Abstract
Background
Adhering to weight loss interventions is difficult for many people. The majority of those who are overweight or obese and attempt to lose weight are simply not successful. The objectives of this study were 1) to quantify overall adherence rates for various weight loss interventions and 2) to provide pooled estimates for factors associated with improved adherence to weight loss interventions.
Methods
We performed a systematic literature review and meta-analysis of all studies published between January 2004 and August 2015 that reviewed weight loss intervention adherence.
Results
After applying inclusion and exclusion criteria and checking the methodological quality, 27 studies were included in the meta-analysis. The overall adherence rate was 60.5% (95% confidence interval [CI] 53.6–67.2). The following three main variables were found to impact adherence: 1) supervised attendance programs had higher adherence rates than those with no supervision (rate ratio [RR] 1.65; 95% CI 1.54–1.77); 2) interventions that offered social support had higher adherence than those without social support (RR 1.29; 95% CI 1.24–1.34); and 3) dietary intervention alone had higher adherence than exercise programs alone (RR 1.27; 95% CI 1.19–1.35).
Conclusion
A substantial proportion of people do not adhere to weight loss interventions. Programs supervising attendance, offering social support, and focusing on dietary modification have better adherence than interventions not supervising attendance, not offering social support, and focusing exclusively on exercise.
Keywords: community based, obesity, social support, program adherence
Introduction
Obesity is a common chronic condition that increases the risk of numerous health problems, including cardiovascular diseases, diabetes, cancer, and mental health issues.1 Substantial weight loss is not required to start to see health benefits. For example, a 5% reduction in body weight is enough to improve health outcomes.1 According to data from the 1998 National Health Interview Survey, 50% of obese men and 58% of obese women in the US are actively trying to lose weight.2 Regrettably, despite the efforts of a large portion of the population, the prevalence of obesity has remained high.3
It is especially difficult to lose weight among those suffering from obesity, as it is a complex condition created by diverse genetic, environmental, cultural, and socioeconomic pathways. For example, a recent study concluded that the chances of returning to a normal weight for someone who is already obese are extremely low: one in 210 chances for men and one in 124 chances for women.4 According to the National Health Interview Study mentioned earlier, the most commonly reported weight loss methods are as follows: 1) calorie restriction alone, 2) eating less fat, and 3) exercising more.2
All of that said, moderate weight loss for health reasons is possible, even among those who are obese. For example, a study of 4,034 obese adults in the US found that 40% lost >5% of their body weight in the past year and 20% lost >10%.5 However, participants need to adhere to evidence-based weight loss methods to lose weight and maintain weight loss in the long term. A meta-analysis of 18 randomized controlled trials (RCTs) found that diet and exercise programs combined were clearly superior to diet programs alone or exercise programs alone.6 A meta-analysis of 29 studies looking at long-term (5 years) weight loss maintenance among those who participated in structured weight loss programs, found that the average individual maintained at least 3 kg of weight loss and at least a 3% reduction of initial body weight. The authors found that those who lost more weight prior to starting the programs were more likely to keep weight off and those who exercised more were able to better maintain their weight loss than those who did not.7
Adhering to healthy weight loss behaviors is required for weight loss initially and in the long term. If participants are unable to adhere to weight loss strategies, they will not lose weight. Problems with attrition and nonadherence exist for intervention programs that are often evaluated in the short term. For example, a meta-analysis of 80 studies on weight loss interventions with a control group found a mean attrition rate of 31%.8 In a meta-analysis of 45 RCTs of nonsurgical weight loss interventions in obese adults, it was found that 28.4% of participants dropped out of the study prior to the maintenance phase and that many of these dropouts were due to not meeting adherence criteria or weight loss criteria during the study phase.9
An important part of advancing weight loss interventions is to understand how to improve adherence to weight loss behaviors. In any health behavior, nonadherence is a problem. The World Health Organization has identified nonadherence as a problem, “of striking magnitude”.10 Behavior change is complex, and even in life-threatening situations, it is difficult for people to adhere to medical advice. For example, a report from Statistics Canada found that among smokers with new diagnoses of chronic diseases: 75% of patients with a recent diagnosis of heart disease, 78% of those with a new cancer diagnosis, 74% of those with stroke, and 96% of those with respiratory disease did not quit smoking.11 Concerning weight-related health risks, more optimistic results have been reported about behavioral changes. For example, a study of 600 participants with a new diagnosis of type II diabetes found that only 20 people were able to change all their cardiovascular disease risk behaviors within 1 year, but many were able to decrease their Body Mass Index (BMI) and decrease their total daily calorie intake.12
In regard to weight loss interventions among those with obesity, there have been no meta-analyses investigating factors that improve adherence rates. The objective of this review and meta-analysis was to quantify adherence rates for various weight loss intervention types and to provide pooled estimates for factors associated with improved adherence to weight loss interventions.
Methods
A systematic literature review was performed, accessing the following databases: Medline, PubMed, ProQuest, CINAHL, Cochrane Central, Global Health, ISI Web of Knowledge, ProQuest, SCOPUS, and EMBASE. Search dates ranged from January 2004 to August 2015.
