ABSTRACT
Many types of tumors are organized in a hierarchy of heterogeneous cell populations. The cancer stem-like cells (CSCs) hypothesis suggests that tumor development and metastasis are driven by a minority population of cells, which are responsible for tumor initiation, growth and recurrences. The inability to efficiently eliminate CSCs during chemotherapy, together with CSCs being highly tumorigenic and invasive, may result in treatment failure due to cancer relapse and metastases. CSCs are emerging as a promising target for the development of translational cancer therapies. Ideal panacea for cancer would kill all malignant cells, including CSCs and bulk tumor cells. Since both chemotherapy and CSCs-specific therapy are insufficient to cure cancer, we propose combination therapy with CSCs-targeted agents and chemotherapeutics for improved breast cancer treatment. We generated in vitro mammosphere of 2 breast cancer cell lines, and demonstrated ability of mammospheres to grow and enrich cancer cells with stem-like properties, including self-renewal, multilineage differentiation and enrichment of cells expressing breast cancer stem-like cell biomarkers CD44+/CD24−/low. The formation of mammospheres was significantly inhibited by salinomycin, validating its pharmacological role against the cancer stem-like cells. In contrast, paclitaxel showed a minimal effect on the proliferation and growth of breast cancer stem-like cells. While combination therapies of salinomycin with conventional chemotherapy (paclitaxel or lipodox) showed a potential to improve tumor cell killing, different subtypes of breast cancer cells showed different patterns in response to the combination therapies. While optimization of combination therapy is warranted, the design of combination therapy should consider phenotypic attributes of breast cancer types.
KEYWORDS: Breast cancer, cancer stem-like cell, combination therapy, mammosphere, salinomycin
Introduction
Most normal tissues in adults are composed of heterogeneous cell populations with a functional hierarchy. The stem cells stand on the top of the hierarchical tree. Stem cells in the steady state are in dormancy. The stem cell niche provides a microenvironment which can send specific signals to induce quiescent stem cells to commit to asymmetric division, leading to generation of both an identical stem daughter cell (known as self-renewal) and a progenitor which undergoes multilineage differentiation. Thus stem cells are able to repopulate whole tissues under certain conditions such as disease and injury, and regenerate cells composed of the tissues via pluripotency.1 Self-renewal and multilineage differentiation are 2 fundamental properties of stem cells.2 Self-renewal allows for maintenance of stem cell population over the entire lifetime of an organism, while multilineage differentiation acts to maintain tissue hemostasis.1
Inspired by the biology of stem cells, Valeriote FA and his co-workers in 1968 began to speculate the existence of similar functional hierarchies within cancer.3 In 1994, John Dick's laboratory first tackled the question with a demonstration of functional hierarchies and the identification of putative cancer stem like cells (CSCs) within acute lymphocytic leukemia through extensive cell cloning.4 They discovered a small subset of cells with phenotype CD34+/CD38− which was able to initiate a new leukemia episode in a SCID mouse model, and possessed self-renewing capacity, that is a critical property of all stem cells.4
These advances with leukemic stem cells generated enthusiasm in the scientific community. Continuing studies focused on the search for a similar subset of cells with capacity to initiate and maintain malignancy in other types of cancers including solid tumors. After two decades, Michael Clark in 2003 reported the first evidence for the presence of putative cancer stem-like cells or cancer-initiated cells within solid breast tumors.5 They isolated and identified a small subpopulation of human breast cancer cells that had the potential for tumorigenesis. Just 200 such breast cancer cells with immunephenotype of CD44+/CD24− were able to initiate new tumors in a nude mouse model, in comparison to at least 106 of unsorted breast cancer cells required to develop a new engrafted tumor.5 Immediately following this milestone in research, putative brain cancer stem cells were reported with positive expression of CD133 and similar attributes to normal neural stem cells.6,7 As low as 100 such CD133+ brain CSCs were capable of driving tumorigenesis in the mouse models.6,7 Recently, rare CSCs have been identified in other types of patient tumors and established cancer cell lines, including ovarian, melanoma, breast, brain, prostate, pancreatic, lung and colon.1,8 Isolation and enrichment of putative CSCs are done mainly through 3 methodologies, including (a) implanting tumor tissues in suitable animal model,5,9 (b) Use of established cell lines containing a minor population of cells with properties similar to those of stem cells 10 and (c) Isolation of CSCs based on the expression of surface markers for cancers.1,8
Discovery of the putative cancer stem cells has radically altered the prospective of cancer biology with this novel proposal of cancer stem cells (CSCs) model, leading to a paradigm shift in oncology. The classic paradigm, where a stochastic model dominated, suggested that all cancer cells are functionally identical with an equal potential for being tumorigenic and with the same possibility to initiate and maintain cancer. In contrast, the emerging cancer stem cell model hypothesized that many types of tumors are organized in functional hierarchies with a heterogeneous cell population, including a small proportion of cancer cells with stem cell-like properties (CSCs) and a majority of tumor bulk cells. The rare CSCs are able to both self-renew and give rise to non-tumorigenic daughter cells that constitute the bulk of a tumor.5,9,11 Other than bulk tumor cells, only these stem-like cancer cells have intrinsic tumorigenic properties. The cancer stem cell model maintains that these rare cancer stem-like cells drive the initiation, expansion, and progression of cancer, and are responsible for cancer recurrence and metastasis.12-15
