ASBTARCT
Adenoviruses mediated cancer gene therapies are widely investigated and show a promising effect on cancer treatment. However, efficient gene transfer varies among different cancer cell lines based on the expression of coxsakie adenovirus receptor (CAR). Hep27, a member of dehydrogenase/reductase (SDR) family, can bind to Mdm2, resulting in the attenuation of Mdm2-mediated p53 degradation. Here we constructed a fiber chimeric adenovirus carrying hep27 gene (F5/35-ZD55-Hep27), in which the fiber protein of 5-serotype adenovirus (Ad5) was substituted by that of 35-serotype adenovirus (Ad35), aiming to facilitate the infection for renal cancer cells and develop the role of hep27 in cancer therapy. We evaluated the CAR and CD46 (a membrane cofactor protein for Ad35) expression in four kinds of renal cancer cells and assessed the relationship between receptors and infection efficiency. 5/35 fiber-modified adenovirus had a much promising infectivity compared with Ad5-based vector in renal cancer cells. F5/35-ZD55-Hep27 had enhanced antitumor activity against human renal cancer cells compared to the other groups. Further, hep27 mediated p53 and cleaved-PARP upregulation and mdm2 downregulation was involved and caused increased apoptosis. Moreover, F5/35-ZD55-Hep27 significantly suppressed tumor growth in subcutaneous renal cancer cell xenograft models. Our data demonstrated that 5/35 fiber-modified adenovirus F5/35-ZD55-Hep27 transferred into renal cancers efficiently and increased p53 to induce cancer cell apoptosis. Thus 5/35 fiber-modified adenoviral vector F5/35-ZD55-Hep27 might a promising vector and antitumor reagent for renal cancer gene therapy.
KEYWORDS: CAR, CD46, fiber chimeric adenovirus, Hep27, renal cell carcinoma (RCC)
Introduction
The incidence and mortality of human renal cell cancer (RCC) is increasing.1 The standard treatment for localized RCC (stages I–III) is surgery; however, 20–30% of patients will relapse and consequently, at least half of all patients with RCC will require systemic treatment of their cancer at some point in time.2,3 There is currently no proven role for neoadjuvant or adjuvant systemic therapy, although several large prospective clinical trials are currently investigating the role of ‘targeted agents’ in patients at medium to high risk of relapse.4 However, despite the high clinical need, few drugs have been useful in metastatic RCC and traditional cytotoxic chemotherapy has not given consistent responses.5 New forms of treatment such as gene therapy are needed.
Recently, oncolytic adenoviruses provided a novel strategy for cancer treatment since they could replicate within cancer cells and destroy them.6 However, the antitumor activity observed in clinical trials has been modest.7 The traditional adenoviral vectors were always developed from adenovirus of serotype 5 (Ad5, which belong to subgroup C). The receptor of Ad5 on cell membrane is coxsakie adenovirus receptor (CAR).8 Ad5 has 12 fiber trimers that are attached to the penton base proteins of the viral capsid. At the distal tip of each fiber monomer is a globular region referred to as the knob domain. It is the knob region that binds to cellular adenoviral receptors. However, Ad5 entry into a multitude of primary tumor cells and tumor in vivo is often restricted due to either the absence or low expression of CAR on several cancer cells.9 In light of this, non-CAR receptors form other Ad serotypes are utilized. Recently, Ad serotype 35(Ad35, which belonged to subgroup B) shows a different pattern of tropism from that of Ad5. Ad35 attaches to cells via a non-CAR receptor, CD46 instead, a membrane cofactor protein.10 CD46 was wildly expressed on most cancer cells. Herein, the adenoviral vector with a chimeric fiber consisting of the knob and shaft of Ad35 relative to Ad5 was constructed. The chimeric fiber adenoviral vector could transduce CAR-negative cell lines more efficiently than the Ad5 vector.11,12 These evidences indicate the chimeric fiber adenoviral vector could be a promising candidate for gene transfer to human renal cancers.
Hep27, also known as SDR25C1 or DHRS2 (dehydrogenase/reductase (SDR family) member 2), is a 258 amino acid protein that localizes to the nucleus and belongs to the short-chain dehydrogenase/reductase (SDR) family.13,14 Functioning as an NADPH-dependent dicarbonyl reductase, hep27 is thought to inhibit cell replication by either converting cortisone in cortisol, or by catalyzing the oxidation of androgen and estrogen.15 Recently, hep27 was reported it has a functional role in promoting growth inhibition.16 The mitochondrial Hep27 translocates to the nucleus, where it binds to Mdm2, resulting in the attenuation of Mdm2-mediated p53 degradation.17 However, the systemic application and potent role of hep27 for cancer treatment had not been investigated.
In the present study, based on Ad5-ZD55 vector, an adenoviral vector deleted with E1B 55KD, we constructed a 5/35 fiber chimeric adenovirus armed with hep27, aiming to evaluate the antitumor activity of hep27 in renal cancer cells. We first examined the expression of CAR and CD46 expression in renal cancer cell lines. Then we determined the ability of the 5/35 adenoviral vector armed with hep27 in vitro. The molecular mechanism of hep27 mediated antitumor effect was further addressed. Finally, the in vivo tumor inhibition capacity of 5/35 fiber adenoviral vector armed with hep27 was investigated using subcutaneous human renal cancer xenograft models.
Results
CAR and CD46 expression are varied in renal cancer cells
CAR and CD46 are two cell surface proteins, and they are the primary cellular receptor for Ad5 and Ad35 to recognize target cells respectively. We quantified expression level of CAR and CD46 in renal cancer cells. qRT-PCR results showed that CAR and CD46 mRNA level are both dramatically high in Ketr-3 human renal cancer cell. CAR mRNA expression is lower in ACHN, 786-O, OSRC-2 human renal cancer cells and human renal tubular epithelial cell HK-2 than that in Ketr-3 cell (Fig. 1A). Meanwhile, all renal cancer cells tested in our experiment had higher CD46 expression (Fig.1B). The western blotting data also showed that CAR had low expression level and CD46 had high level in the tested renal cancer cells except Ketr-3 (Fig.1C). Both CAR and CD46 expression was also evaluated by flow cytometry (Fig. 1D) in HK-2 and four human renal cancer cell lines. The CAR expression was very low in HK-2, ACHN and OSRC-2 cells, whereas 786-O showed median expression level and Ketr-3 showed higher levels of expression. In contrast, CD46 was highly expressed in all renal cancer cell lines but not for HK-2. These findings coincided with qRT-PCR and protein gel blot data. Accordingly, these findings suggest that the use of Ad35 fiber to modify binding tropism may confer more effective transduction than Ad5 fiber in all the renal cancer cells.