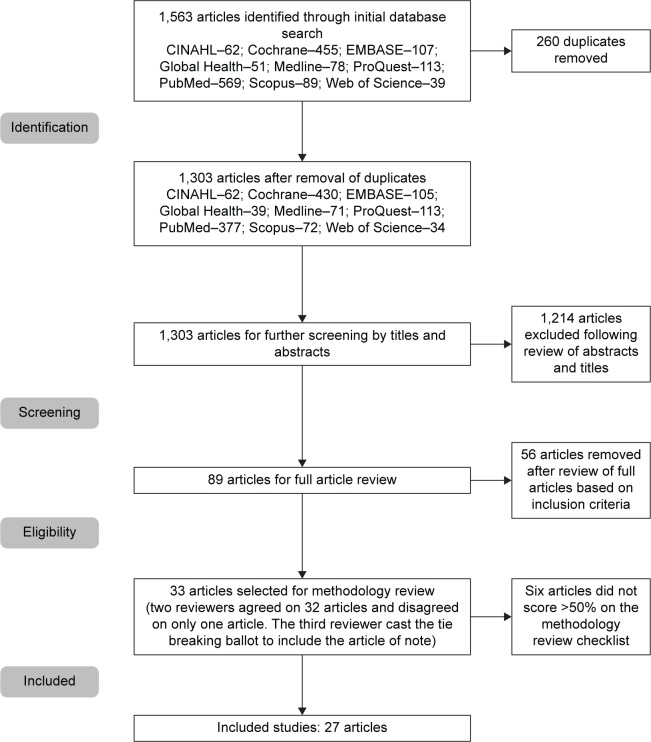
Subject search descriptors included terms listed in Figure 1. Search terms included relevant weight loss or reduction interventions and adherence or behavior modification (Supplementary material). Adherence was defined as completion of the weight loss program or, in certain cases, was assessed by the level of consistency with the weight loss intervention of interest. Reference sections of each article were reviewed for additional articles. Unpublished articles were not included in our search.
Figure 1.
Flow diagram for included studies.
The following inclusion criteria were used in the search:
Article should clearly describe adherence to a weight loss program, and the said program should be neither pharmacological nor surgical.
Article should have quantifiable data describing the effect size (ie, some absolute or relative measure of program adherence).
Article should describe a study that is prospective in nature (ie, an RCT, a quasiexperimental, or a cohort study).
Article should be publicly available.
Article should be published in the English language.
The search strategies excluded opinion articles, letters to the editor, case reports, and case studies.
Titles were initially reviewed for relevance and to remove duplication. The articles that remained were then subjected to full abstract review in order to apply inclusion and exclusion criteria. Finally, the remaining articles were subjected to full review and methodological quality evaluation by a panel of two reviewers (CN and ML). Unanimous agreement was sought; however, when there was disagreement, a tie breaker was used with a third author (JM).
We used methodological quality checklists for experimental and quasiexperimental designs from Greenhalgh et al.13 These checklists are a validated modification of the Cochrane Effective Practice and Organization of Care checklist and contain ten questions covering six areas of methodological rigor (eleven questions for quasiexperimental designs). The lists made provisions for assessing bias with the different study designs. A score of six was required in order to be accepted for review (ie, a score >50%).14,15 The checklists are presented in Tables 1 and 2.
Table 1.
Methodology checklist for experimental designs
| Authors: | ||
|---|---|---|
|
| ||
| Study title: | ||
|
| ||
| Yes | No | |
| A. Research question and design | ||
| 1. Was there a clear research question and was this important and sensible? | ||
| 2. If the study was nonrandomized, could a randomized controlled design have been used? | ||
| B. Baseline comparability of groups | ||
| 3. [RCTs only]: Was allocation adequately concealed by a rigorous method (eg, random number)? | ||
| 4. Were appropriate measures of baseline characteristics taken in all groups before the intervention and were study groups shown to be comparable in all characteristics likely to influence outcome? | ||
| C. Outcome measures | ||
| 5. Was the primary outcome measure valid (ie, do two independent raters agree that this was a sensible and reasonable measure of performance or outcome)? | ||
| 6. Was the primary outcome measure reliable (ie, do two independent raters agree on the nature and extent of change)? | ||
| D. Protection against contamination | ||
| 7. Is it unlikely that the control unit of allocation (professional, practice, institution, and community) received the intervention through contamination? | ||
| E. Protection against bias | ||
| 8. Were outcomes measured by “blinded” observers or were they objectively verified (eg, quantitative measures recorded prospectively and independently)? | ||
| F. Follow-up | ||
| 9. Was there complete follow-up of participants (ideally >80%) | ||
| 10. Was follow-up continued for long enough for the primary outcome measure to show an impact and for sustainability to be demonstrated? | ||
Note: Passing score for experimental designs =6/10.
Abbreviation: RCT, randomized controlled trial.
Table 2.
Quasiexperimental designs
| Authors: | ||
|---|---|---|
|
| ||
| Study title: | ||
|
| ||
| Yes | No | |
| A. Research question and design | ||
| 1. Was there a clear research question, and was this important and sensible? | ||
| 2. If the study was nonrandomized, could a randomized controlled design have been used? | ||
| B. Protection against secular changes | ||
| 3. Was the intervention independent of other changes over time? | ||
| 4. Were there sufficient data points to enable reliable statistical inference? | ||
| 5. Was a formal statistical test for trend correctly undertaken? | ||
| C. Outcome measures | ||
| 6. Was the primary outcome measure valid (ie, do two independent raters agree that this was a sensible and reasonable measure of performance or outcome)? | ||
| 7. Was the primary outcome measure reliable (ie, do two independent raters agree on the nature and extent of change)? | ||
| D. Protection against detection bias | ||
| 8. Was the intervention unlikely to affect data collection (eg, sources and methods of data collection were the same before and after the intervention)? | ||
| 9. Were outcomes measured by “blinded” observers or were they objectively verified (eg, quantitative measures recorded prospectively and independently)? | ||
| F. Completeness of data set and follow-up | ||
| 10. Does the data set cover all or most of the episodes of care (or other unit of analysis) covered by the study (ideally >80%)? | ||
| 11. Was follow-up continued for long enough for the primary outcome measure to show an impact and for sustainability to be demonstrated? | ||
Note: Passing score for quasiexperimental designs =6/11.