Although CSCs are emerging as a novel and translationally relevant target for improved cancer therapy, accumulating evidences show these cancer stem-like cells (CSCs) or tumor initiating cells (TICs), are highly resistant to standard chemo- and radiotherapies, and they persist following treatment.12-14 CSCs are quiescent and may escape cell cycle and proliferation checkpoints of conventional chemo- and radiotherapies, which only target and eradicate cycling differentiated cancer cells. Therefore, conventional chemo- and radiotherapy targeting only the bulk replicating cancer cells may achieve clinical tumor reduction at the beginning of treatment, but are insufficient to cure cancers due to ineffective inhibition of CSCs.11 These drug-resistant CSCs that have evaded chemotherapy, may become highly proliferative and aggressive after receiving specific stimuli from the stem cell niche. Thriving CSCs under the influence of the stem cell microenvironment, lead to tumor recurrence and disease relapse and even promote formation of distant metastases, ultimately leading to treatment failure following chemo-and radiotherapy.12,13,16
Given the inefficiency of standard chemo- and radiotherapies against CSCs, a searching for an alternative therapy specific for CSCs-targeting is warranted. Salinomycin is a promising CSCs-targeting agent as it selectively inhibits cancer stem-like cells in a variety of different cancer types (including breast cancer) with diverse mechanisms.17,18 Salinomycin treatment also reduces metastatic tumor burden by hampering cancer cell migration.19 The mode of action of salinomycin targeting and elimination of CSCs may include triggering of apoptosis,20 inhibition of ABC transporters of CSCs,21 interference with the Wnt/β-Catenin signaling pathway, which confers resistance of CSCs to radiation22,23 and to anticancer drugs,24 elimination of CSCs via reduction in oxidative phosphorylation in mitochondria,25 interference with cytoplasmic and mitochondrial K+ effiux, and promotion of differentiation of CSCs and epithelial reprogramming of cells that had undergone an epithelial to mesenchymal transition (EMT).16,17
While the importance of CSCs as a promising target for cancer therapy is valued, neither conventional chemotherapy nor CSCs specific therapy is alone sufficient to cure cancer. An ideal panacea for breast cancer treatment must kill all malignant cells, including CSCs and bulk tumor cells. Here, we hypothesize that combination therapy with CSCs-targeted agents and conventional cytotoxic drugs will improve cancer treatment. Two types of compounds with different pharmacological mechanisms are expected to work in concert to eradicate CSCs by CSCs-specific agents (saliomycin) and bulk tumor cells by standard chemotherapy (paclitaxel or lipodox). In the study, we focused on breast cancer and testing on 2 different breast cancer cell lines, including MCF-7, which represents hormone receptor-positive breast cancer, and MDA-MB-231, which is triple-negative breast cancers (TNBC). We first established in vitro cancer stem cell mammosphere systems to isolate and grow breast cancer CSCs in vitro, followed by validation of the cultivating system by characterization of breast cancer stem-like cell properties, including (1) self-renewal, (2) CD44+/CD24−/low expression of the CSCs biomarkers:, and (3) their potential for differentiation. The effects of salinomycin and paclitaxel on breast stem-like cells were compared, and the tumor cell killing with combination therapies was evaluated using these 2 breast cancer cell lines using different combinations, including paclitaxel with salinomycin and lipodox with salinomycin at varied concentrations and treatment times.
Results
Formation of two breast cancer mammospheres in vitro
In vitro mammosphere culture is a method for the isolation and enrichment of CSCs based on the ability of CSCs able to grow in a undifferentiated condition without attachment to culture plates, whereas differentiated bulk tumor cells fail to survive under the same conditions.1,8,28 MCF-7 or MDA-MB-231 single cell suspension was cultivated in a low-adherent substrate in a serum-free medium containing growth factors bFGF and EGF. While majority of the seeded cells died, we observed the formation of floated, spherical and tight mammospheres with a 3D multicellular structure after 10–14 d culturing (Fig. 1A), indicating that a minor population of MCF breast cells survived and underwent proliferation in a non-differentiated condition in an anchorage-independent manner. Rare MDA-MB-231 cells were also proliferative but formed a relatively loose and flattened shape sphere under the same culture conditions even after more than 14 d. In modified and optimized medium compositions, we observed that rare MDA-MB-231 cells formed tight and round mammospheres (Fig. 1B) in the suspension culture with a modified undifferentiated medium supplemented with a low concentration of serum (1%).
Figure 1.
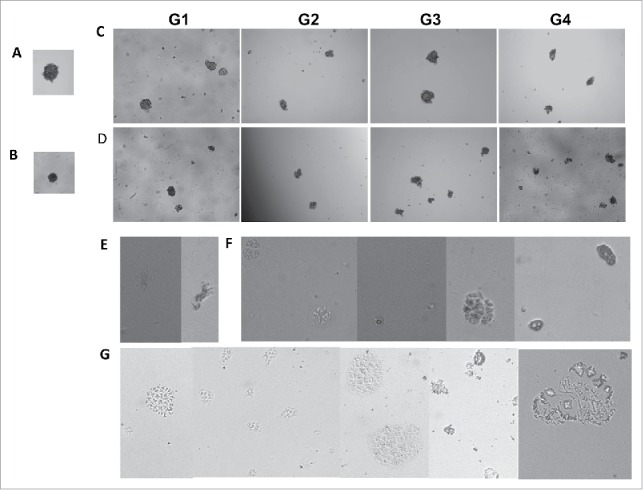
The self-renewal (A-D) and the differential (E-G) potential of stem-like cells enriched in vitro as cancer mammospheres of 2 breast cancer cell lines. (A) MCF-7 and (B) MDA-MB-231 grown under undifferentiated conditions in an anchorage-independent manner to form floating 3D mammospheres of generation 1 (G1). In vitro serial passages of mammosphere of MCF-7 (C) and MDA-MB-231 (D) up to generation 4 (G4), indicating cells with stem cell-like properties and self-renewal potential. The differential potential of breast cancer stem cell-like cells from mammospheres tested on a 3 dimensional clonal cultivation system containing Matrigel under differentiated conditions. MCF-7 showing branched, ductal-acinar structure (E) and acinar structures (F); MDA-MB-231 showing mixed lineage colonies with existence of clear branched, ductal-acinar structure (G). Photographs were taken at 10x magnification.
Assessment of the self-renewal potential of stem-like cells enriched in in vitro cancer mammospheres
Similar to normal mammary stem cells, breast tumorigenic cells with stem cell properties have been reported to propagate as floating mammospheres in vitro.1,8 In the study, the first generation mammospheres of both breast cell lines were subsequently subjected to enzymatically and mechanically dissociated to single cells, followed by continued culturing to give rise to secondary mammospheres. This procedure was repeated at 10–14 day interval, leading to an extensive propagation as floating mammospheres for up to 4 passages in vitro, suggesting that the mammosphere culture contained self-renewing cells which is a crucial attribute of stem cells (Fig. 1C, D). The ability to self renewal of this in vitro mammosphere system-validated suitability in the use of the experimental systems for studying breast cancer stem-like cells, and to challenge them with molecularly targeted agents that interfere specifically with self-renewal and survival of breast cancer stem-like cells.
Assessment of the differential potential of stem-like cells enriched in in vitro mammospheres
In order to explore the morphogenic differentiation potential of these cells enriched in mammospheres, we set up a 3D clonal cultivation system with Matrigel to act as a reconstituted basement membrane. In vitro Matrigel cultivation systems are able to produce the physiological signals necessary for normal mammary morphogenesis.30 The cultivation of both, human primary and immortalized mammary cells in Matrigel generated colonies with morphogenic differentiation showing bilineage potential for production of 2 basic multicellular structures: small acinus-like structures originating from luminal epithelial, and solid spherical colonies derived from myoepithelial cells.27,31 More complicated, branched ductal-acinar structures originated from cell aggregates.32
After 3–4 weeks of differentiated Matrigel culture of cells dissociated from mammospheres, we observed mixed lineage colonies with the existence of similar branched, ductal-acinar (Fig. 1E) and acinar structures (Fig. 1F) for MCF-7, and ductal-acinar structures for MDA-MB-231 (Fig. 1G), suggesting that cells proliferated within in vitro mammospheres maintained multilineage differentiation potential. Single cell suspensions dissociated from mammosphere differentiated in Matrigel culture and rebuilt spatial orientations and ductal-alveolar structures similar to the in vivo mammary tree.
Characterization of stem-like cells enriched in in vitro mammospheres based on the expression of biomarkers CD44 and CD24
Mammopheres culture has been reported to enrich CSCs from several cancer cell lines, including breast cancer.29 We tested the CSC enrichment by mammosphere culture of MCF-7 and MDA-MB-231 human breast adenocarcinoma cells. Immunostaining of cell surface marker is one of a widely-used approaches to characterize and identify CSCs. It has been reported that breast cancer stem-like cells are enriched within the cellular fraction with immunophenotype of CD44+/CD24− low, which identified tumorigenic cells that displayed stem/progenitor cell properties.1,5 We probed breast cancer CSC surface markers CD44 and CD24 in both adherent monolayers and anchorage-independent mammosphere culture with different generations.
While negative stain was detected in the isotype control groups, we observed a heterogeneous cell population with a different level of expression with CD44 and CD24 in the case of the adherent MCF-7 monolayers, in which the majority of cells had a phenotype of CD44+/CD24+. Only 4.9% of cells detected displayed breast cancer stem-like cell biomarkers with the phenotypic expression of high CD44 and low CD24 (CD44+ /CD24−low). This rate was increased slightly to 5.9% when MCF-7 cells were cultivated as the first generation of mammospheres which showed different characteristics of cells in terms of expression level of CD44 and CD 24. However, no statistically significant CSCs enrichment was observed between the first generation mammoshpere and adherent monolayer. Notably, the third MCF-7 mammoshpere generation significantly enhanced enrichment from 5% to 56% of CD44+/CD24−low breast cancer stem-like cells in comparison to adherent MCF-7 monolayer cells, suggesting the MCF-7 mammosphere model enriches and expands MCF-7 CSCs (Fig. 2).
Figure 2.
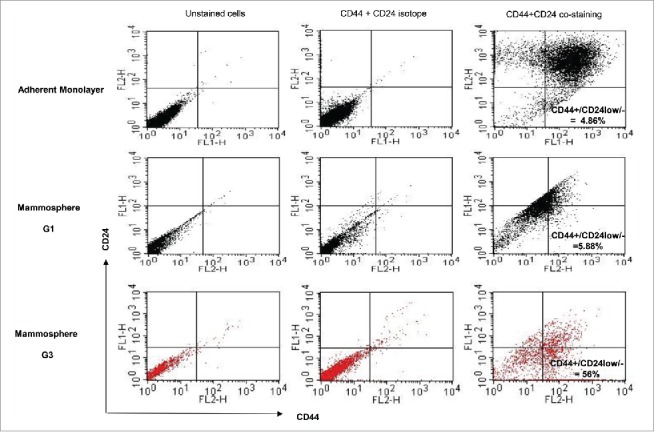
Expression of biomarker CD44 and CD24 of a MCF-7 adherent monolayer culture and different generations of mammospheres, showing that breast cancer stem-like cells with CD44+/CD24−/1ow were enriched by mammosphere passage to the third generation.
In contrast to MCF-7 breast cancer, almost all of MDA-MB-231 cells cultivated in both the adherent monolayer and anchorage-independent mammosphere (up to the fourth generation) were immunostained positively for CD44 and negatively for CD24 (CD44+ /CD24−low) (Fig. 3).
Figure 3.
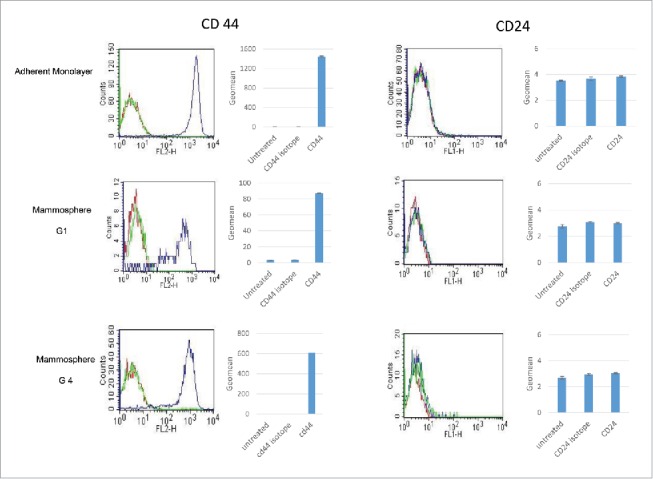
Examination of breast cancer stem-like marker CD44 and CD24 of MDA-MB-231, showing high CD44 and low CD24 expression in both, monolayers and mammospheres of different generations. Red: Unstained cells; Green: Cell with CD44 or CD 24 isotype Antibody stained; Blue: Cells with CD44- or CD 24 Antibody staining.
Drug effects on the formation of MCF-7 mammospheres after 5 day treatment
Recent research showed that salinomycin selectively inhibits growth of human breast cancer stem cells in vitro.17 We examined the effect of CSC-targeted agents, salinomycin, and a cytotoxic chemotherapeutic, paclitaxel, on the formation of mammospheres. Clearly, salinomycin treatment inhibited the formation of mammospheres remarkably (Fig. 4). Cells in exposure to paclitaxel still generated mammospheres, although their sizes were slightly smaller when compared to untreated group (Fig. 4). These results suggest that paclitaxel had only a minor effect on the cell population of CSCs. However, salinomycin inhibited CSCs development and diminished the self-renewing property of the CSC population.
Figure 4.
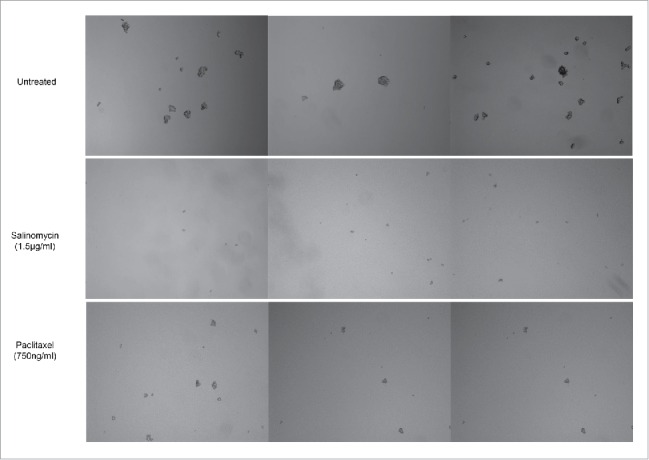
Effect of drug treatment on the formation of MCF-7 mammospheres. Salinomycin treatment inhibited the formation of mammospheres, while paclitaxel treatment generated mammospheres, which were smaller compared to the untreated group.
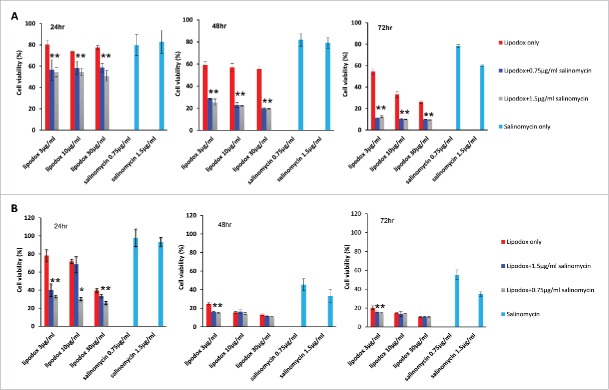
Viability of breast cancer cells exposed to monotherapy and combinatorial therapy of lipodox with salinomycin
After MDA-MB-231 breast cancer cells were exposed to either monotherapy (lipodox alone or salinomycin alone) or combination therapy of lipodox and salinomycin for 24 hr, 48 hr or 72 hrs, a time-dependent tumor cell killing was observed (Fig. 5A). The combinatorial therapy of lipodox and salinomycin produced a synergic effect and led to a significantly higher cytotoxicity than the use of either lipodox or salinomycin alone at all concentrations and time points tested. When compared to lipdox alone, the combination treatment enhanced tumor cell killing by 20–30% at 24 hrs 30% at 48h and around 20–50% at 72 hrs. When compared to salinomycin, alone the combination treatment lowered the cell viability by around 30% at 24 hrs, 50–60% at 48 hrs and 70% at 72 hrs. No significant difference in tumor cell killing was observed between the groups with either 0.75 µg/ml or 1.5 µg/ml salinomycin in combination with lipodox (Fig. 5A).
Figure 5.
Viability of breast cancer cells after exposure to monotherapy or a combination therapy of lipodox with salinomycin. Combination therapy of salinomycin with lipodox produced synergistic effects on the viability of (A) MDA-MB-231 cells and (B) MCF-7 cells. Mean ± SD, n = 5. * P < 0.05.
MCF-7 were more sensitive to treatment with salinomycin at the concentration tested. After short term of exposure to treatment for 24h, the combinatorial therapy of lipodox and salinomycin had a significant higher tumor cell killing in comparison to the use of either lipodox or salinomycin alone. With prolonged treatment for 48 hr and 72 hr, only co-treatment with low concentration lipodox (3 µg/ml) with salinomycin improved cytotoxicity and achieved tumor cell killing similar to the use of high concentration of lipodox (30 µg/ml) alone, implying that systemic toxicity would be reduced by the combination therapy due to a decrease in the lipodox doses used (Fig. 5B).
Viability of breast cancer cells exposed to monotherapy and combinatorial therapy of paclitaxel with salinomycin
MDA-MB-231 and MCF-7 breast cancer cells were exposed to either monotherapy (paclitaxel alone or salinomycin alone) or combination therapy of paclitaxel and salinomycin for 48 hr and 72 hr. With co-treatment with salinomycin and paclitaxel for 48 hr at all concentrations tested, MDA-MB-231 cells had a significantly higher cytotoxicity with paclitaxel alone rather than salinomycin alone. After extension of co-treatment for 72 hr, both compounds: paclitaxel and salinomycin, worked in concert to improve efficacy against MDA-MB-231 in vitro (Fig. 6A)
Figure 6.