Figure 1.
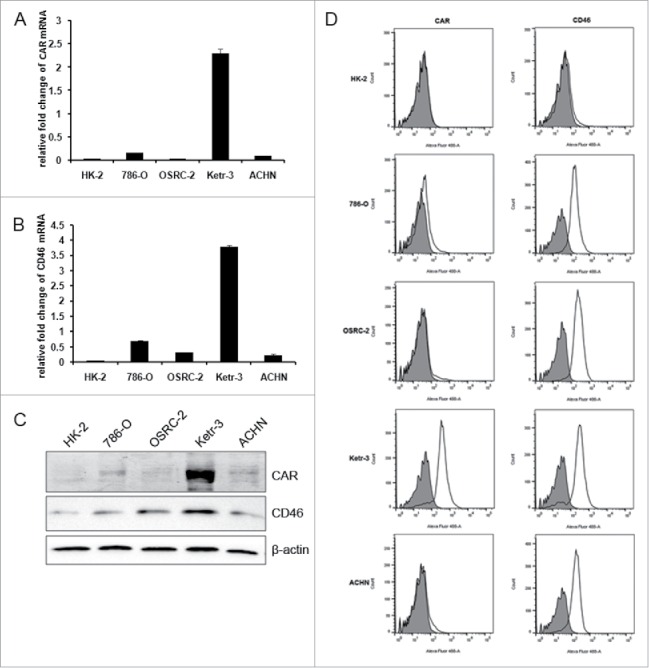
The expression profiles of CAR and CD46 in renal cancer cells. The relative CAR and CD46 mRNA in renal cancer cells 786-O, OSRC-2, Ketr-3, ACHN and human renal tubular epithelial cell HK-2 were detected by qRT-PCR (A&B). The value was normalized to that of GAPDH. (C) Immunoblotting assay was performed to measure CAR and CD46 protein. β-actin was used as loading control. (D) Cell surface expression of CAR and CD46 in human renal cells and normal renal cells were evaluated by flow cytometry. Open traces represent signals and shaded traces represent negative controls.
F5/35-ZD55-EGFP had high infectivity in renal cancer cells
High-level expression of CD46 in renal cancer cells indicates Ad35 as a suitable gene vector. Next, we constructed the 5/35 fiber-modified adenoviral vector to transfer the therapeutic gene into renal cancer cells. The schematic diagram of 5/35 fiber-modified adenovirus F5/35-ZD55-EGFP and F5/35-ZD55-hep27 were illustrated in Fig. 2A. The successful construction of these adenoviruses was confirmed by PCR assay with specific primers (data not shown).To determine the efficiency of 5/35 fiber-modified adenoviral vector to renal cancer cells, Ad5-ZD55-EGFP and F5/35-ZD55-EGFP at a MOI of 10 infected four renal cancer cells and HK-2 cells for 48h, respectively. The percentages of EGFP-positive cells were detected by flow cytometry assay. As shown in Fig. 2B, C, higher percentages of EGFP-positive cell population were observed in F5/35-ZD55-EGFP treatment group than that in Ad5-ZD55-EGFP group in renal cancer cells. There was low EGFP-positive cell population in HK-2 cells infected with Ad5-ZD55-EGFP or F5/35-ZD55-EGFP viruses. These data showed that 5/35 fiber-modified adenovirus had a much promising infectivity in renal cancer cells compared with Ad5-based vector.
Figure 2.
Schematic diagrams of adenovirus vectors and infectivity of Ad5-ZD55-EGFP and F5/35-ZD55-EGFP for renal cancer cells. (A) Schematic diagram of adenoviral constructs of Ad5, Ad5-ZD55, Ad5-ZD55-hep27 and F5/35-ZD55-hep27. The maps are not draw to scale. Ad5-ZD55 was with an E1B 55KD deletion. Ad5/35 fiber: 5 and 35 type adenoviral chimeric fiber. ITR: inverted terminal repeats. Schematic diagrams of Ad5-ZD55-EGFP and F5/35-ZD55-EGFP (not shown) have the same structure as Ad5-ZD55-hep27 or F5/35-ZD55-hep27, with a hep27 replaced by EGFP. (B, C) Ketr-3, OSRC-2, 786-O, ACHN and HK-2 cells were infected with Ad5-ZD55-EGFP and F5/35-ZD55-EGFP at a MOI of 10 for 48h, respectively. The EGFP expression was quantified using flow cytometric analysis. Uninfected cells were selected as the negative control. Representive data were shown in the paragraph B (bottom). Con: uninfected cells. 5-E: Ad5-ZD55-EGFP; 5/35-E: F5/35-ZD55-EGFP. Three independent trials were performed and the quantification of EGFP positive cells was shown in C.
The 5/35 fiber-modified virus vectors replicate more efficiently
Next, we studied the replication ability of 5/35 fiber-modified virus in renal cancer cells. Ad5-ZD55-hep27 or F5/35-ZD55-hep27 adenovirus infected Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells at the indicated doses for 48 h. First, adenoviral E1A mRNA and protein levels were detected. qRT-PCR and western blotting data showed there was higher E1A expression level in all F5/35-ZD55-hep27-infected renal cancer cells (Fig. 3A, B). Further, the production of infective viral progeny was quantified by the TCID50 method. As shown in Fig. 3C, F5/35-ZD55-hep27-infected renal cancer cells produced much higher titers of viral progeny than that in Ad5-ZD55-hep27 treatment group. However, the replication rate of adenoviruses was low in HK-2 cells.
Figure 3.
