ABSTRACT
Transmembrane tumor necrosis factor-α (tmTNF-α) is known to induce the activation of NF-κB to protect tumor cells. Upregulation of tmTNF-α leads to resistance to apoptosis and induces drug resistance in breast cancer. However, the expression of tmTNF-α in colorectal cancer (CRC) and its association with clinical outcome in CRC have remained unclear. In this study, we examined the tmTNF-α expression in CRC by immunohistochemistry and western blotting, assessed the prognostic value of tmTNF-α related to the recurrence/metastasis and survival of stage II/III CRC by the Kaplan-Meier survival curve and Cox regression model, and also explored the role of tmTNF-α expression on the chemotherapeutic efficacy of 5-Fluorouracil by flow cytometry assay and cell counting kit-8 (CCK-8) in vitro. Overall, we found that 77 (78.6%) out of 98 patients exhibited higher tmTNF-α expression in the CRC tissues comparing with the adjacent tissues. The tmTNF-α expression was correlated with Differentiation (P = 0.019), TNM stage (P = 0.039), Lymph nodes metastasis (P = 0.024) and Lymphovascular invasion (P = 0.027) but not related with Age (P = 0.617), Gender (P = 0.625), Tumor location (P = 0.138), Perforation/Obstruction (P = 1.000), Depth of invasion (P = 0.327), and microsatellite instability status (P = 0.150). The prognostic analyses showed that high tmTNF-α expression patients was significantly associated with decreased Disease-Free Survival (P = 0.0209) and Overall Survival (P = 0.0163). CCK-8 results suggested that the tmTNF-α influenced the chemotherapeutic effect of 5-Fluorouracil on colon cancer cells. Altogether, these data indicated the stageII/III CRC patients with high tmTNF-α expression were more likely to have a worse prognosis than patients with low tmTNF-α expression and tmTNF-α may influence the chemotherapeutic effect of 5-Fluorouracil. The mechanism for these observations warrants further study.
KEYWORDS: Colorectal cancer, tmTNF-α, recurrence/metastasis, survival, prognosis
Introduction
Colorectal Cancer (CRC) still remains a major health problem with cancer-related deaths despite remarkable improvements in patient survival achieved in recent decades in the world.1-3 Epidemiologic evidence suggests that to date still approximately 50% of CRC patients present distant metastases at diagnosis, and about 35% of the stage II-III patients who received curative resection have tumor recurrence within the first 3 y post-surgery.4-6 Widely known, the TNM staging is still exclusively the gold standard to judge prognosis and adjuvant chemotherapy, but the Disease-Free Survival (DFS) and Overall Survival (OS) may be completely different in tumor with the same TNM stage. Therefore, the most accurate prognostic information should be provided by the combination of clinical pathology and molecular grading. As yet, few have been proven adequate enough although many efforts have been made to find molecular markers to identify the risk degree of disease and to guide personalized therapy.
Tumor necrosis factor-α (TNF-α), mainly produced in activated T cells, monocytes/macrophages and NK cells, is a pleiotropic cytokine that participates in chronic inflammation and plays an important role in tumor progression.7-10 TNF-α exists in 2 biological forms, namely a 26 kD transmembrane TNF-α (tmTNF-α) and a 17 kD secreted TNF-α (sTNF-α). TmTNF-α is the precursor of sTNF-α. Increasing studies indicate that tmTNF-α mediate a broader tumorcidal spectrum than sTNF-α, because it can kill the tumor cells tolerant to sTNF-α by mainly inducing apoptosis of target cells. It has been documented that tmTNF-α as a bidirectional cytokine can promote but also inhibits tumor growth.11-14 On one hand, tmTNF-α as a highly expressed receptor can induce the activation of NF-κB, and protect tumor cells through the reverse signaling. On the other hand, tmTNF-α as a ligand binding with relevant receptors can down-regulate the expression of NF-κB and induce the apoptosis of tumor cells through the positive signaling, which makes tmTNF-α as a potential target for cancer therapy.12,15 Studies revealed that tmTNF-α can enhance tumor cells to the sensitivity of apoptosis, and induce Fas/FasL to inhibit the tumor growth.16 Subsequent results suggested that the breast cancer cells with high tmTNF-α could resist to apoptosis and induce drug-resistance12,17,18 Furthermore, it was found that tmTNF-α levels were increased in inflammatory bowel disease (IBD) patients,19 blocking the TNF-α could weaken tissue damage in chronic IBD20,21 and reduce the occurrence of CRC associated with chronic colitis,22 suggesting tmTNF-α may play an important role in the malignant transformation of chronic colitis developing into CRC. Nevertheless, little is known about the tmTNF-α expression in CRC and the relationship with its status and the clinical characteristics.
