ABSTRACT
The bone marrow microenvironment plays an important role in the development and progression of AML. Leukemia stem cells are in a hypoxic condition, which induces the expression of HIF-1α. Aberrant activation of HIF-1α is implicated in the poor prognosis of patients with acute myeloid leukemia (AML). Herein, we investigated the expression of HIF-1α in AML and tested 2-methoxyestradiol (2ME2) as a candidate HIF-1α inhibitor for the treatment of AML. We found that HIF-1α was overexpressed in AML. HIF-1α suppression by 2ME2 significantly induced apoptosis of AML cells, and it outperformed traditional chemotherapy drugs such as cytarabine. At the same time, 2ME2 downregulated the transcriptional levels of VEGF, GLUT1 and HO-1 in cellular assays. Additionally, 2ME2 displayed antileukemia activity in bone marrow blasts from AML patients, but showed little effect on normal cells. 2ME2-induced activation of mitochondrial apoptotic pathway is mediated by reactive oxygen species (ROS), which decreased the slight effect of drug on normal cells. Our data show that supression of HIF-1α expression significantly reduced the survival of AML cell lines, suggesting that 2ME2 may represent a powerful therapeutic approach for patients with AML.
KEYWORDS: 2-methoxyestradiaol, acute myeloid leukemia, Hypoxia-inducible factor 1α, hypoxia
Introduction
The outcome of patients with acute myeloid leukemia (AML), the most common adult leukemia, remains poor.1 It is cured in 35–40% of patients ≤60 years old and only 5–15% >60 years old. Older patients who may not be strong enough for intensive chemotherapy have a median survival of just 5–10 months.2 Although some patients can achieve complete remission, many eventually relapse.3 Recently, studies have suggested that chemotherapy resistance is the main reason for the failure of chemotherapy and disease recurrence for AML patients.4-5 The bone marrow microenvironment plays a critical role in the development, progression, and relapse of AML.6 Haematopoietic stem cells (HSCs) and leukemia-initiating cells (LICs) reside in a hypoxic bone marrow niche. This hypoxic microenvironment is thought to contribute to quiescence and self-renewal and, in the case of leukemia, to chemotherapy resistance as well.7
Hypoxia-inducible factor (HIF) is a major molecular response to hypoxia and regulates numerous processes such as angiogenesis, metabolism, and proliferation.8 HIF proteins are composed of 2 different subunits: an oxygen-labile α subunit and a constitutively expressed β subunit. There are 3 different α subunits: HIF-1α, HIF-2α, HIF-3α. HIF-1α is expressed ubiquitously, whereas HIF-2α, HIF-3α exhibit a tissue-restricted mRNA expression pattern.9 In the bone marrow, the expression of HIF-1α is highest in long-term haematopoietic stem cells (HSCs), the stem cells that give rise to all other blood cells through haematopoiesis.9-10 HIF-1α is overexpressed in patients with AML, and is associated with poor survival in normal karyotype adult acute myeloid leukemia.Targeting HIF-1α can increase apoptosis of AML cells.11-14 To activate hypoxia-inducible transcription, HIF-1α recruits the transcriptional adapter/histone acetyltransferase protein, p300 /CBP (EP300, E1A binding protein p300), to the promoters of target gene.15 including those of vascular endothelial growth factor (VEGF), C-X-C chemokine receptor type 4 (CXCR4,) and glucose transporter-1(GLUT1).16-17 All of these genes are associated with chemoresistance. In addicion, HIF-1α alters cancer cells so that they use glycolysis rather than oxidative phosphorylation toproduce energy. This consequently reduces the generation of ROS and increases the survival of tumor cells.18 Because of the multitude of effects of HIF-1α to enhance leukemia, it is essential to identify effective HIF-1 inhibitors.
2-methoxyestradiol (2ME2), an inhibitor of hypoxia-inducible factors (HIFs) and a promising anticancer agent,19 is a naturally occurring estrogen metabolite that exhibits antiproliferative and antiangiogenic activity by inducing apoptosis as well as by inhibiting HIF-1a, microtubules, and tumor angiogenesis.20 2ME2 preferentially kills tumor cells over normal cells by causing reactive oxygen species (ROS) accumulation in cancer cells. Because it is a well-tolerated small molecule and is orally active, it is superior to other AML chemotherapy drugs.21 The goal of this work was to analyze whether HIF-1α expression is correlated with AML and evaluate the efficacy and mechanisms of 2ME2 for the treatment of AML.
Result
Expression of HIF-1α in AML primary cells
HIF-1α was reported to affect leukemia progression in the BM microenvironment, as the expression of HIF-1α regulated the survival of leukemia cells.22 To further analyze the function of HIF-1α in AML, BM samples of AML patients established at newly diagnosis, and samples from healthy donors were evaluated for HIF-1α expression. HIF-1α protein and mRNA levels were significantly up-regulated in AML patients compared with the healthy donors. HIF-1α expression of AML M2 subtype was significantly higher than other subtypes (0.087 ± 0.02 VS 0.071 ± 0.02, 0.035 ± 0.005, P < 0.05; Fig. 1A–B). In addition, we also determined HIF-1α expression in AML cell lines. Both HIF-1α mRNA and protein expression were higher in HL-60 and Kasumi-1 cells compared to other types of cell lines in normoxia conditions (Fig. 1C–D). These results are consistent with other studies that suggested HIF-1α may play a crucial role in AML progression.
Table 1.
The Clinical features of patients with AML.
| Patients | Sex | Age(years) | BM/PB | FAB | WBC(109L) | HB(g/L) | PLT(109L) | %Blasts(BM) | Cytogenetics | Mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| AML1 | M | 16 | BM | normal | 6.86 | 143 | 256 | — | Normal | Not |
| AML2 | M | 42 | BM | normal | 4.25 | 142 | 230 | — | Normal | Not |
| AML3 | M | 34 | BM | normal | 5.62 | 136 | 205 | — | Normal | Not |
| AML4 | F | 36 | BM | normal | 6.23 | 130 | 215 | — | Normal | Not |
| AML5 | M | 34 | BM | normal | 8.27 | 160 | 177 | — | Normal | Not |
| AML6 | F | 31 | BM | normal | 5.64 | 128 | 175 | — | Normal | Not |
| AML7 | M | 27 | BM | M2 | 4.32 | 62 | 13 | 48.19 | Normal | Not |
| AML8 | M | 35 | BM | M2 | 4.32 | 57 | 9 | 52 | t(8,21) | Not |
| AML9 | F | 61 | BM | M2 | 2.75 | 76 | 26 | 30.96 | Normal | Not |
| AML10 | M | 65 | BM | M2 | 5.44 | 60 | 38 | 54.94 | t(8,21) | Not |
| AML11 | M | 19 | BM | M2 | 8.39 | 77 | 44 | 40.25 | t(8,21) | FLT3-ITD |
| AML12 | F | 45 | BM | M4 | 7.45 | 119 | 93 | 26.24 | Normal | Not |
| AML13 | F | 73 | BM | M2 | 2.49 | 69 | 88 | 33 | Normal | Not |
| AML14 | F | 38 | BM | M2 | 66.59 | 108 | 87 | 88.67 | Normal | Not |
| AML15 | M | 28 | BM | M2 | 21.97 | 91 | 21 | 66.8 | Normal | Not |
| AML16 | F | 46 | BM | M2 | 28.21 | 80 | 19 | 81.95 | Normal | Not |
| AML17 | M | 38 | BM | M2 | 62.16 | 91 | 37 | 96 | Normal | Not |
| AML18 | F | 17 | BM | M2 | 4.61 | 83 | 393 | 59.7 | Normal | Not |
| AML19 | F | 45 | BM | M4 | 18.56 | 82 | 20 | 63.69 | Normal | Not |
| AML20 | F | 30 | BM | M4 | 62.44 | 64 | 32 | 72 | Normal | Not |
| AML21 | M | 20 | BM | M4 | 1.97 | 98 | 46 | 20.44 | Normal | Not |
| AML22 | M | 61 | BM | M4 | 35.44 | 34 | 18 | 83.66 | Normal | Not |
| AML23 | M | 22 | BM | M4 | 0.23 | 82 | 31 | 71.22 | −8 | Not |
| AML24 | F | 60 | BM | M4 | 0.85 | 54 | 67 | 41.26 | Normal | Not |
| AML25 | F | 52 | BM | M4 | 2.9 | 79 | 12 | 56.1 | Normal | Not |
| AML26 | F | 61 | BM | M5 | 110.7 | 86 | 16 | 94.35 | Normal | CBFβ-MYH |
| AML27 | F | 53 | BM | M5 | 59.96 | 66 | 9 | 85.34 | Normal | Not |
| AML28 | M | 75 | BM | M5 | 1.13 | 61 | 17 | 32.06 | Normal | Not |
| AML29 | F | 27 | BM | M5 | 53.12 | 45 | 28 | 79.15 | Normal | Not |
| AML30 | M | 39 | BM | M5 | 2.17 | 87 | 49 | 43.48 | Normal | Not |
| AML31 | M | 15 | BM | M5 | 23.4 | 65 | 18 | 56.02 | Normal | Not |
| AML32 | F | 18 | BM | M5 | 11.09 | 76 | 67 | 65.39 | Normal | Not |
Abbreviations: FAB,French-American-British classification; WBC, white blood cells; Hb,hemoglobin;PLT,platelets.
Figure 1.
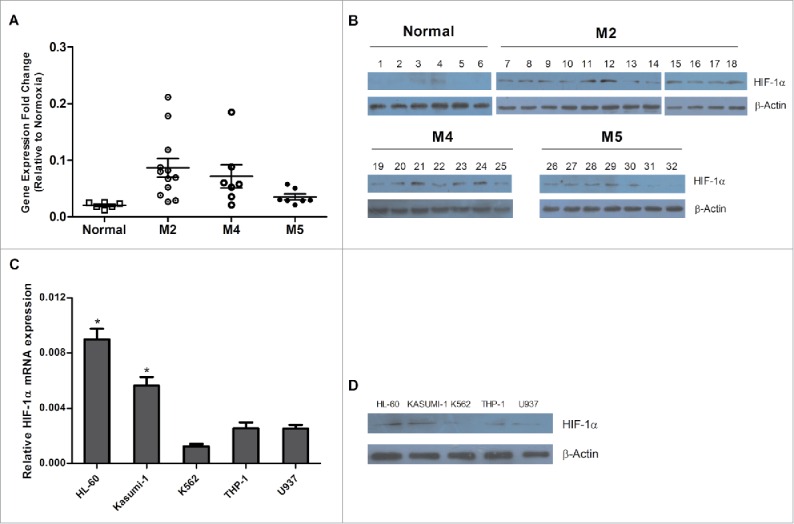
Expression of HIF-1α in AML primary cells. (A) and (B) Six healthy donors were selected as negative controls. HIF-1α mRNA and protein expressions were detected in patients with different subsets of AML by qPCR and western blot analysis. The patient information is listed on Table 1 (C) and (D) HIF-1α mRNA and protein expressions in leukemia cell lines were determined by qPCR and Western blot. 2−ΔCT is the value of relative mRNA expression. Error bars represent ± SEM.
2ME2-dependent inhibition of HIF-1α in acute myeloid leukemia cell lines
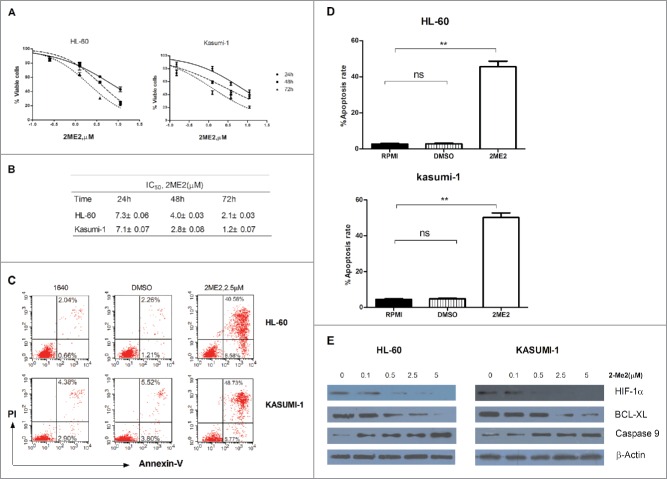
Because of the evident HIF-1α overexpression in AML cell lines, we next assessed the potential antileukemia activity using an inhibitor of HIF-1α. The small-molecule HIF-1α inhibitor, 2ME2, was designed and synthesized using a structure-based design approach. To determine the IC50 of 2ME2 in AML cell lines, we performed dose-response experiments. AML cell lines were treated with increasing doses of 2ME2 for 24 h, 48 h, 72 h, and assessed for effects on proliferation and survival (Fig. 2A). 2ME2 showed a significant antiproliferative activity in both cell lines in a dose- and time-dependent manner; the corresponding IC50 values are summarized in Fig. 2B. Next, the apoptosis of AML cell lines was assessed after incubation with the indicated concentration of 2ME2 for 48 hours. Apoptosis was detected in 40.58% and 48.73% of the HL-60 and Kasumi-1 cells, respectively(Fig. 2C–D). To verify whether 2ME2-induced apoptosis is associated with HIF-1α suppression, we treated HL-60 and Kasumi-1 cells with different concentrations of 2ME2 (0.1-5 μM) for 24 hours. The data showed that HIF-1α expression was decreased in HL-60 and Kasummi-1 cells after 2ME2 incubation. At the molecular level, 2ME2 also down-regulated BCL-XL expression and activated caspase 9 expression (Fig. 2E). Collectively, these data demonstrate that 2ME2 has a potent antiproliferative activity in AML cell lines associated with induction of apoptosis.
Figure 2.
2ME2-induced death by apoptosis in AML cell lines. (A) Two AML cell lines were incubated with increasing doses of 2ME2 (0.08-11.25μM). After 24, 48, or 72 hours, cell viability was detected by an MTT assay, each value represents the mean ± SEM of the results of 3 independent experiments. The concentration of 2ME2 (X)=log(X). IC50 for 2ME2 is shown in (B). (C) AML cell lines were incubated with 2.5μM of 2ME2 for 48 hours. Apoptosis was detected by annexin V-FITC/propidium iodide double staining. Percentages of cells showing apoptosis (combined right quadrants) are shown in the boxes. (D) Summary of the results of annexin V–FITC/7-AAD double staining from 3 independent experiments. **P < .001. Ns indicates no significant. (E) HL-60 and Kasumi-1 cells were treated with different concentration of 2ME2 (0.1, 0.5, 2.5, or 5μM) for 24 hours or diluent control, and intracelluar protein levels were examined by Western blot.
2ME2 downregulated expression of HIF-1α target genes at the transcriptional level
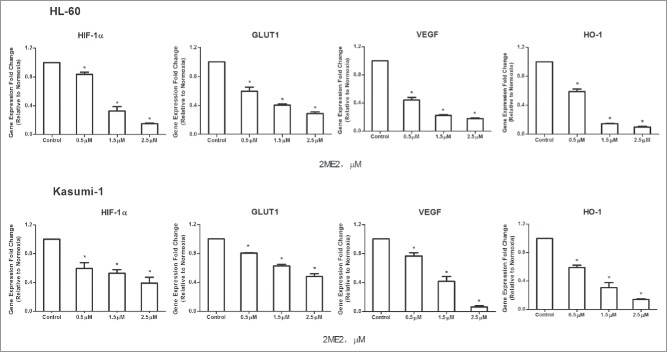
It is generally accepted that chemoresistance is the main cause of chemotherapy failure and relapse of AML.23 The expression of the HIF-1α target genes VEGF, GLUT1 and HO-1 has been associated with drug-resistance, tumor angiogenesis and metastasis. Thus, we next aimed to understand the molecular mechanism of 2ME2-mediated suppression of drug-resistance gene expression by investigating the effect of the inhibitor on the expression of these genes. AML cell lines were treated with different concentrations of 2ME2 and gene expression was analyzed. The transcriptional level of VEGF, GLUT1 and HO-1 was strongly down-regulated in the 2ME2-treated AML cell lines (Fig. 3). HIF-1α-dependent transcription is regulated by two additional proteins, CITED2 (CBP/p300 interacting transactivator with ED-rich tail) and p300/CBP. CITED2, a hypoxia- and growth factor-induced transcription factor, is a negative regulatory factor to HIF-1α that acts to disrupt the interaction between HIF-1α and p300.15 However, it has been reported that CITED2 expression has a functional role in AML maintence,24 attenuation glycolytic metabolism, and elevation of mitochondrial activity,25 similar to HIF-1α. Although CITED2 and HIF-1 are competitive in the regulation of hypoxia, they have similar functions in the maintenance of HSCs. Thus, we next investigated whether 2ME2 had a role in regulating the activity of CITED2. Interestingly, we found that 2ME2 does act to suppress CITED2 expression in addition to HIF-1α expression.(Fig. 3)
Figure 3.
2ME2 down-regulated expression of HIF-1α target genes at the transcriptional level. AML cell lines were treated with different concentrations of 2ME2 (0.1, 0.5, 2.5, or 5 μM) for 24 hours or vehicle alone, and the mRNA expression of VEGF, GLUT1,HO-1 and CITED2 was analyzed with q-PCR. Each value represents the mean of 3 independent experiments performed.2−ΔΔCT is the value of relative mRNA expression. Error bars represent ± SEM. *P < .05.
2ME2 performed better than traditional chemotherapy drugs for treatment of AML
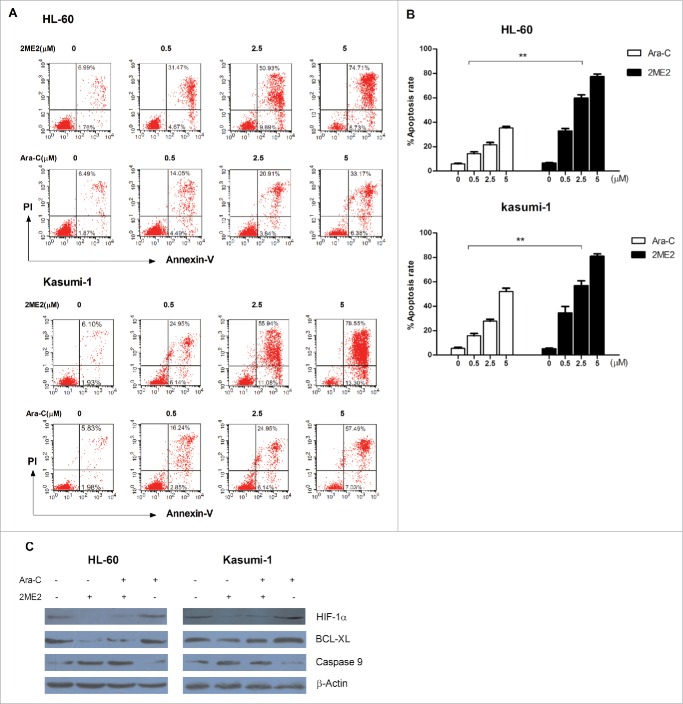
Cytarabine is one of the main chemotherapy agent used in the treatment of AML. On the basis of our observations that 2ME2 exhibited very potent antileukemia activity, we next compared the sensitivity of AML cell lines to 2ME2 and to cytarabine.. AML cell lines were incubated with increasing concentrations of 2ME2 and cytarabine and the percentages of apoptotic cells were determined after 24 hours. As shown in Fig. 4A–B, 2ME2 and cytarabine induced cell death in a dose-dependent way. Surprisingly, the percentages of apoptotic cells induced by 2ME2 treatment was significantly higher than those treated with cytarabine at same concentration.To further investigate the molecular mechanism involved in this phenomenon, the apoptosis-associated proteins were detected in the AML cell lines treated with 2ME2, cytarabine, or a combination of 2ME2 and cytarabine. We clearly observed that 2ME2 induced the activation of caspase 9 and downregulated BCL-XL, but saw no effect on expression of these genes by cytarabine (Fig. 4C). Collectively, these data suggest that the antileukemia activity of 2ME2 is stronger than that of the raditional chemotherapy drug cytarabine.
Figure 4.
Compared with traditional chemotherapy drug, 2ME2 has potent antileukemia activity. (A) AML cell lines were treated with increasing concentration of 2ME2 and cytarabine (0.5, 2.5, or 5μM) for 48 hours. Apoptosis was detected by annexin V-FITC/propidium iodide double staining. (B) Summary of the results of annexin V–FITC/7-AAD double staining from 3 independent experiments. **P < .01. (C) Cells were incubated with 2.5μM of 2ME2 or 5 μM cytarabine or in combination for 48 hours. The protein level of Bcl-xl and caspase 9 were then evaluated by Western blot.
The effect of 2ME2 on normal bone marrow progenitor cells and AML progenitor cells
`Traditional chemotherapy drugs for the treatment of leukemia have significant side effects, including damage to normal haematopoietic stem cells. Next, we compared the effect of 2ME2 and cytarabine on the viability of normal bone marrow progenitor cells and AML progenitor cells, to test whether 2ME2 also had damaging effects on normal cells. Fig. 5 demonstrated that exposure to 2ME2(IC50= 2.08 ± 0.019) induced greater loss of viability in a CD34+ enriched population of AML progenitor cells at relatively low dose, compared with cytarabine (IC50= 25.34±0.019). In CD34+ normal progenitor cells, however, the survival of 2ME2-treated cells was higher than that of cytarabine-treated cells (Fig. 5). Therefore, 2ME2 increased the apoptosis of AML cells, but the toxicity to normal haematopoietic stem cells was small, compared with traditional chemotherapy drugs.
Figure 5.
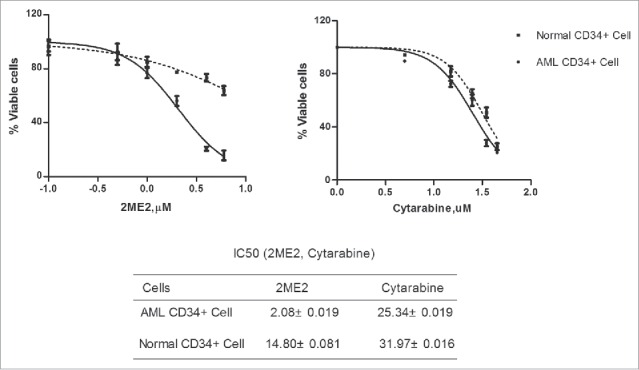
The effect of 2ME2 on normal bone marrow progenitor cells and AML progenitor cells. Bone marrow from 3 patients with AML, CD34+ cells enriched from 4 patients, and CD34+ cells from 3 normal donors were treated with the indicated concentrations of 2ME2 or cytarabine for 24 hours. The concentration of the drug was determined by the IC50 value of the leukemia cells. The percentages of viable cells for each drug were determined by MTT assay. The concentration of drugs (X)=log(X).
HIF-1α inhibition by 2ME2 activates the mitochondrial apoptotic pathway in AML cell lines
As it has been confirmed that 2ME2-induced apoptosis is associated with the increase of reactive oxygen species (ROS).26 So next we examined whether the production of ROS was related to the inhibition of HIF-1α. DMOG, a α-ketoglutarate antagonist that induces HIF-1 activity, stabilized HIF-1α expression even under normoxic conditions. AML cell lines were treated with the indicated concentrations of 2ME2 and DMOG for 8 hours before measuring intracellular ROS. Expectedly, 2ME2 increased the production of ROS, while DMOG reduced its production (Fig. 6A).
Figure 6.
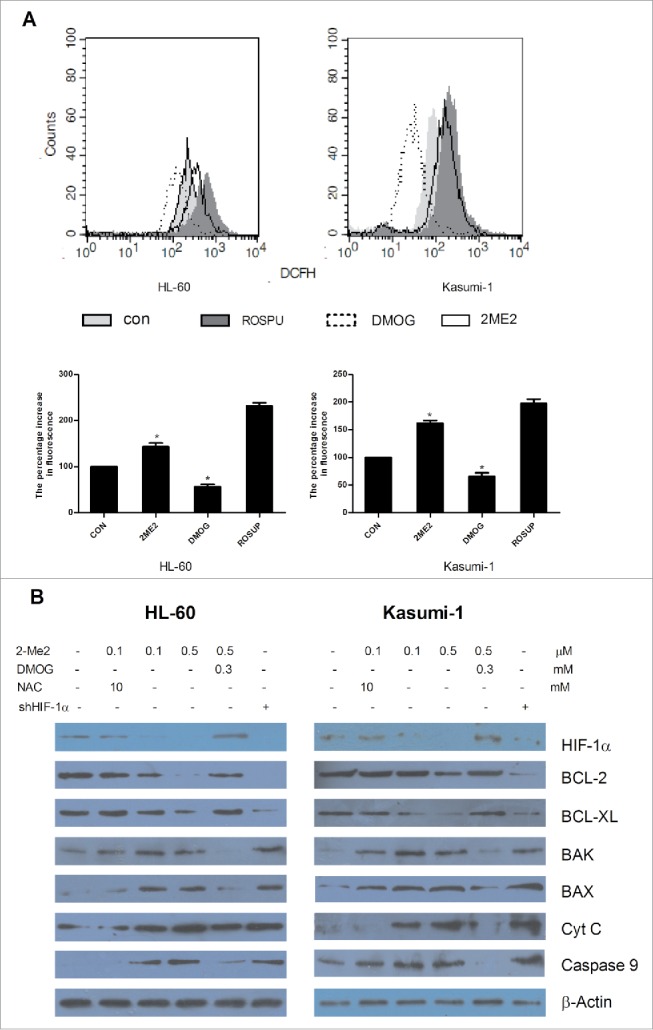
HIF-1α inhibition by 2ME2 activates the mitochondrial apoptotic pathway. (A) AML cell lines were treated with 2.5μM of 2ME2 or 0.8mM of DMOG for 8 hours before measuring the intracellular ROS by dichlorodihydrofluorescein diacetate staining using flow cytometry. (B) AML cell lines were treated with the indicated concentrations of 2ME2, DMOG, shHIF-1α, or a combination of 2ME2 with NAC or DMOG. The relative protein expression of the genes were detected by Western blot.
ROS is closely related to mitochondrial apoptosis.27 To further understand the molecular mechanism of HIF-1α inhibition by 2ME2 in AML cell lines, we examined the expression of mitochondrial-associated proteins after treatment with different drugs and reagents. As shown in Fig. 6B, as HIF-1α expression decreased in AML cell lines, the expression levels of pro-apoptotic Bcl-2 family members (Bax, Bak) and capase9 were increased as was the release of cytochrome C, but expression of anti-apoptotic Bcl-2 family members (Bcl-2, Bcl-xl) decreased after exposure to 2ME2. Similarly, when we inhibited the expression of HIF-1α in AML cells using a short hairpin RNA, we observed that changes in the expression of Bcl-2 family protein was similar to that of cells treated with 2ME2. However, in the presence of DMOG, the function of 2ME2 was obviously weakened and DMOG restored the expression of HIF-1α and mitochondrial-associated proteins in the presence of 2ME2. Based on the above results, we suggest that the silence of HIF-1α expression by 2ME2 induced apoptosis occurs via the mitochondrial apoptotic pathway.
We have previously demonstrated that 2ME2 had an effect on the production of ROS. Hence, we next investigated whether ROS plays a crucial role in 2ME2-induced activation of the mitochondrial apoptotic pathway. AML cell lines were treated with the antioxidant N-acetylcysteine (NAC) in the presence of 2ME2. The combined treatment of NAC and 2ME2 did not activate the mitochondrial pathway, compared with treatment with only 2ME2 at the same concentration (Fig. 6B). These data indicated that NAC eliminated the effect of 2ME2 on the mitochondrial apoptotic pathway and that the mitochondrial apoptosis induced by 2ME2 was mediated by ROS.
Discussion
Leukemia stem cells reside in a hypoxic bone marrow niche that is resistant to treatment and responsible for leukemia initiation and relapse. HIF-1α is a master regulator of hypoxia and plays an important role in angiogenesis, metabolic reprogramming, epithelial-mesenchymal transition, stem cell maintenance, invasion, metastasis, and the resistance to therapy.8 In our study, we demonstrated that both at the RNA and protein level, HIF-1α was overexpressed in patient samples with different subtypes of AML compared to normal haematopoietic cells. Our results are consistent with previous studies that reported high HIF-1α protein levels in mice bearing human or mouse leukemia,28 and that HIF-1α expression is closely related to the prognosis and chemoresistance of AML.29 Other groups reported different findings, and Talia et al suggested that HIF-1α status does not influence the initiation/progression of leukemia.7 In agreement with our effect of HIF-1α inhibition, another group demonstrated that the HIF-1α inhibitor echinomycin can be used to treat relapsed AML without affecting host HSCs,1 and reported a 50% reduction in leukemia cell engraftment in the HIF-1α-shRNA MSC-derived extramedullary bones compared with NS-shRNA MSC-derived bones.22 Our results support the model that HIF-1α affects the survival of AML cells and is a reasonable target for therapeutic development. To further examine the function of HIF-1α in AML, 2ME2 was used to decrease HIF-1α expression. We demonstrated that altered expression of HIF-1α affected the proliferation of the AML cell line. 2ME2 acted as an effective HIF-1α inhibitor against AML cell lines in vitro, with an IC50 range from 1 μM to 7 μM. The antiproliferative activity involved the suppression of HIF-1α, caspase activation, and induction of apoptosis.
2-methoxyestradiol, an agent being clinically evaluated as a new anticancer agent, is currently in phase I/II clinical studies to investigate its safety, efficacy, and pharmacokinetics.30 In this study, we descibe the molecular activity of 2ME2 in AML cell lines. VEGF, GLUT1 and HO-1, are target genes of HIF-1α and are associated with tumor growth and progression in solid tumors and hematological neoplasms.31-33 As HIF-1α expression decreased with the treatment of 2ME2 in a dose-dependent manner, the expression of VEGF, GLUT1 and HO-1 was also inhibited at transcriptional level. This effect increases the antiproliferative activity of 2ME2 on AML cell lines to some extent. In addition, our results showed that 2ME2 downregulated CITED2 mRNA expression, which plays a critical role in HSC metabolic regulation.25 Suppression of CITED2 may increase apoptosis and impaire leukemia stem cell function. However, the details of the mechanism remain to be elucidated.
To further examine the function of 2ME2, we investigated the antileukemia activity of 2ME2 compared with cytarabine. Experimental results showed that the sensitivity of AML cell lines to 2ME2 was higher than the sensitivity to cytarabine at the same concentration, and the effect of 2ME2 on normal haematopoietic stem cells was weak compared to traditional chemotherapy drugs. This suggests that 2ME2 is a promising anticancer drugs because it potently induced apoptosis of AML cell lines and but only a minor side effect. However, the molecular mechanism of 2ME2-induced apoptosis remains unclear.
HIF-1α changes the way that cancer cells provide energy and reduces the generation of ROS.18 The inhibition of HIF-1α expression induces mitochondrial ROS production, resulting in the death of AML cells. To test this model, we altered the expression of HIF-1α. Experimental results show that the expression of HIF-1α reduced the generation of ROS in AML cell lines. It was previously reported that 2ME2- induced apoptosis in tumor cells is associated with an increase in ROS.26 This is consistent with our results, as the inhibitor of HIF-1α increased the generation of ROS and then induced apoptosis in AML cell lines. AML cells are more susceptible to oxidative stress than normal cells and a subset of primary AML cells were found to be more sensitive to drug-induced mitochondrial ROS production compared with normal haematopoietic cells.34 We suggest that this explains why normal cells are not susceptible to 2ME2. Finally, we demonstrated that 2ME2 induced activation of the mitochondrial apoptotic pathway, but was blocked by NAC. This finding further verified that inhibition of HIF-1α by 2ME2 induced apoptosis is mediated by ROS.
In conclusion, our data confirmed previous results of high expression of HIF-1α in human AML cell lines. We propose that inhibition of HIF-1α by 2ME2 has a potent antileukemia activity through activation of the mitochondrial apoptotic pathway mediated by ROS, and is not cytotoxic to normal cells. Therefore, 2ME2 is a potential candidate for the treatment of AML.
Materials and methods
Reagents and antibodies
2ME2 and Dimethyloxalylglycine (DMOG) were purchased from MedChemExpress (New Jersey, USA). N-acetylcysteine (NAC) was purchased from Beyotime Biotechnology. Antibodies specific for HIF-1α, Bcl-2, Bak, Bax, Cytochrome C were purchased from Beyotime Biotechnology (Shanghai; China). Antibodies to Bcl-xl, caspase 9 and β-actin were from Proteintech (Chicago, USA).
Cell lines and cell culture
Human myeloid leukemia cell lines Kasumi-1, HL-60 were obtained from the Laboratory of Hematology, Guiyang Hospital of Guiyang Medical University. These cell lines were cultured at 37°C in a 5% humidified atmosphere in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, Gibco BRL, MD, USA), penicillin (100 units/mL) and streptomycin (100 μg/mL).
Patient samples
Primary human AML samples were isolated from peripheral blood or marrow samples from consenting patients with AML. AML cells were isolated by percoll density centrifugation. Except where otherwise noted, primary normal haematopoietic cells refer to normal mononuclear cells obtained from healthy consenting volunteers donating peripheral blood stem cells (PBSCs) for allogeneic stem cell transplantation after G-CSF mobilization. The CD34+ cells were purified immediatedly by magnetic-activated cell sorting (MACS; Miltenyi Biotech, Auburn CA) using mononuclear cells from patients with AML and healthy donors. The purity of the cells (≥80%) was assessed by flow cytometry. CD34+ cells were seeded in 96-well plates at a density of 5000 cells/well after exposure to different concentrations of 2ME2 and cytarabine for 12 hours and then cell viability was detected. Institutional Review Board approval was obtained from Guiyang Medical University Hospital (Guiyang,Guizhou, China). The study was performed in accordance with the modified Helsinki Declaration, and the protocol was approved by our ethical review boards before study initiation. Informed consent was obtained from the patients and healthy volunteers.
shRNA experiments
Recombinant lentivirus short hairpin RNA (shRNA) sequence shRNA-HIF-1α (5′-CCGGTCTTAAGGCACGCGGAATAAACTCGAGTTTATTCCGCGTGCCTTAAGATTTTTG-3′) targeted human HIF-1α. PLKO.1-TRC/Lenti-EGFP was used as a negative control for shRNA expression. Recombinant lentivirus /shRNA-encoding viruses were cotransfected into 293FT cells. For infection, cells were plated onto 12 well plates at the density of 2.5 × 105 cells/well, infected with lentiviral stocks at an MOI of 10 in the presence of polybrene, and then analyzed by q-PCR after 48 h of infection.
In vitro proliferation assay
Cell viability was determined using the MTT (4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Briefly, cells were seeded in 96-well plates at a density of 5000 cells/well. The cell lines were exposed to different concentrations of 2ME2 (0, 0.08, 0.25, 1.25, 3.75, or 11.25 μM) for 24, 48 or 72h. After the treatment, the cells were incubated with 20 μl MTT (5 mg/ml; Sigma, USA) at 37°C for 4 hrs. The MTT solution was carefully aspirated and the purple formazan crystals produced by the mitochondrial dehydrogenase enzymes were dissolved in DMSO. The optical density (OD) was measured using a microplate reader Epoch (BioTek, Winooski, VT, USA) at 570 nm wavelength. The experiment was repeated three times. Survival rate (SR) was determined using the following equation: SR (%) = (A Treatment /A Control) × 100%. The concentration that produced 50% cytotoxicity (IC50) was determined using GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA).
Apoptosis assay
Apoptosis was determined by annexin V-FITC and propidium iodide doube staining according to the manufacture's instructions (7sea biotech, Shanghai, China). Data were collected on a FACSCalibur flow cytometer (BD, San Jose, CA, USA). Results represent the mean value of 3 independent experiments.
RNA isloation and reverse transcription-polymerase chain reaction
Total RNAs from cell lines and primary mononuclear cell samples were extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. qPCR was performed using a SYBR Green PCR Master Mix (TianGen, Biotech, Beijing, China) and a PRISM 7500 real time PCR detection system (ABI, USA). The levels of genes expression were analyzed relative to the levels of β-actin gene transcript. The primers for qPCR were:
HIF-1α-F5′-CTTGGATGGTTTTGTTATGGTTCTC-3′; HIF-1α-R5′-ATTTCTCTCATTTCCTCATGGTCA-3′; GLUT1-F5′-CTAAAGATGGTGCGGAATGAGAAG-3′; GLUT1-R5′-CTCAAACGTCATGCAGATGTGGT-3′; HO-1-F5′-ACCCATGACACCAAGGACCAGA-3′; HO-1-R5′-GTGTAAGGACCCATCGGAGAAGC-3′;
CITED 2-F5′-GCAAAAACGGAAGGACTGGAA-3′;
CITED 2-R5′-GCGCCGTAGTGTATGTGCT-3′;
β-actin-F5′-GAGACCTTCAACACCCCAGC-3′;
β-actin-R5′-ATGTCACGCACGATTTCCC-3′;
The cDNA samples were mixed with primers and SYBR Master Mix in a total volume of 20 μL. The thermal cycling conditions used in the protocol were 1 min at 94°C, followed with 40 cycles at 94°C for 10 s and at 60°C for 15 s.
Western blot analysis
Cells from different groups were harvested. After being washed twice in ice-cold PBS, the cells were lysed by sonication in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 1% Triton X-100, 2% SDS, and 1% sodium deoxycholate) containing 1 mM phenylmethanesulfonyl fluoride (Solarbio Science & Technology, Beijing, China). The lysate was transferred into EP tubes and centrifuged at 12000 ×g for 10 min at 4°C. The supernatant was collected and mixed with loading buffer. The final solution was boiled for 10 min and aliquots were stored at −80°C before use. An equal amount of protein (50-100 μg) was loaded and separated by 10% SDS-PAGE gel and transferred onto PVDF membrane (Millipore Corporation, Milford, MA, USA) which was then blocked in 5% non-fat milk in Tris buffer at 4°C overnight. The membrane was blotted with the relevant antibody for 2 h. After washing, the blot was incubated with secondary antibodies (HRP-conjugated goat anti-rabbit or anti-mouse; Beyotime, Shanghai; China) for 1 h at room temperature. The protein bands were visualized on film by enhanced chemiluminescence (7sea Biotech, Shanghai, China) following the protocol of the manufacturer. All tests were repeated three times.
Intracellular ROS measurement
DCFH-DA was used for ROS detection. DCFH-DA is cleaved intracellularly by nonspecific esterases to form 2′, 7′-dichlorodihydrofluorescein (DCFH), which is further oxidized by ROS to form the highly fluorescent compound 2′,7′-dichlorodihydrofluorescein (DCF). The medium was removed and replaced with serum-free medium DCFH-DA (Beyotime Institute of Biotechnology, Jiangsu, China). The stock solution (10 mM) was diluted 1000-fold in serumfree medium and was added to each well of 96-well plates (10 uM). The 1×106 cells were incubated for 25 min and then DCF fluorescence was determined at 520 nm following excitation with 488 nm light from an argon laser with a FACSCalibur. The results were expressed as relative fluorescence intensity per 104 cells. Data were expressed as percentage of the control (untreated cells).
Statistical analysis
Data were statistically analyzed using GraphPad Prism 5.0 software. All data were presented as mean ± standard error of the mean. Statistical analyses were performed using analysis of variance (ANOVA) and t text. P values less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Clinicians of Affiliated Hospital of Guiyang Medical College for collecting clinical samples.
Funding
This study was supported by the National Science Foundation of China (No. 81070444, No. 81270636, No. 81360501 and No. 30760276), International Cooperation Project of Guizhou Province (No. 2011-7010 and No. 2012-7043), Social Project of Guizhou Province (No. 2011-3012 and No. 2012-3138), and Provincial Governor Special Fund of Guizhou Province (No. 2010-84).There have no conflicts of interest among the authors.
References
- 1.Wang Y, Liu Y, Tang F, Bernot KM, Schore R, Marcucci G, Caligiuri MA, Zheng P, Liu Y. Echinomycin protects mice against relapsed acute myeloid leukemia without adverse effect on hematopoietic stem cells. Blood 2014. August 14; 124(7):1127-35; PMID:24994068; http://dx.doi.org/ 10.1182/blood-2013-12-544221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med 2015. September 17; 373(12):1136-52; PMID:26376137; http://dx.doi.org/ 10.1056/NEJMra1406184 [DOI] [PubMed] [Google Scholar]
- 3.Marjerrison S, Antillon F, Bonilla M, Fu L, Martinez R, Valverde P, Vasquez R, Howard SC, Ribeiro RC, Sung L. Outcome of children treated for relapsed acute myeloid leukemia in Central America. Pediatr Blood Cancer 2014; 61(7):1222-6; PMID:24443303; http://dx.doi.org/ 10.1002/pbc.24942 [DOI] [PubMed] [Google Scholar]
- 4.Zhe N, Wang J, Chen S, Lin X, Chai Q, Zhang Y, Zhao J, Fang Q. Heme oxygenase-1 plays a crucial role in chemoresistance in acute myeloid leukemia Hematol 2015. August; 20(7):384-91; PMID:26218201; http://dx.doi.org/ 10.1179/1607845414Y.0000000212 [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Cao C, Gong X, Rong L. Inhibition of ERK5 enhances cytarabine-induced apoptosis in acute myeloid leukemia cells. Int J Clin Exp Med 2015; 8(4):6446-55; PMID:26131272 [PMC free article] [PubMed] [Google Scholar]
- 6.Rashidi A, Uy GL. Targeting the microenvironment in acute myeloid leukemia. Curr Hematol Malig Rep 2015. June; 10(2):126-31; PMID:25921388; http://dx.doi.org/ 10.1007/s11899-015-0255-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velasco-Hernandez T, Hyrenius-Wittsten A, Rehn M, Bryder D, Cammenga J. HIF-1α can act as a tumor suppressor gene in murine acute myeloid leukemia. Blood 2014. December 4; 124(24):3597-607; PMID:25267197; http://dx.doi.org/ 10.1182/blood-2014-04-567065 [DOI] [PubMed] [Google Scholar]
- 8.Hutt DM, Roth DM, Vignaud H, Cullin C, Bouchecareilh M. The histone deacetylase inhibitor, Vorinostat, represses hypoxia inducible factor 1 α expression through translational inhibition. PLoS One 2014. August 28; 9(8):e106224; PMID:25166596; http://dx.doi.org/ 10.1371/journal.pone.0106224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du J, Chen Y, Li Q, Han X, Cheng C, Wang Z, Danielpour D, Dunwoodie SL, Bunting KD, Yang YC. HIF-1α deletion partially rescues defects of hematopoietic stem cell quiescence caused by Cited2 deficiency Blood 2012. March 22; 119(12):2789-98; PMID:22308296; http://dx.doi.org/ 10.1182/blood-2011-10-387902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, et al.. Regulation of the HIF-1 α level is essential for hematopoietic stem cells. Cell Stem Cell 2010. September 3; 7(3):391-402; PMID:20804974; http://dx.doi.org/ 10.1016/j.stem.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 11.Hatfield KJ, Bedringsaas SL, Ryningen A, Gjertsen BT, Bruserud O. Hypoxia increases HIF-1α expression and constitutive cytokine release by primary human acute myeloid leukaemia cells. Eur Cytokine Netw 2010. September; 21(3):154-64; PMID:20729179; http://dx.doi.org/ 10.1684/ecn.2010.0204 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 2011. April 8; 8(4):399-411; PMID:21474104; http://dx.doi.org/ 10.1016/j.stem.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song K, Li M, Xu XJ, Xuan L, Huang GN, Song XL, Liu QF. HIF-1α and GLUT1 gene expression is associated with chemoresistance of acute myeloid leukemia. Asian Pac J Cancer Prev 2014; 15(4):1823-9; PMID:24641416; http://dx.doi.org/10.7314 [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Li B, Huang P, Wan X, Qin Y, Zhou L, Liu H, Bai H, Gao Y, Wang C, et al.. Citrate induces apoptosis of the acute monocytic leukemia U937 cell line through regulation of HIF-1α signaling. Mol Med Rep 2013. November; 8(5):1379-84; PMID:24064771; http://dx.doi.org/ 10.3892/mmr.2013.1702 [DOI] [PubMed] [Google Scholar]
- 15.Fox SB, Bragança J, Turley H, Campo L, Han C, Gatter KC, Bhattacharya S, Harris AL. CITED4 inhibits hypoxia-activated transcription in cancer cells, and its cytoplasmic location in breast cancer is associated with elevated expression of tumor cell hypoxia-inducible factor 1alpha. Cancer Res 2004. September 1; 64(17):6075-81; PMID:15342390; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-0708 [DOI] [PubMed] [Google Scholar]
- 16.Tabe Y, Konopleva M. Role of Microenvironment in Resistance to Therapy in AML. Curr Hematol Malig Rep 2015. June; 10(2):96-103; PMID:25921386; http://dx.doi.org/ 10.1007/s11899-015-0253-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Niu X, Liao L, Cho EA, Yang H. The contributions of HIF-target genes to tumor growth in RCC. PLoS One 2013. November 18; 8(11):e80544; PMID:24260413; http://dx.doi.org/21208194 10.1371/journal.pone.0080544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spégel P, Malmgren S, Sharoyko VV, Newsholme P, Koeck T, Mulder H. Metabolomic analyses reveal profound differences in glycolytic and tricarboxylic acid cycle metabolism in glucose-responsive and -unresponsive clonal β-cell lines. Biochem J 2011. April 1; 435(1):277-84; PMID:21208194; http://dx.doi.org/ 10.1042/BJ20100655 [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Jiang X, Zhu H, Jiang W, Dong X, Qiao H, Sun X, Jiang H. 2ME2 inhibits the activated hypoxia-inducible pathways by cabozantinib and enhances its efficacy against medullary thyroid carcinoma. Tumour Biol 2016; 37(1):381-91; PMID:26219898; http://dx.doi.org/ 10.1007/s13277-015-3816-1 [DOI] [PubMed] [Google Scholar]
- 20.Chow JM, Liu CR, Lin CP, Lee CN, Cheng YC, Lin S, Liu HE. Downregulation of c-Myc determines sensitivity to 2-methoxyestradiol-induced apoptosis in human acute leukemia. Exp Hematol 2008. February; 36(2):140-8; PMID:18206725; http://dx.doi.org/ 10.1016/j.exphem.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Chen YY, Yeh CH, So EC, Sun DP, Wang LY, Hsing CH. Anticancer drug 2-methoxyestradiol protects against renal ischemia/reperfusion injury by reducing inflammatorycytokines expression. Biomed Res Int 2014; 2014:431524; PMID:25229058; http://dx.doi.org/ 10.1155/2014/431524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Jacamo R, Shi YX, Wang RY, Battula VL, Konoplev S, Strunk D, Hofmann NA, Reinisch A, Konopleva M, et al.. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood 2012. May 24; 119(21):4971-80; PMID:22490334; http://dx.doi.org/ 10.1182/blood-2011-11-389957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho BS, Zeng Z, Mu H, Wang Z, Konoplev S, McQueen T, Protopopova M, Cortes J, Marszalek JR, Peng SB, et al.. Antileukemia activity of the novel peptidic CXCR4 antagonist LY2510924 as monotherapy and in combination with chemotherapy. Blood 2015. July 9; 126(2):222-32; PMID:26031918; http://dx.doi.org/ 10.1182/blood-2015-02-628677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korthuis PM, Berger G, Bakker B, Rozenveld-Geugien M, Jaques J, de Haan G, Schuringa JJ, Vellenga E, Schepers H. CITED2-mediated human hematopoietic stem cell maintenance is critical for acute myeloid leukemia. Leukemia 2015. March; 29(3):625-35; PMID:25184385; http://dx.doi.org/ 10.1038/leu.2014.259 [DOI] [PubMed] [Google Scholar]
- 25.Jinwei Du, Qiang Li, Tang F, Puchowitz MA, Fujioka H, Dunwoodie SL, Danielpour D, Yang YC. Cited2 is required for the maintenance of glycolytic metabolism in adult hematopoietic stem cells. Stem Cells Dev 2014. January 15; 23(2):83-94; PMID:24083546; http://dx.doi.org/ 10.1089/scd.2013.0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow JM, Liu CR, Lin CP, Lee CN, Cheng YC, Lin S, Liu HE. Downregulation of c-Myc determines sensitivity to 2-methoxyestradiol-induced apoptosis in human acute myeloidleukemia. Exp Hematol 2008. February; 36(2):140-8; PMID:18206725; http://dx.doi.org/ 10.1016/j.exphem.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 27.You BR, Kim SH, Park WH. Reactive oxygen species, glutathione, and thioredoxin influence suberoyl bishydroxamic acid-induced apoptosisin A549 lung cancer cells. Tumour Biol 2015. May; 36(5):3429-39; PMID:25537089; http://dx.doi.org/ 10.1007/s13277-014-2978-6 [DOI] [PubMed] [Google Scholar]
- 28.Forristal CE, Brown AL, Helwani FM, Winkler IG, Nowlan B, Barbier V, Powell RJ, Engler GA, Diakiw SM, Zannettino AC. Hypoxia inducible factor (HIF)-2α accelerates disease progression in mouse models of leukemia and lymphomabut is not a poor prognosis factor in human AML. Leukemia 2015. October; 29(10):2075-85; PMID:25921247; http://dx.doi.org/ 10.1038/leu.2015.102 [DOI] [PubMed] [Google Scholar]
- 29.Chen P, Jiang X, Huang HF, Yuan Q, Wu JY, Guo YF, Chen YZ. Expression of HIF-1α in primary acute myeloid leukemia cells and its relationship with prognosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2015. February; 23(1):19-23; PMID:25687039; http://dx.doi.org/ 10.7534/j.issn.1009-2137 [DOI] [PubMed] [Google Scholar]
- 30.Kang SH, Cho HT, Devi S, Zhang Z, Escuin D, Liang Z, Mao H, Brat DJ, Olson JJ, Simons JW. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res 2006. December 15; 66(24):11991-7; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1320 [DOI] [PubMed] [Google Scholar]
- 31.Medinger M, Passweg J. Angiogenesis in myeloproliferative neoplasms, new markers and future directions. Memo 2014; 7:206-210; PMID:25544863; http://dx.doi.org/ 10.1007/s12254-014-0142-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao H, Wang Z, Deng Z, Ren H, Li X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. Int J Clin Exp Med 2015. June 15; 8(6):8948-57; PMID:26309547 [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Fang Q, Chen S, Zhe N, Chai Q, Yu M, Zhang Y, Wang Z, Wang J. Heme oxygenase-1 suppresses the apoptosis of acute myeloid leukemia cells via the JNK/c-JUN signaling pathway. Leuk Res 2015. May; 39(5):544-52; PMID:25828744; http://dx.doi.org/ 10.1016/j.leukres.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 34.Sriskanthadevan S, Jeyaraju DV, Chung TE, Prabha S, Xu W, Skrtic M, Jhas B, Hurren R, Gronda M, Wang X, et al.. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidativement abolic stress. Blood 2015. March 26; 125(13):2120-30; PMID:25631767; http://dx.doi.org/ 10.1182/blood-2014-08-594408 [DOI] [PMC free article] [PubMed] [Google Scholar]