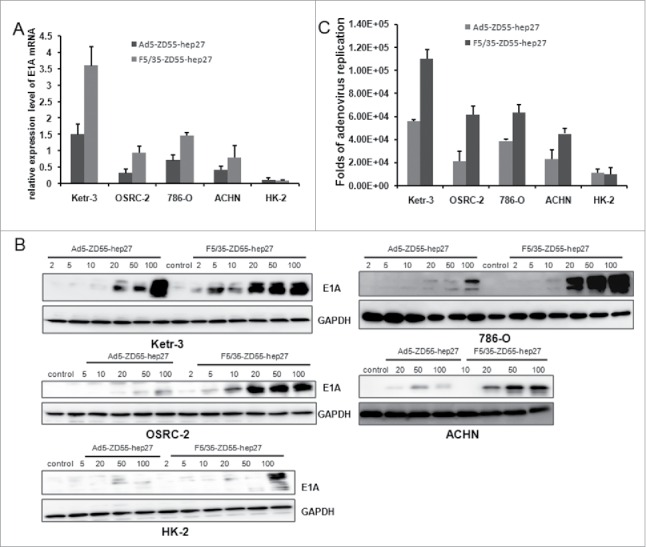
Adenoviral replication in renal cancer cells. Ketr-3, OSRC-2, 786-O, ACHN and HK-2 cells were infected with Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at an MOI of 20 for either 2 h or 48 h. (A) Expression level of adenoviral E1A mRNA were quantified by qRT-PCR. GAPDH mRNA expression was used as the endogenous control. (B) E1A protein in infected cells was determined by western blotting. GAPDH was used as the loading control. (C) The viral burst sizes were obtained in HEK293 cells by TCID50 methods. The ratio of viral progeny form cells infected for 48 h was normalized to that of cells infected for 2 h.
F5/35-ZD55-Hep27 had enhanced antitumor activity of against human renal cancer cells
The tumor-suppressing ability of the 5/35 fiber-modified virus was investigated by MTT assay. Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells were infected with Ad5-ZD55, F5/35-ZD55, Ad5-ZD55-Hep27 or F5/35-ZD55-Hep27 at a MOI = 0.1, 1, 10, 50,100 for 48h, respectively. Cell viability was measured using the MTT assay. The data showed that F5/35-ZD55-Hep27 had higher antitumor capacity for renal cancer cells than other treatment groups (Fig. 4). There was a low 50% inhibitory concentration (IC50) for F5/35-ZD55-Hep27 in each renal cancer cells.
Figure 4.
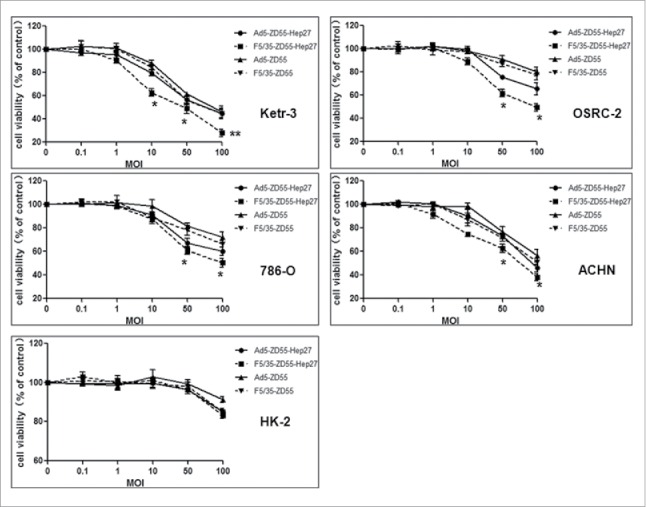
Adenoviral-mediated antitumor capacity in renal cancer cells. Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells were exposed to Ad5-ZD55, F5/35-ZD55, Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at a various MOIs (0.1, 1, 10, 50 and 100) for 48 h, respectively. Cell viability was measured using the MTT assay. Relative cell viability was calculated compared to the control group, which was set at 1. The data were expressed as mean values + SD (n = 6). Statistical significance was determined using Student's t test. The data were representative of three separate experiments. * means P < 0.05, ** means P < 0.01.
In addition, the cytopathic effect (CPE) induced by F5/35-ZD55-Hep27 was evaluated by crystal violet staining. Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells were infected with Ad5-ZD55, F5/35-ZD55, Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at a MOI = 0.1, 1, 10, 50,100, respectively. After 4 days of culture, oncolytic potential of different adenoviruses was evaluated by crystal violet staining. F5/35-ZD55-Hep27 induced more potent cytopathic effect than Ad5-ZD55-Hep27 and mock in renal cancer cells (Fig. 5). We also found that significant CPE was not observed in HK-2 cells infected with all the viruses. Above all, our data suggested that 5/35 fiber-modified virus F5/35-ZD55-Hep27 might eliminate renal cancer cells more effectively than Ad5-based vector.
Figure 5.
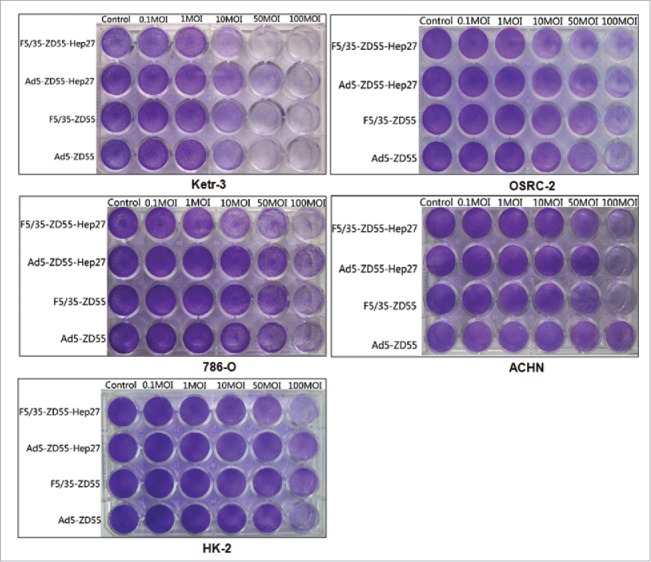
Adenoviral-induced cytotoxicity in renal cancer cells. Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells were infected with Ad5-ZD55, F5/35-ZD55, Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at an MOI = 0.1, 1, 10, 50 and 100, respectively. After 4 days of culture, oncolytic potential of different adenoviruses was evaluated by crystal violet staining.