In this study, we aimed to analyze the expression level of tmTNF-α in human CRC, assess the correlates of tmTNF-α expression with MSI status and other clinical-pathological factors, and then comprehensively evaluate its prognostic value related to Recurrence/Metastasis and Survival of stageII/III CRC; Finally, we explored the influence of tmTNF-α expression on the chemotherapeutic effect of 5-fluorouracil (5-FU) in vitro.
Subjects and methods
Patient selection and clinical pathological materials
Data were obtained from the chart reviews of stageII-III CRC patients in the Department of General Surgery, from Jan. 2009 to Jan. 2010 of the First affiliated Hospital of Zhengzhou University. All patients underwent successful tumor curative resection with pathologically confirmed negative margins and regional lymph node dissection. The TNM classification was based on the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC). The stage, grade and histological types were defined after preoperative diagnostic imaging and histopathologic examinations, and subgroups were constructed and compared. Only patients with available tissue specimens from adjuvant therapy trials of 5-FU with levamisole were included in our analysis, and therefore, none of the study patients received oxaliplatin or others as adjuvant therapy. Patients in the study group had no history of hereditary familial CRC and other cancer or critical chronic disease. According to the inclusion criteria, 98 CRC cases with the integral clinic-pathologic data and routine pathological tissue sections were enrolled. Clinical materials were collected from the case management system in the medical record room of our hospital. CRC recurrence data were prospectively collected and recorded in the study. Median follow-up on living patients was 5 y. The informed consents of 98 cases with CRC were obtained from the patients or their families. The study was approved by the Ethics Committee of the First affiliated Hospital of Zhengzhou University and strictly followed the principle of the Helsinki declaration and no damaging the interests of the patients.
Microsatellite instability (MSI) testing
Tumor specimens from 98 patients were analyzed for the MSI status. DNA was extracted from 10% formalin-fixed paraffin-embedded tissue blocks that best represented each tumor. Extracted DNA was amplified by immune fluorescence PCR technology with a set of 5 standardized microsatellite markers (BAT25, BAT26, D2S123, D5S346 and D17S250) recommended by the National Cancer Institute reference, and then was analyzed by GeneMapper software to determine the MSI status. Tumors MSI instability with ≥ 2 markers were defined as MSI-high (MSI-H), whereas those with 1 marker or showing no instability were defined as MSI-low (MSI-L) and microsatellite stable (MSS), respectively.23,24
IHC analysis of tmTNF-α expression
To validate tmTNF-α expression in the cancer tissues of CRC and to determine its exact localization, IHC was performed using standard staining procedures. All the cases were confirmed by way of histopathology, no distant metastasis, and no chemotherapy before operation. We performed IHC staining using the CRC specimens with the surgical resection, Formalin fixation and paraffin embedding, and drawn the regions enriched of tumor cells (> 50% tumor cells).