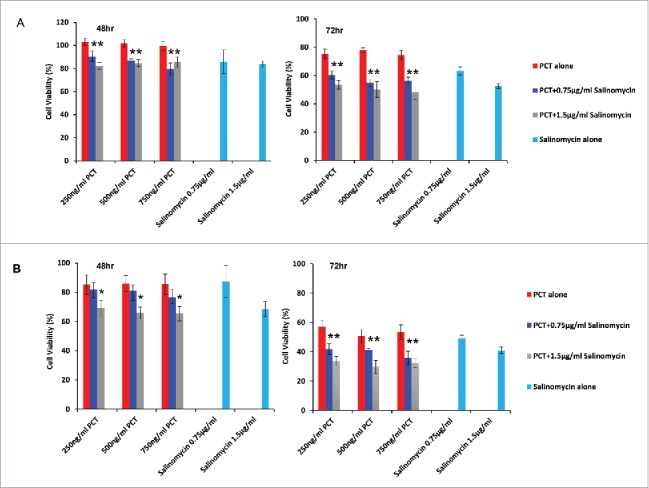
Viability of breast cancer cells exposed to monotherapy or with a combination therapy of paclitaxel with salinomycin. (A) MDA-MB-231; (B) MCF-7 cells. Mean ± SD, n = 5. * P < 0.05.
Similar to results with MDA-MD-231, a significantly lower survival rate was observed with MCF-7 cells treated for 48 hr with 1.5 µg/ml salinomycin in combination of paclitaxel at the 3 varied concentrations tested than monotherapy with paclitaxel, whereas the levels of tumor cell killing by combination therapy were similar to corresponding monotherapy with salinomycin. However, synergy between paclitaxel and salinomycin for growth inhibition were seen with a longer term drug incubation for 72 hr in all of 6 combination treatment groups, indicating that combination therapy enhanced antitumor activity if prolonged exposure of drugs was used (Fig. 6B).
Discussion
Many type of tumors are organized in a hierarchy with heterogeneous cell population. The cancer stem cell (CSC) hypothesis suggests that tumor development and metastasis are driven by a minority population of cells, which are responsible for tumor initiation, growth and recurrences. The inability to efficiently eliminate CSCs during conventional therapy, together with CSCs being both highly tumorigenic and invasive, may result in the treatment failure due to cancer recurrence and metastases. Numerous studies reported a link between CSCs and epithelial-to-mesenchymal transition (EMT),33 and recently the concept of “migrating cancer stem cells” has been proposed.34 Since EMT is critical for metastasis, it implies that CSCs have been invoked as the seed for distant metastases, which typically are responsible for end-stage disease and ultimately death.33
Eradication of all malignant cells within a patient's cancer, including CSCs and their progeny, is essential to prevent cancer relapse and metastasis. Standard chemo- and radiotherapy may have clinical benefits on tumor regression in advanced stages of cancer as a result of their killing the bulk tumor population, but disease relapse is highly likely to occur due mainly to their minimal effect on the CSC population. In contrast, a CSC-targeted therapy may have substantial clinical benefit in early stages of cancer as well as in neo-adjuvant and adjuvant clinical settings.11 However, their modest effect on tumor growth of the bulk tumor population limits their use for advanced stages of cancer. Accumulated evidence obtained on both in vitro cell-based tests and in human cancer mouse xenografts, supports the conclusion that CSC targeting agents are most effective in eradicating CSCs and their progeny when these agents are in combination with conventional cytostatic drugs and/or novel CSCs-targeted drugs.35,36,37 Breast cancer CSC-targeting therapies have been under investigation by numerous research groups worldwide. Targeted strategies being developed include direct inhibition of the self-renewal of breast cancer stem-like cells, indirect modulation of the microenvironment and direct induction of death of breast cancer stem cells by chemical agents that trigger differentiation of CSCs, immunotherapy and oncolytic viruses.28
To develop breast cancer stem-like cell targeted therapies, a pivotal step for successful outcomes is to isolate and identify the breast cancer stem-like cells to acquire starting materials for all subsequent tests. Three methods have been developed so far to enrich breast cancer stem-like cells, including (1) fluorescence-activated cell sorting (FACS) based on breast cancer stem cell markers such as CD44, CD24 and CD133;38 (2) sorting of the side population (SP) that effluxes Hoechst 33342;39 and (3) mammosphere or tumorsphere assay.1,8 Every method has its own advantages and disadvantages. The 3D mammosphere or tumorsphere culture system has been valued as an extremely useful model for the study of stem cell of epithelial tissues and tumor initial cells in solid tumors. It has been utilized initially to enrich and expand normal neural stem cells and later to enrich brain tumor stem cells in vitro. Doutu et al,27 developed a mamosphere system to enrich mammary stem cells under undifferentiated conditions by culturing cells in an anchorage-independent way. Breast cancer stem cells were also propagated and enriched in such a mammosphere culture system.27 The principle behind the approach is that when a single cell suspension is seeded in defined serum-free media, containing growth factors and mitogen (to sustain pluriptency) in an anchorage-independent fashion (using a low attachment plate), only stem cells can form 3D spherical colonies of cells in suspension, but most epithelial bulk tumor cells cannot survive and undergo anoikis due to lack of “a substratum,” an absolute condition for the growth of epithelial cells. Notably, that one of the limitations in the use of these techniques is the enriched breast cancer stem cell population often contains phenotypes with differences in the expression level of CD44 and CD24 or may contain a small population that do not exhibit the CD44+CD24−/low phenotype.
In this study, we selected a 3D mammosphere culture system for in vitro isolation of breast cancer CSCs. We demonstrated its ability to enrich and expand cells with stem-like properties, including self-renewal and differentiation. The combined expression of 2 cell surface markers: CD44+/CD24−/low.5, was first utilized to identify and isolate tumorigenic CSC from non-tumorigenic cancer cells in breast cancer.5 We used the same methodology to validate mammospheres by examining the expression of the CSCs' phenotype in 2 breast cancer cell lines. We detected less than 5% CSCs with phenotype of CD44+/CD24−/low in MCF-7 breast cancer cell monolayer culturing and the 3rd generation of mammospheres enriched CD44+/CD24−/low stem-like cells up to 56%. However MDA-MB-231 constantly expressed a high percentage of CD44+/CD24−/low in both monolayer culturing and float mammospheres of different generations. These results highlight the biological heterogeneity of breast cancer. Indeed, breast cancer based on molecular profile, has been classified into 4 major subtypes, including (1) the luminal A with the expression of hormonal receptors (both estrogen (ER) and progesterone ( PgR); (2) a luminal B with ER and PgR expression together with HER2 overexpression; (3) the HER2-OE subtype with presence of HER2 and absence of hormonal receptors, and (4) basal-like tumors expressing diverse and distinct basal markers.40
Our results confirmed MDA-MB-231 as being characterized as a basal/ mesenchymal breast cancer cell line, bearing an intrinsically higher percentage of the tumor cell population with the CSC phenotype of CD44+CD24−/low. This is consistent with tests on primary breast carcinomas38 and in vitro data, which showed a predominant CD44+CD24−/low CSC phenotype in basal-like breast cancer.41 In general, CD44 is a positive indicator characteristic of cancer stem cells, and has significant expression in invasive basal-like cell lines and basal-like tumors with poor prognosis. CD44 is associated with a mesenchymal stem cell-like profile with enrichment for genes involved in cell motility, proliferation and angiogenesis. CD44 positivity is related to decrease in patient survival.42 However, CD24 expression reflects differentiated properties of epithelial cells.43 CD24−/low cells show stem or progenitor-like properties.38,42,43 The majority of the basal-like tumors were characterized to be CD24−/low. The mesenchymal cell lines, including MDA-MB-231, showed lower levels or no expression of CD24.44,45
In terms of luminal A tumors, most of them maintain an epithelial phenotype, enriched with a cell population with positive expression of CD24, indicating CD24+ cells are related to more differentiated cell lines, tumors or tissues.41 Our results showed that a high percentage of CD24+ is prevalent in MCF-7-adherent monolayers. We also detected a MCF-7 adherent monolayer with mainly positive CD44 cells, which seems more characteristic of basal/epithelial breast cancer cells, since the epithelial luminal breast cancer cell line is thought to enrich the CD44− cell populations. Most importantly, the in vitro MCF-7 mammospheres established in the study showed the ability to enrich breast cancer stem-like cell with phenotype of CD44+/CD24−.
Saliomycin showed significant effect on the formation of mammospheres, validating its role in targeting breast cancer stem cells, while paclitaxel was ineffective again breast cancer stem cells. Both compounds in the combination therapy showed a potential to improve tumor cell killing presumably by working in concert to eradicate CSCs by the CSCs-specific agent salinomycin and bulk tumor cells by standard chemotherapy, such as paclitaxel or lipodox. Certainly, the optimization of the combination therapy regimen is warranted in further studies. In comparison with monotherapy, that the combination treatments we achieved similar or better effects on tumor cell killing by the replacement trial with lower doses, suggesting combination therapy could reduce dose-limiting toxicity and improve the systemic profile of safety and thus therapeutic index. Furthermore, we observed that different types of breast cancer can show different patterns in response to combination therapies, suggesting that the design of combination therapy should consider the phenotypic attributes of breast cancers.
Although salinomycin demonstrated promise as a CSCs-targeted agent as it selectively inhibits cancer stem cells17 in a variety of different cancers.18 However, salinomycin had considerable toxicity in mammals, which is an important reason for its limited use as a coccidistat and growth promoter in livestock for more than 30 years.18 To repurpose its use in cancer therapy, it is necessary to develop strategies to mitigate its systemic toxicity. While combination therapy could lower the dose of salinomycin, our next effort will be focused on design of nanocarrier systems for delivery of salinomycin and for its use in human drug development for CSC targeting.
Although, the real practical potential of this approach should be confirmed in the in vivo experiments we are planning for the future, the data obtained and presented in this paper clearly point at high efficacy of the suggested combination.
In sum, we have developed and optimized protocols to generate in vitro mammosphere cancer models of 2 breast cancer cell lines. The mammosphere models are able to grow and enrich cancer cells with stem cell-like properties, including self-renewal, multilineage differentiation and enrichment of cells expressed breast cancer stem cell biomarkers. Combination therapy with CSCs-targeted agents and conventional cytotoxic drugs is promising as a strategy to improve outcome for cancer treatment with a favorable therapeutics index.
Materials and methods
Material and reagents
Ultra low-attachment 6-well plates were purchased from Corning Costar; Human Epithelia growth factor (EGF) was from Sigma-Aldrich; DMEM/F12, Human recombinant basal fibroblast growth factor (bFGF) and 50x B27supplement minus Vitamin A were purchased from Life Technologies ; 40 µm cell strainer cap filters were purchased from Fisher Scientific. Salinomycin was purchased from Cayman Chemical; Paclitaxel was purchased from Cedarburg Pharmaceuticals, and lipodox was purchased from SUN Pharmaceutical Ind, Ltd. PE mouse anti-human CD44 and FITC Mouse Anti-Human CD24, FITC Mouse IG2a, k isotype control and PE Mouse IG2b, k isotype control were purchased from BD Sciences. MCF-7(ATCC number: HTB-22) and MDAMB-231 cells (ATCC number: HTB-26) were purchased from ATCC. A CellTiter-Blue assay kit was from Promega.
Methods
Adherent monolayer cell culture
MCF-7 and MDAMB-231 breast cancer cells were grown with the completed MEM or DMEM supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics in a humidified incubator at 37°C and 5% CO2.
In vitro mammosphere culture
In vitro mammosphere cultures were prepared according to a described protocol.26. Briefly, MCF-7 single cell suspensions were grown in serum-free DMEM/F12 medium supplemented with 20ng/ml EGF, 20ng/ml bFGF, and 1X B27supplement. For growth and proliferation of MDA-MB-231 single cell suspensions, cells were grown in DMEM/F12 medium containing 1% FBS (V/V) without addition of EGF, bFGF, and 1X B27supplement. The single cell suspension was plated in 6-well ultralow attachment plates at a density of 5,000–10,000 cells per well depending on the cell line and cultured for 10–14 d at 37°C and 5% CO2. To obtain the single cell suspension, a 40 µm cell strainer cap filter was used to remove cell aggregates.
In vitro serial passage of mammosphere culture
To assess the self-renewal potential of stem-like cells enriched in vitro cancer mammosphere, mammospheres harvested by centrifugation at 130 g and room temperature, were dissociated into a single cell suspension using a P1000 pipette and chemically using pre-warmed trypsin followed by passage through a 40 µm cell strainer cap filter. The single cell suspension was plated in 6-well ultralow attachment plates at a density of 5,000–10,000 cells per well and cultured for 10–14 d at 37°C and 5% CO2. At the end of experiments, mammospheres were photographed under a microscopy at 10x magnification.
Identification of the self-renewal potential of breast cancer stem cells enriched in mammospheres
The first generation of mammospheres was collected by gentle centrifugation at 130 g and room temperature, dissociated into single cell suspensions, and cultured under the conditions described above for another 10–14 days, followed by another round of mammosphere passage.
Assessment of the differential potential of breast cancer stem-like cells from mammospheres
To assess the potential for lineage differentiation, a 3-dimensional (3D) Matrigel-based culture was established under a differentiating condition according to a previous description.27 For the 3D cultures, single cell suspensions from dissociated mammospheres of MCF-7 and MDA-MB-231 breast cancer cells were plated in 24-well plates separately at clonogenic densities of 120 cells/ 300 µl of Matrigel and cultivated in medium containing DMEM/F12 supplemented with 5% FBS and 1% antibiotics. After 3–4 weeks, photographs were taken with an optical microscopy at 10x magnification.
Expression of stem cell surface markers of breast cancer stem cells enriched in mammospheres
For probing and identifying the cell-surface immunophenotypes of MCF-7 and MDA-MB-231 breast cancer cells in an adherent monolayer culture as well from a serial passages of mammospheres, cells were dissociated into a single cell suspension mechanically using a P1000 pipette and chemically using pre-warmed trypsin followed by passing through a 40 µm cell strainer cap filter. Cells were washed with a cold washing buffer composed of Phosphate Buffered Saline (PBS) and 3% FBS, harvested by centrifugation and re-suspended at a cell density of 1 × 106 cells in100 µl of staining buffer containing 10% FBS in PBS and incubated for 10 min at 4°C. Cells collected by centrifugation were subjected to immunostaining with PE mouse anti-human CD44 and FITC Mouse Anti-Human CD24, or stained with their isotype controls, including a PE Mouse IG2b, k isotype control and a FITC Mouse IG2a, k isotype control, for 45 min at 4°C in the dark. The samples were then washed 3 times with washing buffer, collected and re-suspended in 500 μL of sheath fluid. Flow cytometry was performed on a flow cytometer (Becton Dickinson, San Jose, CA, USA).
The effect of drugs on mammosphere formation
A MCF-7 single cell suspension dissociated from generation 2 of mammospheres, was seeded into 6-well ultralow attachment plates and cultivated under undifferentiating condition as described above. MCF-7 cells were treated with 750ng/ml PCT and 1.5 µg/ml Salinomycin immediately after seeding. Plates were be analyzed for the formation of mammospheres at day +5 using an optical microscopy at 10x magnification.
Cytotoxicity
MCF-7 and MDA-MB-231 breast cancer cells were seeded into 96-well microplates at a density of 5 × 103 cells in complete medium per well. After growth to 60–70% confluence, each cell line was treated with a variety of different monotherapy and combination therapies, including various concentrations of monotherapy: PCT (250 ng/ml, 500 ng/ml and 750 ng/ml) or lipodox alone (3,10,30 μg/ml), salinomycin (0.75,1.5 μg/ml) alone, combination treatment of lipodox (3,10,30 μg/ml) and salinomycin (0.75,1.5 μg/ml) or of PCT (250 ng/ml, 500 ng/ml and 750 ng/ml) and salinomycin (0.75,1.5 μg/ml). After the cell lines was exposed to treatment for 24, 48 or 72h, cell viability was measured by CellTiter-Blue Cell Viability assay as described in the manufacturer's manual. Briefly, at the end of treatment, drug-contained medium were removed, and cells were incubated with fresh complete medium containing the CellTiterBlue assay reagent (20 μl/well), at 37°C for 1h. The fluorescence intensity was measured by a multidetection microplate reader (Bio-Tek) with excitation/emission wavelengths of 525/590 nm.
Statistical analysis
SPSS (version 16) with ANOVA followed by a Bonferroni post hoc test was used to analyze the statistical significance of the results and differences between experimental groups. The p value less than 0.05 indicated a statistically significant result.
Disclosure of potential of conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We greatly thank Dr. William Hartner for his valuable advice in the manuscript preparation.
Funding
This work was supported by NIH grant #1U54CA151881 to Vladimir P. Torchilin.
Reference
- 1.Bapst S, ed. Cancer Stem Cells. New Jersey: A John Wiley & Sons, Inc.; 2009 [Google Scholar]
- 2.McCulloch EA, Till JE. Perspectives on the properties of stem cells. Nat Med 2005; 11:1026-8; PMID:16211027; http://dx.doi.org/ 10.1038/nm1005-1026 [DOI] [PubMed] [Google Scholar]
- 3.Bergsagel DE, Valeriote FA. Growth characteristics of a mouse plasma cell tumor. Cancer Res 1968; 28:2187-96; PMID:5723963 [PubMed] [Google Scholar]
- 4.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367:645-8; PMID:7509044; http://dx.doi.org/ 10.1038/367645a0 [DOI] [PubMed] [Google Scholar]
- 5.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100:3983-8; PMID:12629218; http://dx.doi.org/ 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature 2004; 432:396-401; PMID:15549107; http://dx.doi.org/ 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 7.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63:5821-8; PMID:14522905 [PubMed] [Google Scholar]
- 8.Yu, John S. (Ed.), editor. Cancer Stem Cells, Methods and Protocols, Methods in Molecular Biology .Volume 568 2009. ISBN: 978-1-58829-938-3 (Print) 978-1-59745-280-9 (Online). Humana Press. New York City. www.springer.com/humana. [Google Scholar]
- 9.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene 2004; 23:7274-82; PMID:15378087; http://dx.doi.org/ 10.1038/sj.onc.1207947 [DOI] [PubMed] [Google Scholar]
- 10.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A 2004; 101:14228-33; PMID:15381773; http://dx.doi.org/ 10.1073/pnas.0400067101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott SP, Wicha MS. Targeting breast cancer stem cells. Mol Oncol 2010; 4:404-19; PMID:20599450; http://dx.doi.org/ 10.1016/j.molonc.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al.. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009; 106:13820-5; PMID:19666588; http://dx.doi.org/ 10.1073/pnas.0905718106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444:756-60; PMID:17051156; http://dx.doi.org/ 10.1038/nature05236 [DOI] [PubMed] [Google Scholar]
- 14.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al.. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008; 100:672-9; PMID:18445819; http://dx.doi.org/ 10.1093/jnci/djn123 [DOI] [PubMed] [Google Scholar]
- 15.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, et al.. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007; 131:1109-23; PMID:18083101; http://dx.doi.org/ 10.1016/j.cell.2007.10.054 [DOI] [PubMed] [Google Scholar]
- 16.Bardsley MR, Horvath VJ, Asuzu DT, Lorincz A, Redelman D, Hayashi Y, Popko LN, Young DL, Lomberk GA, Urrutia RA, et al.. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology 2010; 139:942-52; PMID:20621681; http://dx.doi.org/ 10.1053/j.gastro.2010.05.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009; 138:645-59; PMID:19682730; http://dx.doi.org/ 10.1016/j.cell.2009.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol 2012; 2012:950658; PMID:23251084; http://dx.doi.org/ 10.1155/2012/950658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp F, Hermawan A, Oak PS, Herrmann A, Wagner E, Roidl A. Salinomycin treatment reduces metastatic tumor burden by hampering cancer cell migration. Mol Cancer 2014; 13:16; PMID:24468090; http://dx.doi.org/ 10.1186/1476-4598-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, et al.. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett 2011; 311:113-21; PMID:21835542; http://dx.doi.org/ 10.1016/j.canlet.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 21.Riccioni R, Dupuis ML, Bernabei M, Petrucci E, Pasquini L, Mariani G, Cianfriglia M, Testa U. The cancer stem cell selective inhibitor salinomycin is a p-glycoprotein inhibitor. Blood Cells Mol Dis 2010; 45:86-92; PMID:20444629; http://dx.doi.org/ 10.1016/j.bcmd.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci 2007; 120:468-77; PMID:17227796; http://dx.doi.org/ 10.1242/jcs.03348 [DOI] [PubMed] [Google Scholar]
- 23.Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A 2007; 104:618-23; PMID:17202265; http://dx.doi.org/ 10.1073/pnas.0606599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun 2010; 392:373-9; PMID:20074550; http://dx.doi.org/ 10.1016/j.bbrc.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 25.Mitani M, Yamanishi T, Miyazaki Y, Otake N. Salinomycin effects on mitochondrial ion translocation and respiration. Antimicrob Agents Chemother 1976; 9:655-60; PMID:131509; http://dx.doi.org/ 10.1128/AAC.9.4.655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel S, Rameshwar P. Cancer Stem cells. Protocol Exchange; 1038/protex.2013.023 [Google Scholar]
- 27.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17:1253-70; PMID:12756227; http://dx.doi.org/ 10.1101/gad.1061803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Pham Phuc, Thanh Vu Binh, Chinh Phan Nhan Lu, et al., eds. Biomedical Tissue Culture,: InTech; 2012; 10.5772/3071 [DOI] [Google Scholar]
- 29.Kondo T. Stem cell-like cancer cells in cancer cell lines. Cancer Biomark 2007; 3:245-50; PMID:17917153 [DOI] [PubMed] [Google Scholar]
- 30.Simian M, Hirai Y, Navre M, Werb Z, Lochter A, Bissell MJ. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development 2001; 128:3117-31; PMID:11688561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev 2002; 16:693-706; PMID:11914275; http://dx.doi.org/ 10.1101/gad.952602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A 1992; 89:9064-8; PMID:1384042; http://dx.doi.org/ 10.1073/pnas.89.19.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al.. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704-15; PMID:18485877; http://dx.doi.org/ 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer 2005; 5:744-9; PMID:16148886; http://dx.doi.org/ 10.1038/nrc1694 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. The eradication of breast cancer and cancer stem cells using octreotide modified paclitaxel active targeting micelles and salinomycin passive targeting micelles. Biomaterials 2012; 33:679-91; PMID:22019123; http://dx.doi.org/ 10.1016/j.biomaterials.2011.09.072 [DOI] [PubMed] [Google Scholar]
- 36.Kim JH, Chae M, Kim WK, Kim YJ, Kang HS, Kim HS, Yoon S. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br J Pharmacol 2011; 162:773-84; PMID:20973777; http://dx.doi.org/ 10.1111/j.1476-5381.2010.01089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Büchler MW, Zöller M, et al.. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res 2010; 70:5004-13; PMID:20530687; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0066 [DOI] [PubMed] [Google Scholar]
- 38.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A, Hegardt C. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 2008; 10:R53; PMID:18559090; http://dx.doi.org/ 10.1186/bcr2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, et al.. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004; 10:5367-74; PMID:15328174; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-0220 [DOI] [PubMed] [Google Scholar]
- 40.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res 2011; 13:215; PMID:21884641; http://dx.doi.org/ 10.1186/bcr2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F, Paredes J. Breast cancer stem cell markers CD44, CD24 and ALDH1: expression distribution within intrinsic molecular subtype. J Clin Pathol 2011; 64:937-46; PMID:21680574; http://dx.doi.org/ 10.1136/jcp.2011.090456 [DOI] [PubMed] [Google Scholar]
- 42.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, et al.. Molecular definition of breast tumor heterogeneity. Cancer Cell 2007; 11:259-73; PMID:17349583; http://dx.doi.org/ 10.1016/j.ccr.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 43.Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res 2010; 16:876-87; PMID:20103682; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008; 10:R25; PMID:18366788; http://dx.doi.org/ 10.1186/bcr1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R Jr, Badve S, Nakshatri H. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 2006; 8:R59; PMID:17062128; http://dx.doi.org/ 10.1186/bcr1610 [DOI] [PMC free article] [PubMed] [Google Scholar]