Cell apoptosis was induced by F5/35-ZD55-Hep27 adenovirus
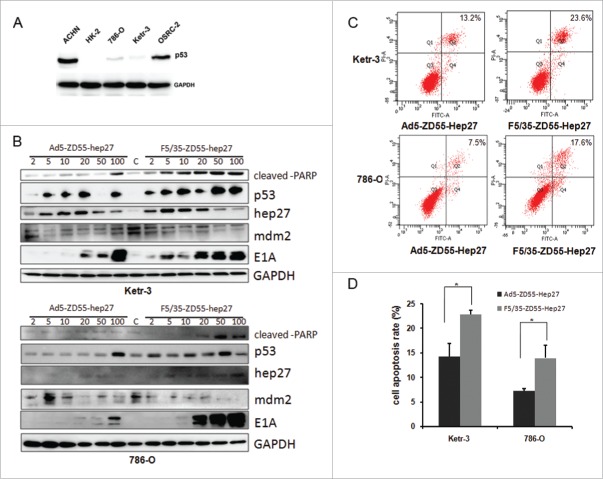
We firstly detected the p53 protein expression level in four renal cancer cell lines and HK-2 cells. As shown in Fig. 6A, there was high p53 in ACHN and OSRC-2, moderate expression in 786-O and Ketr-3, but low p53 expression in HK-2cells.Hep27 could bind to the central domain of Mdm2, thereby promoting p53 accumulation and stabilization [17]. Then we want to investigate whether the hep27-mdm2-p53 pathway played a role in F5/35-ZD55-Hep27 mediated antitumor activity. Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 infected Ketr-3 and 786-O at a MOI of 2, 5, 10, 20, 50 and 100 for 48h. Western blot assay was performed to determine the expression of hep27, p53, mdm2, cleaved-PARP and E1A (Fig. 6B). Both Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 expressed an increased hep27 and E1A level in a dose-dependent manner. The hep27 expression decreased in cells treated with high MOIs (50 or 100) because of cells detached from the dish bottom and cells were lysed, compared with that low MOI. It showed that hep27 up regulated p53 and cleaved-PARP, however, mdm2 expression was down regulated. These data was also identical with aforementioned reports. Moreover, 5/35 fiber-modified F5/35-ZD55-Hep27 had higher E1A expression level than Ad5-ZD55-Hep27 in the renal cancer cells.
Figure 6.
Adenoviral-expressed hep27 inhibited mdm2 and increased p53 and cleaved-PARP expression to induce cell apoptosis. (A) Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells were lysed and lysates were to detect p53 expression by protein gel blotting. GAPDH served as the protein loading control. (B) Ketr-3 and 786-O cells were infected with Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at an MOI of 2, 5, 10, 20, 50 and 100 for 48h respectively. Lysates were harvested for western blotting assay. The level of cleaved PARP, p53, mdm2, E1A and hep27 was analyzed. GAPDH served as the protein loading control. (C) Ketr-3 and 786-O cells were infected with Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at an MOI of 20 for 48h. The analysis of Annexin V/PI double stained cells was performed to quantify cell apoptosis by FACS. Representative flow cytometric data are shown. (D) The mean percentage of cell apoptosis was calculated based on three independent experiments. * means P < 0.05.
Cell apoptosis induced by over-expression hep27 was also determined by FACS assay. Ketr-3 and 786-O cells were infected with Ad5-ZD55-Hep27 or F5/35-ZD55-Hep27 at MOI = 20. Forty-eight hours later, analysis of Annexin V/PI double stained cells was performed to quantify cell apoptosis by FACS. As shown in Fig. 6C, D, F5/35-ZD55-Hep27 induced more prophase and advanced stage cell apoptosis compared to Ad5-ZD55-Hep27 group in renal cancer cells.
Antitumor efficacy of 5/35 fiber-modified F5/35-ZD55-Hep27 was confirmed for renal cancer in nude mice
Next, the antitumor effects of F5/35-ZD55-Hep27 in a renal cancer cell Ketr-3 xenograft model were examined. Ketr-3 cells (2 × 106) were subcutaneously inoculated into the right flank of 4–6 weeks-old female nude mice obtained from the Institute of Animal Center (Chinese Academy of Sciences, Shanghai). Animal welfare and experimental procedures were carried out strictly in accordance with the Guide for the Care and Use of Laboratory Animals. About when tumor diameters reached about 5–8mm, mice were randomly divided into five groups (six mice per group) and treated by intratumor injections of F5/35-ZD55-Hep27, Ad5-ZD55-Hep27, F5/35-ZD55, Ad5-ZD55 at a dose of 7×108 plaque forming unit (PFU) per mouse every other day for three times or with PBS as a control. During the therapeutic days, tumor size was measured every 7 days and the mice were sacrificed 35 days later. The data of tumor volumes showed that F5/35-ZD55-Hep27 was able to inhibit the growth of renal cancer cells (Fig. 7A, B). TUNEL assay was done and the results showed that predominant apoptosis was observed in the F5/35-ZD55-Hep27 group (55.8 ± 4.9%), which were more significant than that in the Ad5-ZD55-Hep27 (29.8 ± 0.5%), F5/35-ZD55 (19.2 ± 2.1%), Ad5-ZD55 (12.7 ± 2.8%) and control groups with PBS (3.6 ± 1.1%) (Fig.7C).
Figure 7.
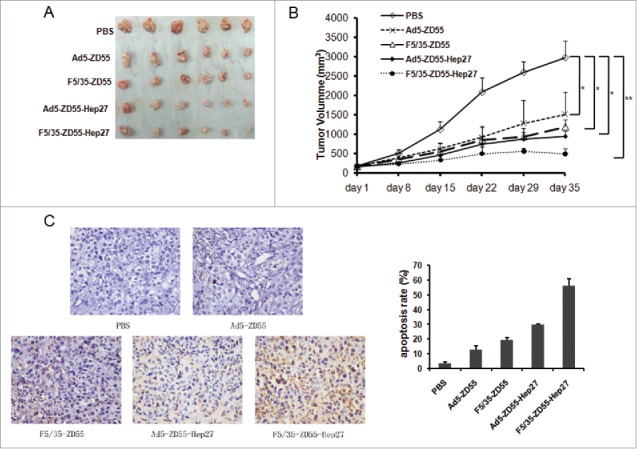
BALB/C nude mice (n = 6 per group) bearing Ketr-3 xenografts were treated with Ad5-ZD55, F5/35-ZD55, Ad5-ZD55-Hep27, F5/35-ZD55-Hep27 and PBS buffer control by intratumor injection five times every other day at a total dose of 100 (1×109pfu). Tumor size was measured every week. After 5 weeks treatment, mice were sacrificed and tumor mass were collected (A). Tumor growth curve was determined based on tumor size measured on the indicated day (B), * means P < 0.05, ** means P < 0.01. TUNEL assay was done to identify cell apoptosis mediated by adenovirus and the results showed that predominant apoptosis was observed. The tumor inhibition rate was also calculated and shown in C.
Discussion
Genetically engineered oncolytic adenoviruses are expected to be a strong solution to treat cancer.18 It has been reported that attachment is one of the critical cell surface events required for adenovirus to enter host cells. The coxsakie adenovirus receptor (CAR) is involved in adenoviral attachment.19 Therefore the expression of CAR is important for efficient gene transfer. However, because of the absence or low expression of CAR on several types of cancer cells always happens,20 the improvement of adenoviral vector in tropism is dramatically needed.
We previously constructed oncolytic adenovirus Ad5-ZD55 that caused tumor cells growth inhibition.21,22 In this study, basing on the previous research, we constructed fiber chimeric adenovirus vectors, in which the fiber protein of 5-serotype adenovirus (Ad5) was substituted by that of 35-serotype adenovirus (Ad35), aiming to facilitate the infection for renal cancer cells. The expression of the cell surface proteins (CAR, correlated with Ad5 infection and CD46, correlated with Ad35 infection) had been investigated in the cultured renal cancer cell lines. As shown in Fig. 1, CAR had low mRNA and protein expression level in most renal cancer cells (786-O, OSRC-2, ACHN) and human renal tubular epithelial cell HK-2. The expression of CD46 was high in the tested renal cancer cells. This different primary cellular receptor for adenovirus may be responsible for infectivity of Ad-based vectors into renal cancer cells. F5/35-ZD55-EGFP had a much higher infectivity in renal cancer cells compared with Ad5-ZD55-EGFP (Fig. 2). There was low EGFP-positive cell population in HK-2 cells infected with either Ad5-ZD55-EGFP or F5/35-ZD55-EGFP viruses. These data clearly showed 5/35 fiber-modified adenoviral vector had efficient infection for renal cancer cells in our experiment, which was consistent with reports from different investigators.10,23,24
Hep27 is a short-chain dehydrogenase/reductase, which is synthesized in human hepatoblastoma HepG2 cells following growth arrest induced by butyrate treatment.15 Hep27 could translocate to the nucleus and bind to Mdm2, resulting in the attenuation of Mdm2-mediated p53 degradation.17 The direct antitumor activity or systemic administration of hep27 was less described. Here we clone hep27 into 5/35 fiber-modified adenoviral vector. Interestingly, the replication of 5/35 fiber-modified adenoviral vector F5/35-ZD55-hep27 was higher than Ad5-based vectors Ad5-ZD55-hep27 (Fig. 3). E1A mRNA and protein level were higher by qRT-PCR and protein gel blotting assays. Further, F5/35-ZD55-hep27-infected renal cancer cells produced much higher titers of viral progeny than that in Ad5-ZD55-hep27 treatment group. Increased replication of 5/35 fiber-modified adenoviral vector F5/35-ZD55-hep27 might contribute to its enhanced antitumor capacity (Fig. 4 and 5). The possible molecular mechanism of hep27-mediated tumor cell growth suppression was also described. Adenoviral-expressed hep27 had inhibited mdm2 and increased p53 and cleaved-PARP expression to induce cell apoptosis (Fig. 6). These data indicated that hep27 may affect the activity of cancer cells, consistent with aforementioned reports. 17Animal models also confirmed the F5/35-ZD55-hep27 had an enhanced antitumor effect for renal cancer cells in nude mice. Based on our findings, we propose that 5/35 fiber-modified adenoviral vector carrying hep27 had improved tumor suppressor activity. In spite of the antitumor activity, hep27 was investigated to induce cell apoptosis through inhibiting mdm2 and increasing p53 and cleaved-parp. The hep27 was confirmed to use in adenovirus mediated gene therapy.
In conclusion, we have demonstrated that the hep27-expressing and 5/35 fiber-modified adenoviral vector F5/35-ZD55-hep27 had a much stronger antitumor effect than that mediated by Ad5, though hep27- induced apoptosis. Hep27 gene transduction by adenovirus should therefore be a promising antitumor therapy for renal cancer cells.
Materials and methods
Cell lines and plasmids
Human renal carcinoma Ketr-3, OSRC-2,786-O, ACHN, human renal tubular epithelial cell HK-2 and HEK (human embryonic kidney)-293 were maintained in DMEM (Dulbecco's modified Eagle's medium) with 10% (v/v) fetal bovine serum (FBS) supplemented with penicillin and streptomycin. Hep27 expressing plasmid pcDNA3-Hep27 was a gift from Dr. Yanping Zhang (University of North Carolina at Chapel Hill, NC, USA). Hep27 gene was cloned into adenoviral shuttle plasmid pCA13 to generate pCA13-Hep27, including an expressing frame CMV promoter +Hep27+polyA. There was two BglII restriction enzyme sites located at upstream and downstream of Hep27 expressing frame respectively. The fragment from pCA13-Hep27 digested with BglII was inserted into the same site of pZD55 to construct pZD55-Hep27. The control plasmid pZD55-EGFP was described previously.25,26
Adenoviruses construction
The shuttle plasmid pZD55 or pZD55-Hep27 were co-transfected into HEK-293 cells with adenoviral cytoskeleton plasmid pBHGE3 (Microbix Biosystems Inc.) or a fiber chimeric adenoviral cytoskeleton plasmid pF5/35 (a gift from professor Changqing Su, The Second Military Medical University, China) using Lipfectamine 2000(Invitrogen) according to manufacturer's instructions respectively. The plaque showed at about 9–14 days after transfection. The transgenes were identified by PCR using specific primers for E1A or Hep27. The adenoviruses Ad5-ZD55-Hep27, F5/35-ZD55-Hep27, Ad5-ZD55 and F5/35-ZD55 were grown in HEK293 cells to amplify and titrate by tissue culture infection dose 50 (TCID50) methods. Virus stocks were stored in adenoviral buffer (Ad buffer) (10mM Tris-HCl, pH 8.0; 2mM MgCl2; 4% sucrose).
Quantitative real-time PCR
Cells treated with adenovirus or without treatment were harvested for extraction of total RNA. The RNA was reversely transcribed into cDNAs using PrimeScriptII reverse kit (Takara,Dalian, China) according to the standard protocol. GAPDH served as the endogenous housekeeping gene. Fold changes were determined by the comparative ΔΔCt method. The sequences of the primers used were as follows: CAR, forward: 5′-GCAGGAGCCTTATAGGAACTTTG-3′, reverse, 5′-GGACCCCAGGGATGAATGAT-3′. CD46, forward: 5′-GCTACCTGTCTCAGATGACG-3′, reverse, 5′-ACCACTTTACACTCTGGAGC-3′, GAPDH, forward: 5′-TCAGTGGTGGACCTGACCTG-3′, reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′ E1A, forward primer: 5′-cccagtgacgacgaggat-3′, reverse primer: 5′-ACTGTAGACAAACATGCCACA-3′.
Cell viability and cytopathic assay
Sub-confluent monolayer cultures were trypsinized, and cells were seeded on 96-well plates at a density of 104 cells/well. Twenty-four hours later, cells were infected with different adenoviruses at multiple of infection (MOI) of 0, 0.1,1,10, 50 or 100 and cell proliferation was analyzed on day 2 post infection by an MTT-based assay. Briefly, MTT at 0.5 mg/ml was added to the medium in each well, and plates were returned to the incubator for 1 h. The medium MTT was then removed, 500 μl of DMSO were added to each well, and the plate was kept in agitation for 5 min in the dark to dissolve the MTT–formazan crystals. The absorbance of the samples was then recorded at 570 nm. Wells containing medium plus MTT but no cells were used as blanks. Data are the average of 3 independent experiments performed in triplicate cultures.
Ketr-3, OSRC-2,786-O, ACHN and HK-2 cells were infected with Ad5-ZD55, F5/35-ZD55, Ad5-ZD55-Hep27 and F5/35-ZD55-Hep27 at a MOI = 0.1, 1, 10, 50,100, respectively. After 4 days of culture, the cytopathic effect (CPE) induced by adenoviruses was evaluated by crystal violet staining. Oncolytic potential of different adenoviruses was evaluated by crystal violet staining. Briefly, at the indicated time, the media was removed and the cells were washed twice with phosphate-buffered saline (PBS). Crystal violet solution was added, which were incubated at room temperature for 15 min, washed with distilled water and then documented with photographs.
Flow cytometric analysis
For infection capacity assay, cells were plated in 6-well plates and infected with Ad5-ZD55-EGFP and F5/35-ZD55-EGFP at a MOI of 10 for 48h, respectively. The percentages of EGFP-positive cells were determined by flow cytometry. Cells were trypsinized, washed with PBS, fixed with cold 70% ethanol in PBS at −20°C and extensively washed in cold 1× PBS. Samples were analyzed on a BD FACS Canto II flow cytometer (Becton Dickinson). Data were analyzed by using FACSDiva (Becton Dickinson) and FlowJo (Tree Star Inc.) software packages. For cell apoptosis detection, Ketr-3 and 786-O cells were infected with Ad5-ZD55-hep27 and F5/35-ZD55-hep27 at a MOI of 20 for 48 h. Cells were harvested for apoptosis analysis. Briefly, cells were washed with cold PBS and then added 300ul Binding Buffer, 10ul Annexin V-FITC and5ul propidium iodide (PtdIns) was added to the cells and incubated for 1 h. Samples were also analyzed on a BD FACS Canto II flow cytometer (Becton Dickinson). For cell surface receptors (CAR and CD46) expression profile analysis, cells were stained with rabbit polyclonal anti-human antibodies for CAR and CD46 (Santa Cruz Biotechnology, Inc.), then with an Alexa Fluor 488-conjugated goat anti-rabbit IgG secondary antibody (A11001; Molecular Probes, Eugene, OR, USA), and analyzed on a BD FACS Canto II flow cytometer (Becton Dickinson) with FlowJo (Tree Star Inc.) software.
Immunoblotting analysis
To prepare whole-cell extracts, cells were washed with cold Tris-buffered saline (TBS) and lysed with a RIPA buffer containing 50 mMTris-HCl (pH 7.4), 150 mMNaCl, 2 mM EDTA (pH 8.0), 1% Nonidet P-40, 0.1% SDS, 50 mMNaF, 1 mM Na3VO4, and 1mM PMSF. Protein concentration was measured by Bradford assay. After SDS-PAGE and semi-dry transfer to polyvinylidenedifluoride membrane (PVDF), the membrane was blocked in a5% nonfat dry milk in TBS with 0.1% Tween 20 for 30min-1h, and then incubated overnight at 4°C with primary antibodies and for 1h at room temperature with the corresponding fluorescence-labeled secondary antibodies (Sigma). Immunoreactive bands were detected with Orddsey (German). Primary antibodies used for detection were: anti-CD46, anti-CAR, anti-hep27, anti-mdm2, anti-p53, anti-cleaved PARP, anti-GAPDH (Santa Cruz Biotechnology, USA), anti-E1A (EMD Millipore, USA) and anti-β-actin (Zhongshan, Beijing, China).