After review of the hematoxylin-eosin stained slides of all surgical specimens, one representative paraffin block was selected from each case. Typical tumor tissues were present in all selected blocks. Successive sections at 4 μm intervals from each block were used for evaluation. The mouse anti-human tmTNF-α monoclonal antibody (homemade) was used for IHC staining. IHC staining was carried out with the streptavidin peroxidase complex method, as previously described.25 We also collected tissues of 43 Gastric Cancer (GC) cases were used as parallel control, which was performed completely consistent with the conditions of the experimental group (including temperature, humidity, equipment, reagents, and operators) to prevent the accidental error. Each stained section was blindly evaluated by 2 investigators unaware of the clinical information. In each immunohistochemistry run, the positive section provided by regent company served as positive control and omission of the primary antibody served as negative control. The percentages of cells with brown-staining indicating the presence of tmTNF-α were determined. The positive cell ratio (integral optical density value/integral area) was calculated by Qianping Image Analysis Software.
Protein extraction and western blotting analysis
Directly following surgical removal and routine pathological evaluation, tissue samples were snap frozen in liquid nitrogen and stored at −80°C. The homogenized CRC samples were lysed in RIPA lysis buffer with 1 mM PMSF and 1× protease inhibitor cocktail (Roche Diagnostics Ltd., Burgess Hill, UK). Cool on ice for 30 minutes, and intermittently oscillate for 15 s each 10 min. Lysates were centrifuged at 12000 g for 30 minutes at 4°C. The protein concentration of the supernatants was determined by the BCA Protein Assay kit (P0012, Beyotime, China). The supernatant was transferred to a fresh eppendorf tube and then subjected to 15% SDS-PAGE and Western blotting analysis followed. Primary antibodies (specific mouse anti-human tmTNF-α) was diluted with the ratio of 1:1000, and anti- mouse (H+L) HRP diluted 1:5000 was the secondary antibody. Equal protein loading was confirmed by mouse anti-humanβ-Actin McAb. To remove bound antibody, membranes were incubated in BSA blocking buffer, drenched thoroughly with stripping buffer (0.1 M glycine, PH 2.0;1% SDS). The enhanced chemiluminescence (ECL) from NEN LIFE Science was used to visualize the antibody reaction. Bands were quantified by a celibrated imaging densitometer (GS-710; Bio-Rad) and analyzed by “Quantity One” software (Bio-Rad). The samples were analyzed in triplicate.
Cell culture
The human CRC cell lines DLD-1, HT-29, LOVO and SW480 were obtained from the American Type Culture Collection (Rockville, MD). The CRC cells were maintained in T-150 flasks (Corning) in a humidified, 37°C incubator in an atmosphere of 90% air/ 10% CO2. The stock medium was DMEM (GIBCO) containing 5% pyrogen-free fetal calf serum (FCS, Sijiqing), 100U/mL penicillin, 100 μg/mL streptomycin, and 2.0 mmol/L L-glutamine. Fresh vials of cells were periodically thawed and used in experiments to ensure that cell lines were unchanged in culture during the study. The stock was passaged weekly at 1:2 and fed 3 times per week. Passages 17–21 were used for the experiments. The cells were routinely checked for mycoplasma and always found to be negative. The cells were kept for FACS and CCK-8 analysis followed.
Fluorescence-activated cell sorting
Fluorescence-activated cell sorting (FACS) was carried out chiefly to analysis the expression of tmTNF-α on the surface of human CRC cell lines DLD-1, HT-29, LOVO and SW480. Cells were detached with trypsin-EDTA (Invitrogen) and resuspended in PBS containing 1% BSA and 0.1% sodium azide. The samples were incubated for 1 hour on ice with tmTNF-α mAb (homemade) or the isotype antibody as negative control. After washing with cold PBS, cell staining was carried out by one hour for incubation with fluorescein isothiocyanate (FITC)-labeled anti-murine immunoglobulin G (Beijing Comwin Biotech Co., Ltd.). Stained cells were analyzed on a FACSCalibur 440 E (Becton Dickinson) using Cell Quest software (BD Biosciences Immunocytometry Systems).
The cell viability assay
The cell counting kit-8 (CCK-8, Dojindo Laboratories, Tokyo, Japan) assay was adopted to detect the killing effect of the chemotherapy drug 5-FU on the 4 colon cancer cell lines. The chemotherapy drug 5-FU was perchased from Haimen pharmaceutical factory, