumed in a satiety state on the onset of the next meal. Appetite. 2000;34(2):161–8. [DOI] [PubMed] [Google Scholar]
- 13.Griffioen-Roose S, Smeets PA, van den Heuvel E, Boesveldt S, Finlayson G, de Graaf C. Human protein status modulates brain reward responses to food cues. Am J Clin Nutr. 2014;100(1):113–22. 10.3945/ajcn.113.079392 [DOI] [PubMed] [Google Scholar]
- 14.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–22. 10.1172/JCI74915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA. 2012;307(1):47–55. 10.1001/jama.2011.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75(4):1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89(1):71–84. [DOI] [PubMed] [Google Scholar]
- 18.Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31(7):665–70. [DOI] [PubMed] [Google Scholar]
- 19.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–66. [DOI] [PubMed] [Google Scholar]
- 20.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101(6):1320S–1329(S). [DOI] [PubMed] [Google Scholar]
- 21.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. 10.1146/annurev-nutr-080508-141056 [DOI] [PubMed] [Google Scholar]
- 22.Gosby AK, Soares-Wynter S, Campbell C, Badaloo A, Antonelli M, Hall RM, et al. Design and testing of foods differing in protein to energy ratios. Appetite. 2010;55(2):367–70. 10.1016/j.appet.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Gonder-Frederick LA, Cox DJ, Clarke WL. Evaluating the accuracy of continuous glucose-monitoring sensors. Diabetes Care. 2004;27(8):1922–8. [DOI] [PubMed] [Google Scholar]
- 24.Chlup R, Jelenova D, Kudlova P, Chlupova K, Bartek J, Zapletalova J, et al. Continuous glucose monitoring—a novel approach to the determination of the glycaemic index of foods (DEGIF 1)—determination of the glycaemic index of foods by means of the CGMS. Exp Clin Endocrinol Diabetes. 2006;114(2):68–74. [DOI] [PubMed] [Google Scholar]
- 25.Wallace AJ, Willis JA, Monro JA, Frampton CM, Hedderley DI, Scott RS. No difference between venous and capillary blood sampling and the Minimed continuous glucose monitoring system for determining the blood glucose response to food. Nutrition Research. 2006;26(8):403–8. [Google Scholar]
- 26.Urbanek S, Bibiko H-J, Iacus M. Wooden Christmas Tree. R version 3.2.3 ed: The R Foundation for Statistical Computing; 2014. [Google Scholar]
- 27.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–63. 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- 28.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67(1):1–48. [Google Scholar]
- 29.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 3.1.3 ed2013.
- 30.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139(2):456–63. 10.1053/j.gastro.2010.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring). 2008;16(3):566–71. [DOI] [PubMed] [Google Scholar]
- 32.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28(3):201–13. [DOI] [PubMed] [Google Scholar]
- 33.Vienberg SG, Brons C, Nilsson E, Astrup A, Vaag A, Andersen B. Impact of short-term high-fat feeding and insulin-stimulated FGF21 levels in subjects with low birth weight and controls. Eur J Endocrinol. 2012;167(1):49–57. 10.1530/EJE-12-0039 [DOI] [PubMed] [Google Scholar]
- 34.Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y, et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125(12):4601–11. 10.1172/JCI83349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab. 2015;4(1):51–7. 10.1016/j.molmet.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garza C, Scrimshaw NS, Young VR. Human protein requirements: evaluation of the 1973 FAO/WHO safe level of protein intake for young men at high energy intakes. Br J Nutr. 1977;37(3):403–20. [DOI] [PubMed] [Google Scholar]
- 37.Morrison CD, Laeger T. Protein-dependent regulation of feeding and metabolism. Trends Endocrinol Metab. 2015;26(5):256–62. 10.1016/j.tem.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pissios P, Hong S, Kennedy AR, Prasad D, Liu FF, Maratos-Flier E. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol Metab. 2013;2(3):306–13. 10.1016/j.molmet.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martens EA, Tan SY, Mattes RD, Westerterp-Plantenga MS. No protein intake compensation for insufficient indispensable amino acid intake with a low-protein diet for 12 days. Nutrition & Metabolism. 2014;11(38). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell CP, Raubenheimer D, Badaloo AV, Gluckman PD, Martinez C, Gosby A, et al. Developmental contributions to macronutrient selection: A randomized controlled trial in adult survivors of malnutrition. Evol Med Public Health. 2016;1:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felton AM, Felton A, Lindenmayer DB, Foley WJ. Nutritional goals of wild primates. Functional Ecology. 2009;23(1):70–8. [Google Scholar]
- 42.Simpson SJ, Raubenheimer D. Perspective: Tricks of the trade. Nature. 2014;508(7496):S66 10.1038/508S66a [DOI] [PubMed] [Google Scholar]
- 43.Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab. 2011;22(3):81–6. 10.1016/j.tem.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 44.Solon-Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Le Couteur DG, Simpson SJ. Macronutrients and caloric intake in health and longevity. J Endocrinol. 2015;226(1):R17–28. 10.1530/JOE-15-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223–41. 10.1146/annurev-physiol-021115-105339 [DOI] [PubMed] [Google Scholar]
- 46.Gardner CD, Offringa LC, Hartle JC, Kapphahn K, Cherin R. Weight loss on low-fat vs. low-carbohydrate diets by insulin resistance status among overweight adults and adults with obesity: A randomized pilot trial. Obesity (Silver Spring). 2016;24(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297(9):969–77. [DOI] [PubMed] [Google Scholar]
- 48.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–30. 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Floegel A, Pischon T. Low carbohydrate-high protein diets. BMJ. 2012;344:e3801 10.1136/bmj.e3801 [DOI] [PubMed] [Google Scholar]
- 50.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026 10.1136/bmj.e4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, et al. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr. 2006;83(2):211–20. [DOI] [PubMed] [Google Scholar]
- 52.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91(8):2913–9. [DOI] [PubMed] [Google Scholar]
- 53.Al Awar R, Obeid O, Hwalla N, Azar S. Postprandial acylated ghrelin status following fat and protein manipulation of meals in healthy young women. Clin Sci (Lond). 2005;109(4):405–11. [DOI] [PubMed] [Google Scholar]
- 54.Smeets AJ, Soenen S, Luscombe-Marsh ND, Ueland O, Westerterp-Plantenga MS. Energy expenditure, satiety, and plasma ghrelin, glucagon-like peptide 1, and peptide tyrosine-tyrosine concentrations following a single high-protein lunch. J Nutr. 2008;138(4):698–702. [DOI] [PubMed] [Google Scholar]
- 55.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39(3):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du H, van der AD, Boshuizen HC, Forouhi NG, Wareham NJ, Halkjaer J, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91(2):329–36. 10.3945/ajcn.2009.28191 [DOI] [PubMed] [Google Scholar]
- 57.Robertson MD, Henderson RA, Vist GE, Rumsey RD. Extended effects of evening meal carbohydrate-to-fat ratio on fasting and postprandial substrate metabolism. Am J Clin Nutr. 2002;75(3):505–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
Data Availability Statement
All relevant data are within the paper.