Mouse xenografts
Animal experiment protocols were approved by the Ethics Review Committee for Animal experimentation of China. BALB/C nude mice, male, 4 weeks old, were purchased from the Shanghai SLAC Laboratory Animal Center of Chinese Academy of Sciences (Shanghai, China). Ketr-3 cells (1×107) were injected subcutaneously into the right flank of nude mice. Viral treatment (at a total dose of 1×109 PFU) was administered for three cycles every 2 days when tumor achieved approximately 5–6mm in diameter. Phosphate buffered saline (PBS) was used as a control. Tumors were measured at least every week and their volume was calculated by the formula V=large diameter x (small diameter) 2 × 0.5. At the end of the experiment tumors were removed from mice, weighed and immediately fixed in formalin and embedded in paraffin. Tumor sections were cut for further assay.
TUNEL assay
Apoptotic cells in tumor tissue sections were quantified using the TdT-mediated dUTP nick end-labeling (TUNEL) assays as instructed by the manufacturer (Beyotime, China). Briefly, formalin-fixed, paraffin-embedded sections were dewaxed before being permeabilized with proteinase K for 15 min at room temperature. Endogenous peroxidase was blocked with 3% H2O2, and sections were incubated with equilibration buffer and terminal deoxynucleotidyl transferase (TdT) enzyme. Finally, the sections were incubated with anti-digoxigenin-peroxidase conjugate. Peroxidase activity in each tissue section was demonstrated by the application of DAB. Under microscopy, six fields were randomly selected from every sample and 100 cells were randomly selected from every field. Apoptotic rate = (number of total apoptoticcells/100)×100%.
Statistical analysis
Data were represented by the mean + standard deviation (SD). Student's t test was used to compare differences between groups. Statistical significance was defined as a P value less than 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This project is supported by grants from the National Natural Science Foundation of China (Nos. 81372460, 81101702), the Natural Science Foundation of Jiangsu Province (Grants Nos. BK20131120, BK2011207), China Postdoctoral Science Foundation funded project (2013M531407), the Education Department of Jiangsu Province (Nos. 13KJB320028, 12KJA320001, 11KJA320002, No.2011-WS-069) and the sponsorship of Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents We thank Dr. Changqing SU (The Second Military Medical University, Shanghai, China) and Dr. Yanping Zhang (The University of North Carolina at Chapel Hill, School of Medicine, Chapel Hill, North Carolina) for the gifts of plasmids.
References
- 1.Kim EH, Strope SA. Postoperative surveillance imaging for patients undergoing nephrectomy for renal cell carcinoma. Urologic Oncol 2015; 33(12):499-502; PMID:26411549; http://dx.doi.org/26085875 10.1016/j.urolonc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacovelli R, Modica D, Palazzo A, Trenta P, Piesco G, Cortesi E. Clinical outcome and prognostic factors in renal medullary carcinoma: A pooled analysis from 18 years of medical literature. Can Urol Assoc J 2015; 9:E172-7; PMID:26085875; http://dx.doi.org/ 10.5489/cuaj.2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, Bjarnason GA, Srinivas S, Pal SK, Yuasa T, et al.. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015; 16:293-300; PMID:25681967; http://dx.doi.org/ 10.1016/S1470-2045(14)71222-7 [DOI] [PubMed] [Google Scholar]
- 4.Johnson AG, Ruiz J, Hughes R, Page BR, Isom S, Lucas JT, McTyre ER, Houseknecht KW, Ayala-Peacock DN, Bourland DJ, et al.. Impact of systemic targeted agents on the clinical outcomes of patients with brain metastases. Oncotarget 2015; 6:18945-55; PMID:26087184; http://dx.doi.org/ 10.18632/oncotarget.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caratozzolo MF, Valletti A, Gigante M, Aiello I, Mastropasqua F, Marzano F, Ditonno P, Carrieri G, Simonnet H, D'Erchia AM, et al.. TRIM8 anti-proliferative action against chemo-resistant renal cell carcinoma. Oncotarget 2014; 5:7446-57; PMID:25277184; http://dx.doi.org/ 10.18632/oncotarget.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang H, Gomez-Manzano C, Rivera-Molina Y, Lang FF, Conrad CA, Fueyo J. Oncolytic adenovirus research evolution: from cell-cycle checkpoints to immune checkpoints. Curr Opin Virol 2015; 13:33-9; PMID:25863716; http://dx.doi.org/ 10.1016/j.coviro.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bressy C, Benihoud K. Association of oncolytic adenoviruses with chemotherapies: an overview and future directions. Biochem Pharmacol 2014; 90:97-106; PMID:24832861; http://dx.doi.org/ 10.1016/j.bcp.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Wang B, Lou J, Yan J, Gao L, Geng R, Yu B. Mutation in fiber of adenovirus serotype 5 gene therapy vector decreases liver tropism. Int J Clin Exp Med 2014; 7:4942-50; PMID:25663991 [PMC free article] [PubMed] [Google Scholar]