A computer program that utilized a random effects model, taken from Fleiss,16 was built to take interstudy heterogeneity into account. The statistical basis and its assumptions have been previously described in detail elsewhere.17–19 At least four articles were required for statistical pooling.
Results
Systematic review
The initial search generated 1,563 articles of which 260 articles were duplicates and removed, leaving 1,303 articles to screen abstracts. After the initial screening, 89 articles were included in the full review with 56 articles removed based on the inclusion/exclusion criteria. The remaining 33 articles were then subjected to full methodological review by two reviewers. There was disagreement on only one article that was included based on tie-breaking vote from a third reviewer. In total, six of the articles did not meet methodological requirements, leaving 27 articles for meta-analysis. Figure 1 depicts the search process. Studies included in the analysis and their detailed results are presented in Table 3.20–46
Table 3.
Included studies in meta-analysis
| Primary authors; | Study | Study | Number of study participants | Type of weight loss intervention | Adherence rate | Quality scores | Baseline BMI | Predictors of adherence | Other moderators (work place intervention, social support, financial incentive, or mandatory intervention) |
|---|---|---|---|---|---|---|---|---|---|
| Acharya et al;20 2009, USA | RCT | 12 months | 127 | Group sessions, energy/calorie goal, and diet | Energy/calorie goal – 10% Attendance – 44% Self-monitor – 22% |
6/10 pass | – | – | – |
| Annunziato et al;212009, USA | RCT | 12 months | 42 | Diet – MR | Attendance – 70% MR – 41.5% |
8/10 pass | 31.9 | – | – |
| Ard et al;22 2008, USA | Pre/post | 5 months | 377 | Calorie reduction, diet, and PA | Attendance – 45.6% PA goal – 36.9% |
8/11 pass | 35.1 | – | – |
| Austin et al;23 2013, USA | Pre/post | 4 months | 82 | LEARN | Attendance – 48.8% Calorie goals – 64.5% Step goals – 80.9% |
6/11 pass | 33.0 | Higher adherence: older age, increasing income, and increasing education Lower adherence: more children living at home, young age, and low socioeconomic status |
Financial incentive |
| Bartfield et al;24 2011, USA | RCT | 18 months | 507 | Group session, DASH diet, and exercise | Food record – 15.7% Exercise – 14% Attendance – 53.8% |
7/10 pass | 33.7 | – | – |
| Befort et al;25 2008, USA | RCT | 4 months | 34 | Diet, PA, motivational interview, and health education | Attendance – 52% | 6/10 pass | 39.8 | – | – |
| Burke et al;26 2009, USA | RCT | 24 months | 210 | Paper record, PDA, and PDA with feedback | Attendance/retention: cohort 1 (n=73) – 90% (24 months) Cohort 2 (n=63) – 76% (18 months) Cohort 3 (n=74) – 92% (6 months) |
6/10 pass | 33.4 | – | – |
| Carson et al;27 2013, USA | RCT | 6 months | 92 | Self-monitoring, goal setting, stimulus control, nutrition education, and cognitive restructuring | Attendance – 64.5% Monitoring – 62.1% |
6/10 pass | 38.1 | Higher adherence: having a social contact | Social support |
| Carter et al;28 2013, UK | RCT | 6 months | 128 | MMM smartphone app | Attendance – 61.7% MMM app – 16% (N=43) |
7/10 pass | 34.0 | Lower adherence: higher BMI and poorer health status | – |
| Church et al;29 2009, USA | RCT | 6 months | 411 | Exercise | 4 kcal/kg/wk (N=139) – 99.5% 8 kcal/kg/wk (N=85) – 99.3% 12 kcal/kg/wk (N=93) – 99.2% |
6/10 pass | 31.7 | – | – |
| Colley et al;30 2008, Australia | Pre/post | 4 months | 29 | PA | PA goals – 31% | 6/11 pass | 36.8 | Higher adherence: older age Lower adherence: higher weight | – |
| Das et al;31 2009, USA | RCT | 12 months | 38 | Dietary ER | 10% ER – 18% (N=9) 30% ER – 23% (n=29) |
6/10 pass | 28.8 | – | – |
| Dutton et al;32 2015, USA | Pre/post | 6 months | 33 | Primary care weight loss intervention + peer coaches | Attendance – 50% Peer calls – 40% |
7/11 pass | 42.9 | – | Social support and financial incentive |
| Dutton et al;33 2014, USA | RCT | 12 months | 66 | Behavioral intervention based on group sizes | Attendance: large group (n=31) – 49% Small group (n=35) – 62% Self-monitoring: large group (n=31) – 56.3% Small group (n=35) – 28.4% |
6/10 pass | 36.5 | – | – |
| Greenberg et al;342009, Israel | RCT | 24 months | 322 | Diet | Attendance – 84.5% Diet – 57% |
7/10 pass | 31.0 | Higher adherence: low carbohydrate diet, postexercise Lower adherence: during holidays, current smoker, female sex, and less initial weight loss |
– |
| Lemstra andRogers;35 2015, Canada | Pre/post | 6 months | 183 | Exercise, diet, and CBT | Exercise – 81% Diet – 71% CBT – 68% |
8/11 pass | 37.7 | Higher adherence: higher education and completion of a social support contract Lower adherence: past negative experiences with physical activity, high school or less than high school education, and depressed mood |
Social support |
| McAndrew et al;362013, USA | RCT | 3 months | 69 | Portion controlled weight loss intervention | Dietary adherence – 87% | 8/10 pass | 39.0 | Higher adherence: self-monitoring of blood glucose | Social support |