- 9.Ballard EN, Trinh VT, Hogg RT, Gerard RD. Peptide targeting of adenoviral vectors to augment tumor gene transfer. Cancer Gene Therapy 2012; 19:476-88; PMID:22595794; http://dx.doi.org/ 10.1038/cgt.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takagi-Kimura M, Yamano T, Tamamoto A, Okamura N, Okamura H, Hashimoto-Tamaoki T, Tagawa M, Kasahara N, Kubo S. Enhanced antitumor efficacy of fiber-modified, midkine promoter-regulated oncolytic adenovirus in human malignant mesothelioma. Cancer Sci 2013; 104:1433-9; PMID:23962292; http://dx.doi.org/ 10.1111/cas.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Wu Y, Liu W, Wang G, Wang X, Yang Y, Chen W, Tai Y, Lu M, Qian Q, et al.. Enhanced antitumor efficacy of a novel fiber chimeric oncolytic adenovirus expressing p53 on hepatocellular carcinoma. Cancer Lett 2011; 307:93-103; PMID:21504839; http://dx.doi.org/ 10.1016/j.canlet.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 12.Iguchi K, Sakurai F, Tomita K, Katayama K, Yamaguchi T, Kawabata K, Tagawa M, Kawabata M, Shirakawa T, Mizuguchi H. Efficient antitumor effects of carrier cells loaded with a fiber-substituted conditionally replicating adenovirus on CAR-negative tumor cells. Cancer Gene Ther 2012; 19:118-25; PMID:22076042; http://dx.doi.org/ 10.1038/cgt.2011.74 [DOI] [PubMed] [Google Scholar]
- 13.Gabrielli F, Donadel G, Bensi G, Heguy A, Melli M. A nuclear protein, synthesized in growth-arrested human hepatoblastoma cells, is a novel member of the short-chain alcohol dehydrogenase family. Eur J Biochem / FEBS 1995; 232:473-7; PMID:7556196; http://dx.doi.org/16685466 10.1111/j.1432-1033.1995.473zz.x [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini S, Censini S, Guidotti S, Iacopetti P, Rocchi M, Bianchi M, Covacci A, Gabrielli F. A human short-chain dehydrogenase/reductase gene: structure, chromosomal localization, tissue expression and subcellular localization of its product. Biochim Et Biophysica Acta 2002; 1574:215-22; PMID:1997086; http://dx.doi.org/16685466 10.1016/S0167-4781(01)00323-2 [DOI] [PubMed] [Google Scholar]
- 15.Shafqat N, Shafqat J, Eissner G, Marschall HU, Tryggvason K, Eriksson U, Gabrielli F, Lardy H, Jörnvall H, Oppermann U. Hep27, a member of the short-chain dehydrogenase/reductase family, is an NADPH-dependent dicarbonyl reductase expressed in vascular endothelial tissue. Cell Mol Life Sci 2006; 63:1205-13; PMID:16685466; http://dx.doi.org/ 10.1007/s00018-006-6013-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monge M, Colas E, Doll A, Gil-Moreno A, Castellvi J, Diaz B, Gonzalez M, Lopez-Lopez R, Xercavins J, Carreras R, et al.. Proteomic approach to ETV5 during endometrial carcinoma invasion reveals a link to oxidative stress. Carcinogenesis 2009; 30:1288-97; PMID:19443906; http://dx.doi.org/ 10.1093/carcin/bgp119 [DOI] [PubMed] [Google Scholar]
- 17.Deisenroth C, Thorner AR, Enomoto T, Perou CM, Zhang Y. Mitochondrial Hep27 is a c-Myb target gene that inhibits Mdm2 and stabilizes p53. Mol Cell Biol 2010; 30:3981-93; PMID:20547751; http://dx.doi.org/ 10.1128/MCB.01284-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth K, Dhar D, Wold WS. Oncolytic (replication-competent) adenoviruses as anticancer agents. Exp Opin Biol Ther 2010; 10:353-68; http://dx.doi.org/ 10.1517/14712590903559822 [DOI] [PubMed] [Google Scholar]
- 19.Puntener D, Engelke MF, Ruzsics Z, Strunze S, Wilhelm C, Greber UF. Stepwise loss of fluorescent core protein V from human adenovirus during entry into cells. J Virol 2011; 85:481-96; PMID:21047958; http://dx.doi.org/ 10.1128/JVI.01571-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki T, Tazawa H, Hasei J, Osaki S, Kunisada T, Yoshida A, Hashimoto Y, Yano S, Yoshida R, Kagawa S, et al.. A simple detection system for adenovirus receptor expression using a telomerase-specific replication-competent adenovirus. Gene therapy 2013; 20:112-8; PMID:22241176; http://dx.doi.org/ 10.1038/gt.2011.213 [DOI] [PubMed] [Google Scholar]
- 21.Fang L, Cheng Q, Bai J, Qi YD, Liu JJ, Li LT, Zheng JN. An oncolytic adenovirus expressing interleukin-24 enhances antitumor activities in combination with paclitaxel in breast cancer cells. Mol Med Rep 2013; 8:1416-24; PMID:24042845; http://dx.doi.org/ 10.3892/mmr.2013 [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Fang L, Cheng Q, Li L, Su C, Zhang B, Pei D, Yang J, Li W, Zheng J. Effects of G250 promoter controlled conditionally replicative adenovirus expressing Ki67-siRNA on renal cancer cell. Cancer Sci 2012; 103:1880-8; PMID:22775978; http://dx.doi.org/ 10.1111/j.1349-7006.2012.02380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hai C, Jin YM, Jin WB, Han ZZ, Cui MN, Piao XZ, Shen XH, Zhang SN, Sun HH. Application of mesenchymal stem cells as a vehicle to deliver replication-competent adenovirus for treating malignant glioma. Chinese J Cancer 2012; 31:233-40; PMID:22429494; http://dx.doi.org/26251031 10.5732/cjc.011.10367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takei Y, Okamoto S, Kawamura K, Jiang Y, Morinaga T, Shingyoji M, Sekine I, Kubo S, Tada Y, Tatsumi K, et al.. Expression of p53 synergistically augments caspases-mediated apoptosis induced by replication-competent adenoviruses in pancreatic carcinoma cells. Cancer Gene Therapy 2015; 22:445-53; PMID:26251031; http://dx.doi.org/ 10.1038/cgt.2015.33 [DOI] [PubMed] [Google Scholar]
- 25.Jiang G, Liu YQ, Wei ZP, Pei DS, Mao LJ, Zheng JN. Enhanced anti-tumor activity by the combination of a conditionally replicating adenovirus mediated interleukin-24 and dacarbazine against melanoma cells via induction of apoptosis. Cancer letters 2010; 294:220-8; PMID:20189296; http://dx.doi.org/ 10.1016/j.canlet.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, Qian QJ, Liu XY. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res 2003; 13:481-9; PMID:14728805; http://dx.doi.org/ 10.1038/sj.cr.7290191 [DOI] [PubMed] [Google Scholar]