| Meffert et al;37 2010, Germany | Pre/post | 12 months | 481 | Metabolic balance nutrition program | Attendance – 61.6% Program adherence – 68% |
6/11 pass | 30.3 | Lower adherence: noncompatibility with job, dissatisfaction with program, and personal counseling, individual nutrition plans | – |
| Shapiro et al;38 2012, USA | RCT | 12 months | 170 | Self-monitoring via daily text messaging | Daily text – 60% | 8/10 pass | 32.2 | – | Financial incentive |
| Steinberg et al;392013, USA | RCT | 6 months | 50 | Self-monitoring via daily text messaging | Attendance – 90% Daily text (n=26) – 49% |
9/10 pass | 35.8 | – | Financial incentive |
| Steinberg et al;402014, USA | RCT | 12 months | 194 | Self-monitoring via IVR |
IVR – 71.6% | 6/10 pass | 30.2 | Higher adherence: older age and increasing education | – |
| Theim et al;41 2013, USA | RCT | 5 months | 101 | FBT | Attendance: FBT – 85% MT – 75% |
7/10 pass | 35.1 | – | Social support |
| Travier et al;42 2014, Spain | Pre/post | 3 months | 42 | Diet and PA | Attendance: diet (n=37) – 92% PA (n=37) – 91% |
7/11 pass | 30.4 | – | – |
| Turner-McGrievyet al;43 2007, USA | RCT | 24 months | 64 | Diet | Vegan (n=31) – 61.3%, NCEP (n=31) – 54.8% | 8/10 pass | – | – | Social support |
| Unick et al;44 2011, USA | RCT | 12 months | 2,503 | Intensive lifestyle/diabetic support and education | Program adherence – 80% | 6/10 pass | 35.8 | – | – |
| van Gool et al;452006, USA | RCT | 18 month | 238 | Diet and PA | Attendance – diet (n=137) 52.3% Physical activity (n=133) 49.9% |
6/10 pass | 34.5 | Higher adherence: Diet – low social participation and single status Exercise – exercising at home and no cardiovascular disease |
– |
| Wang et al;46 2012, USA | RCT | 12 months | 210 | Diet and PA self-monitoring | Paper record (n=72) – diet 34.4% and PA 29.7% PDA (n=68) – diet 57.8% and PA 59.4% PDA + feedback message (n=70) – diet 71.9% and PA 68.8% |
6/10 pass | 34.0 | – | – |
Abbreviations: CBT, Cognitive Behavioral Therapy; DASH, Dietary Approach to Stop Hypertension; ER, energy restriction; MMM, my meal mate; MR, meal replacement; MT, maintenance therapy; NCEP, National Cholesterol Education Program; PA, physical activity; PDA, personal digital assistant; RCT, randomized controlled trial; BMI, Body Mass Index; FBT, Family-Based Treatment; LEARN, Lifestyle, Exercise, Attitudes, Relationships, Nutrition; IVR, Interactive Voice Response.
Operationalizing adherence
Intervention adherence ranged significantly from 10%20 to 99.5%.29 Adherence was operationalized in 13 studies, where threshold rates were given to determine adherence to particular intervention behaviors.20,22–24,29–32,34,35,37,38,43
Study design
Twenty of the articles were RCTs with 5,576 participants (ranging from 34 to 2,503). The remaining seven articles were observational intervention studies with 1,227 participants (ranging from 29 to 481), for a total of 6,803 participants included in the overall analysis. Among RCTs, the mean adherence was 63.1%, while the mean adherence for the observational intervention studies was 59.6%.
Study length of time and adherence
The duration of nine of the studies was 12 months, with a sample size of 3,831 participants.20,21,31,33,37,38,40,44,46 The duration of 13 studies was <12 months with a sample size of 1,631 participants,22,23,25,27–30,32,35,36,39,41,42 and the duration of five studies was ≥18 months with a sample size of 1,341 participants24,26,34,43,45 (no articles had study durations between 12 months and 18 months). Interventions lasting <12 months had a mean adherence rate of 69.9%, while those lasting ≥12 months had a mean adherence rate of 53.0%.
Factors that affect adherence
Only ten of the 27 studies discussed factors that affected adherence.23,27,28,30,34–37,40,45 One study23 found associations between higher adherence and older age, higher income, and higher education. Older age was also associated with better adherence in two other studies,30,40 and increasing education levels were associated with higher adherence in two other studies.35,40 Social support contracts increased adherence rates in two studies27,35 and six studies in total, including a social support aspect to the intervention.27,32,35,36,41,43
Six studies discussed factors associated with lower adherence.23,28,30,34,35,37 These included lower socioeconomic status (education and income),23,35 higher weight,28,30 poor health,28 dissatisfaction with the program or weight loss results,34,37 smoking status,34 and depressed mood.35
Subgroup analysis
Subgroup analysis is presented in Table 4. Interventions with a duration of <12 months had higher adherence rates than interventions lasting ≥12 months (RR 1.32; 95% CI 1.28–1.36). Those interventions that included social support improved adherence rates by 29% compared to those interventions that did not include social support (95% CI 1.24–1.34).
Table 4.
Adherence rate and rate ratio for multifactor subgroup analysis across baseline body mass index, study duration, incentive, social support, age, study design, and intervention type
| Factor | Subgroup 1 | N | Adherent (%) | Subgroup 2 | N | Adherent (%) | Adherence rate ratio (1 vs 2) | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Baseline BMI | Overweight/moderate obesity | 5,321 | 63.39 | Severe/morbid obesity | 4,598 | 61.56 | 1.03 | 1.00–1.06 |
| Study duration | <12 months | 2,771 | 69.88 | ≥12 months | 7,591 | 53.01 | 1.32 | 1.28–1.36 |
| Financial incentive | Financial incentive | 538 | 61.79 | No financial incentive | 9,804 | 60.33 | 1.02 | 0.96–1.10 |
| Social support | Social support | 1,144 | 73.43 | No social support | 9,218 | 57.11 | 1.29 | 1.24–1.34 |
| Age | Predicted by older age | 469 | 60.97 | Not predicted by older age | 9,893 | 60.50 | 1.01 | 0.94–1.09 |
| Study design | RCT | 2,680 | 63.05 | Pre/post | 7,682 | 59.57 | 1.06 | 1.02–1.10 |
| Intervention type | Supervised program | 5,600 | 68.59 | Diet | 1,933 | 63.73 | 1.08 | 1.04–1.12 |
| PA | 1,388 | 50.21 | Self-monitoring | 1,258 | 41.50 | 1.21 | 1.11–1.32 | |
| Supervised program | 5,600 | 68.59 | Self-monitoring | 1,258 | 41.50 | 1.65 | 1.54–1.77 | |
| Diet | 1,933 | 63.73 | PA | 1,388 | 50.21 | 1.27 | 1.19–1.35 | |
| Supervised program | 5,600 | 68.59 | PA | 1,388 | 50.21 | 1.37 | 1.29–1.44 | |
| Diet | 1,933 | 63.73 | Self-monitoring | 1,258 | 41.50 | 1.54 | 1.43–1.65 |
Notes: Potential moderators of exercise adherence were identified apriori and used as factors for a subgroup analysis. Subgroup 1 and 2 were used (for analytical purposes) to represent the sub-categories within each pre-determined factor. Adherence rates were estimated for each sub-category (subgroup) of the pre-determined factors. The adherence rates for each subgroup represents the pooled estimate for a particular sub-category of a given factor. Pairwise rate ratios were computed for all sub-categories of each factor. “Supervised program” and “Self-monitoring” as used above, refer to whether the participants were directly monitored by the investigators or not, respectively. Subgroup analysis by intervention type aims to determine which of supervised monitoring, self monitoring, diet alone, or physical activity alone had a greater effect on adherence to weight loss programs.
Abbreviations: N, number of participants in subgroup; PA, physical activity; RCT, randomized controlled trial; CI, confidence interval.
Self-monitoring programs had the lowest adherence rate (41.5%), and supervised interventions had the highest adherence rate (68.6%). When attendance was monitored (supervised) by a researcher or intervention leader, participants were more adherent compared to programs that used self-monitoring interventions (RR 1.65; 95% CI 1.54–1.77), diet interventions, or physical activity interventions without supervision or attendance tracking. Participants were more adherent to diet interventions alone than exercise interventions alone (RR 1.27; 95% CI 1.19–1.35) or self-monitoring interventions (RR 1.54; 95% CI 1.43–1.65).
Interpretation
It is not possible to lose weight without actually adhering to weight loss protocols, such as exercise and dietary interventions. In weight loss interventions, nonadherence rates are usually high. In this article, we have included studies with interventions using methods, such as education, self-monitoring (electronic or nonelectronic), group-based or individual exercise or diet interventions, peer support, and lifestyle interventions, which included both diet and exercise. For many studies, adherence rates were low; as low as 10% in a 12-month study.20 Different intervention strategies reported various adherence rates within different study designs. We performed a pooled subgroup analysis to determine intervention characteristics that increase the likelihood of participant adherence.
High adherence rates were observed for interventions that incorporated aspects of social support. Social support is an important determinant in overall health. The Public Health Agency of Canada has listed social support as the second most important determinant of health,47 and the results from a number of studies indicate that those with social supports in place even have a reduced risk of premature mortality.48–50
Social support in the studies reviewed in this article ranged from group sessions to peer coaches to social support contracts to “buddy” programs. In the existing literature, multiple studies have indicated that social support (whether through family, friends, peers, or providers) is important for successful behavioral change. For example, one study found that those who received guided support for weight loss were 37% more likely to maintain weight loss than those who participated in self-directed strategies.51
Participation in weight loss interventions that allow friends or family to participate may have important implications for weight loss and weight loss maintenance. A previous study compared a weight loss intervention in which participants attended alone, with three friends, or with family members and found that at 6-month follow-up, those who attended with family or friends were more likely to maintain weight loss after program completion than those who attended alone.52 Social support contracts also allow ongoing support outside of the program. These contracts can help maintain a stronger commitment to a weight loss plan and have been shown to also improve rates of weight loss when compared to those who try to lose weight on their own.53 As such, it is important to incorporate aspects of social support into weight loss interventions and to provide social support when participants have none. Utilizing existing social networks may be one way to do this, while using existing technology to deliver social support via the web or smartphones is another. These social tools may also act as a motivational tool for participants to remain engaged in the weight loss intervention.54
Our analysis also determined that programs supervising and monitoring attendance improved adherence rates by 65% compared to self-monitoring programs and had the highest adherence rates overall. Monitoring attendance likely improves adherence rates since participants are more accountable for their behaviors. Supervision has been found to increase physical activity intervention adherence in other conditions. For example, a mixed methodology study on adherence to a physical activity program in patients with chronic obstructive pulmonary disorder found that participants felt that they would not be able to maintain the behaviors if not for the group dynamic of the classes and the required reporting to the physiotherapist.55
Supervised programs also allow participants to access health-care provider knowledge and feedback. Working without guidance can be discouraging and, at times, dangerous. Increasing knowledge transfer through access to an expert supervisor may help improve self-efficacy.56 Furthermore, a good relationship between supervisors and participants can increase adherence to additional health advice.56
Better adherence was also seen when comparing randomized with nonrandomized studies. This result has been observed in other health intervention studies, including a meta-analysis on adherence to statin therapy, where 90% of patients in RCTs were adherent to statin therapy, while only 49% were adherent in observational studies.57 This may be attributed in large part to the structured and specific nature of the inclusion and exclusion criteria used in RCTs. As such, participants in RCTs tend to be “less sick, younger, better educated, and of higher socioeconomic status”.58 It has been estimated that the average RCT excludes 90% of patients.59 There are other key differences in RCTs when compared to observational studies that could improve adherence, including differences in patient populations, different intervention intensities or therapeutic regimens, control of confounding factors, potentially more rigorous follow-up, among others.60 Additionally, due to the funding structure of RCTs and their need to evaluate efficacy instead of real-world effectiveness, these studies tend to be more heavily supervised. As such, the finding that randomized trials have higher adherence than observational studies is likely due to supervised programming and the recruitment of more “ideal” participants. For those offering clinical weight loss interventions, it is important to have participants engage in activities in which they are held accountable for their attendance or adherence to a group, a care provider, or a social support individual.
We also found better adherence to dietary interventions alone than exercise interventions alone. This could be due to a number of reasons. First, in cases where diet adherence was captured via self-report, poor recall or false information could be a possibility.61 Second, it may be due in part to participants seeing more weight loss from restrictive diets than from exercise interventions, which is supported by the literature.6,62 However, it has been previously stated that dietary and exercise programming needs to be combined in order to be most effective.6,62 Physical activity is important to overall health and overall weight loss maintenance63 and should be encouraged as a part of a weight loss intervention along with dietary change.
Financial incentives for weight loss programs are discussed throughout the literature, and four studies were included in our analysis.23,32,38,39 However, when comparing interventions that utilized financial incentives with those studies that did not, there were no significant differences in adherence rates. Among the studies using financial incentives, the participant pool was among the smallest in our analysis.
Future research
Weight loss intervention studies targeting obese individuals should take into account and measure factors that predict adherence. Few studies were found in this systematic review that had acceptable methodological rigor. Given the high prevalence of obesity and the importance of adherence in order to lose weight and maintain weight loss, this is an important area to continue to study.
Study limitations
Due to the variability between studies, the pooling of data, while helpful in increasing the sample size, does introduce a level of uncertainty, with regard to the study conclusion due to possible sampling errors or unmeasured covariates. Also, there were only ten studies in which the authors set out to measure factors that predict adherence to weight loss programs. Therefore, it is possible that the remaining studies may not have given proper consideration to potential con-founders for adherence or made the necessary adjustment as needed.
Conclusion
It is unlikely that there is a single solution to reverse the rising prevalence of obesity observed globally. A comprehensive approach is needed to address this complex issue. Supervising weight loss programs and adding social supports help improve adherence to weight loss programs. It is expected that with better adherence, overweight or obese persons can lose more weight and help keep it off in the long term. Evaluating the views of participants and adherence rates to weight loss programs is critical as it can offer insight, expand our current knowledge, and provide evidence in support of designing and implementing more effective interventions.
Supplementary materials
Search terms
Weight loss programs
Weight loss intervention
Weight loss regimen
Weight loss plan
Weight reduction programs
Weight reduction intervention
Weight reduction regimen
Weight reduction plan
Adherence
Behavior modification
Search strategy
(((((((((Weight loss programs) OR weight loss intervention) OR weight loss regimen) OR weight loss plan) OR weight reduction programs) OR weight reduction intervention) OR weight reduction regimen) OR weight reduction plan)) AND ((adherence) OR “behavior modification”).
Databases searched
Medline
PubMed
Cochrane Library
CINAHL
Global Health
ISI Web of Knowledge
ProQuest
SCOPUS
EMBASE
Inclusion criteria
Article should be in English language.
Article should have a prospective design.
Article should have quantifiable data describing the effect size.
Article should describe participation in a supervised weight loss program that is neither pharmacologic nor surgical and assess adherence to the said program.
Article is publicly available.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.National Institutes of Health . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Institutes of Health; 1998. NIH Publication No. 98-4083. [PubMed] [Google Scholar]
- 2.Kruger J, Galusk DA, Serdula MK, Jones DA. Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med. 2004;26(5):402–406. doi: 10.1016/j.amepre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fildes A, Charlton J, Rudisill C, Littlejohns T, Gulliford MC, Prevost AT. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Pub Health. 2015;105(9):e54–e59. doi: 10.2105/AJPH.2015.302773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicklas JM, Huskey K, Davis RB, Wee CC. Successful weight loss among obese U.S. adults. Am J Prev Med. 2012;42(5):481–485. doi: 10.1016/j.amepre.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10(3):313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74(5):579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 8.Franz MJ, van Wormer JJ, Crain AL, et al. Weight-loss outcomes: systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long-term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization [webpage on the Internet] Adherence to Long-Term Therapies. Evidence for Action. Geneva: World Health Organization; 2003. [Accessed April 22, 2016]. Available from: http://whqlibdoc.who.int/publications/2003/9241545992.pdf. [Google Scholar]
- 11.Statistics Canada [webpage on the Internet] Health Behaviour Changes After Diagnosis of Chronic Illness Among Canadians Aged 50 or Olde; 2012) [Accessed April 22, 2016]. Available from: http://www.statcan.gc.ca/pub/82-003-x/2012004/article/11740-eng.pdf. [PMC free article] [PubMed]
- 12.Long GH, Cooper AJM, Wareham NJ, Griffin SJ, Simmons RK. Healthy behavior change and cardiovascular outcomes in newly diagnosed Type 2 diabetic patients: a cohort analysis of the ADDITION-Cambridge study. Diabetes Care. 2014;37(6):1712–1720. doi: 10.2337/dc13-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenhalgh T, Robert G, Bate P, et al. Diffusion of Innovations in Health Service Organizations: A Systematic Literature Review. Malden, MA: Blackwell Publishing Ltd; 2005. [Google Scholar]
- 14.Lemstra M, Alsabbagh MW. Proportion and risk indicators of nonadherence to antihypertensive therapy: a meta-analysis. Patient Prefer Adherence. 2014;8:211–218. doi: 10.2147/PPA.S55382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsabbagh W, Lemstra M, Eurich D, et al. Socioeconomic Status and Non Adherence to Anti-Hypertensive Drugs: A Systematic Review and Meta-Analysis. Value in Health; 2014. [Accessed April 22, 2016]. webpage on the Internet. Available from: http://www.ispor.org/publications/value/articlesin-press.asp. [DOI] [PubMed] [Google Scholar]
- 16.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2(2):121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 17.Lemstra M, Bennett N, Neudorf C, et al. A meta-analysis of school based marijuana and alcohol prevention programs in targeting adolescents aged 10–15 years old. Addict Res Theory. 2010;18(1):84–96. [Google Scholar]
- 18.Lemstra M, Bennett N, Neudorf C, et al. A systematic review of drug and alcohol use by socioeconomic status in adolescents aged 10–15 years. Can J Pub Health. 2008;99(3):172–177. doi: 10.1007/BF03405467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemstra M, Neudorf C, D’Arcy C, Kunst A, Warren L, Bennett N. A systematic review of depressed mood and anxiety by socioeconomic status in adolescents aged 10–15 years. Can J Pub Health. 2008;99(2):125–129. doi: 10.1007/BF03405459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharya S, Elci O, Sereika S, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151–160. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annunziato R, Timko C, Crerand C, et al. A randomized trial examining differential meal replacement adherence in a weight loss maintenance program after one-year follow-up. Eat Behav. 2009;10(3):176–183. doi: 10.1016/j.eatbeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Ard J, Kumanyika S, Stevens V, et al. Effect of group racial composition on weight loss in African Americans. Obesity. 2008;16(2):306–310. doi: 10.1038/oby.2007.49. [DOI] [PubMed] [Google Scholar]
- 23.Austin J, Smith J, Gianini L, Campos-Melady M. Attitudinal familism predicts weight management adherence in Mexican American women. J Behav Med. 2013;36(3):259–269. doi: 10.1007/s10865-012-9420-6. [DOI] [PubMed] [Google Scholar]
- 24.Bartfield J, Stevens V, Jerome G, et al. Behavioral transitions and weight change patterns within the PREMIER Trial. Obesity. 2011;19(8):1609–1615. doi: 10.1038/oby.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Befort C, Nollen N, Ellerbeck E, Sullivan D, Thomas J, Ahluwalia J. Motivational interviewing fails to improve outcomes of a behavioral weight loss program for obese African American women: a pilot randomized trial. J Behav Med. 2008;31(5):367–377. doi: 10.1007/s10865-008-9161-8. [DOI] [PubMed] [Google Scholar]
- 26.Burke L, Styn M, Glanz K, et al. SMART trial: a randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemp Clin Trials. 2009;30(6):540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carson T, Eddings K, Krukowski R, Love S, Harvey-Berino J, West D. Examining social influence on participation and outcomes among a network of behavioral weight-loss intervention enrollees. J Obes. 2013;2013:1–8. doi: 10.1155/2013/480630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter M, Burley V, Nykjaer C, Cade J. Adherence to a smart-phone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(4):e32. doi: 10.2196/jmir.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Church T, Martin C, Thompson A, Earnest C, Mikus C, Blair S. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colley R, Hills A, O’Moore-Sullivan T, Hickman I, Prins J, Byrne N. Variability in adherence to an unsupervised exercise prescription in obese women. Int J Obes Relat Metab Disord. 2008;32(5):837–844. doi: 10.1038/sj.ijo.0803799. [DOI] [PubMed] [Google Scholar]
- 31.Das S, Saltzman E, Gilhooly C, et al. Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity. 2009;17(11):2019–2024. doi: 10.1038/oby.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutton G, Phillips J, Kukkamalla M, Cherrington A, Safford M. Pilot study evaluating the feasibility and initial outcomes of a primary care weight loss intervention with peer coaches. Diabetes Educ. 2015;41(3):361–368. doi: 10.1177/0145721715575356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutton G, Nackers L, Dubyak P, et al. A randomized trial comparing weight loss treatment delivered in large versus small groups. Int J Behav Nutr Phys Act. 2014;11(1):123. doi: 10.1186/s12966-014-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg I, Stampfer M, Schwarzfuchs D, Shai I, DIRECT Group Adherence and success in long-term weight loss diets: the dietary intervention randomized controlled trial (DIRECT) J Am Coll Nutr. 2009;28(2):159–168. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- 35.Lemstra M, Rogers M. The importance of community consultation and social support in adhering to an obesity reduction program: results from the Healthy Weights Initiative. Patient Preference and Adherence. 2015;9(9):1473–1480. doi: 10.2147/PPA.S91912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAndrew L, Napolitano M, Pogach L, et al. The impact of self-monitoring of blood glucose on a behavioral weight loss intervention for patients with Type 2 diabetes. Diabetes Educ. 2012;39(3):397–405. doi: 10.1177/0145721712449434. [DOI] [PubMed] [Google Scholar]
- 37.Meffert C, Gerdes N. Program adherence and efectiveness of a commercial nutrition program: the metabolic balance study. J Nutr Metab. 2010;2010:1–8. doi: 10.1155/2010/197656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro J, Koro T, Doran N, et al. Text4Diet: a randomized controlled study using text messaging for weight loss behaviors. Prev Med. 2012;55(5):412–417. doi: 10.1016/j.ypmed.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg D, Levine E, Askew S, Foley P, Bennett G. Daily text messaging for weight control among racial and ethnic minority women: randomized controlled pilot study. J Med Internet Res. 2013;15(11):e244. doi: 10.2196/jmir.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg D, Levine E, Lane I, et al. Adherence to self-monitoring via interactive voice response technology in an ehealth intervention targeting weight gain prevention among Black women: randomized controlled trial. J Med Internet Res. 2014;16(4):e114. doi: 10.2196/jmir.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theim K, Sinton M, Goldschmidt A, et al. Adherence to behavioral targets and treatment attendance during a pediatric weight control trial. Obesity. 2013;21(2):394–397. doi: 10.1038/oby.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travier N, Fonseca-Nunes A, Javierre C, et al. Effect of a diet and physical activity intervention on body weight and nutritional patterns in overweight and obese breast cancer survivors. Med Oncol. 2014;31(1):783. doi: 10.1007/s12032-013-0783-5. [DOI] [PubMed] [Google Scholar]
- 43.Turner-McGrievy G, Barnard N, Scialli A. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet. Obesity. 2007;15(9):2276–2281. doi: 10.1038/oby.2007.270. [DOI] [PubMed] [Google Scholar]
- 44.Unick J, Beavers D, Jakicic J, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and Type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34(10):2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gool C, Penninx B, Kempen G, et al. Determinants of high and low attendance to diet and exercise interventions among overweight and obese older adults. Contemp Clin Trials. 2006;27(3):227–237. doi: 10.1016/j.cct.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Sereika S, Chasens ER, Ewing LJ, Matthews JT, Burke LE. Effect of adherence to self-monitoring of diet and physical activity on weight loss in a technology-supported behavioral intervention. Patient Prefer Adherence. 2012;6:221–226. doi: 10.2147/PPA.S28889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Public Health Agency of Canada [webpage on the Internet] What Makes Canadians Healthy or Unhealthy? [Accessed December 7, 2015]. Available from: http://www.phac-aspc.gc.ca/ph-sp/determinants/determinants-eng/php.
- 48.Ben-Shlomo Y, Smith GD, Shipley M, Marmot MG. Magnitude and causes of mortality differences between married and unmarried men. J Epidemiol Community Health. 1993;47(3):200–205. doi: 10.1136/jech.47.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beasley JM, Newcomb PA, Trentham-Dietz A, et al. Social networks and survival after breast cancer diagnosis. J Cancer Surviv. 2010;4(4):372–380. doi: 10.1007/s11764-010-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brantley PJ, Stewart DW, Myers VH, et al. Psychosocial predictors of weight regain in the weight loss maintenance trial. J Behav Med. 2014;37(6):1155–1168. doi: 10.1007/s10865-014-9565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wing RR, Jeffery RW. Benefits of recruiting participants with friends and increasing social support for weight loss and maintenance. J Consult Clin Psychol. 1999;67(1):132–138. doi: 10.1037//0022-006x.67.1.132. [DOI] [PubMed] [Google Scholar]
- 53.Harris MB, Bruner CG. A comparison of a self-control and a contract procedure for weight control. Behav Res Ther. 1971;9(4):347–354. doi: 10.1016/0005-7967(71)90047-7. [DOI] [PubMed] [Google Scholar]
- 54.Aguilar-Martinez A, Sole-Sedeno JM, Mancebo-Moreno G, Medina FX, Carreras-Collado R, Saigi-Rubio F. Use of mobile phones as a tool for weight loss: a systematic review. J Telemed Telecare. 2014;20(6):339–349. doi: 10.1177/1357633X14537777. [DOI] [PubMed] [Google Scholar]
- 55.O’Shea SD, Taylor NF, Paratz JD. …But watch out for the weather: factors affecting adherence to progressive resistance exercise for persons with COPD. J Cardiopulm Rehabil Prev. 2007;27(3):166–174. doi: 10.1097/01.HCR.0000270686.78763.c8. [DOI] [PubMed] [Google Scholar]
- 56.Avers D, Wharton M. Improving exercise adherence: instructional strategies. Top Geriatr Rehabil. 1991;6:62–73. [Google Scholar]
- 57.Lemstra M, Blackburn D, Crawley F, Fung F. Proportion and risk indicators of non-adherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC. 2008;1(3):211–217. doi: 10.1016/j.jcin.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen JT, Lash TL, Rothman KJ. Beyond randomized controlled trials: a critical comparison of trials with nonrandomized studies. Hepatology. 2006;44(5):1075–1082. doi: 10.1002/hep.21404. [DOI] [PubMed] [Google Scholar]
- 60.Ting HH, Sjojania KG, Montori VM, Bradley EH. Quality improvement – science and action. Circulation. 2009;119(14):1962–1974. doi: 10.1161/CIRCULATIONAHA.108.768895. [DOI] [PubMed] [Google Scholar]
- 61.Dhurandhar NV, Schoeller D, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2014;39(7):1109–1113. doi: 10.1038/ijo.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mill WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise, or diet plus exercise intervention. Int J Obes. 1997;21(10):941–947. doi: 10.1038/sj.ijo.0800499. [DOI] [PubMed] [Google Scholar]
- 63.Kruger J, Blanck HM, Gillespie C. Dietary and physical activity behaviors among adults successful at weight loss maintenance. Int J Behav Nutr Phys Act. 2006;3:17–26. doi: 10.1186/1479-5868-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]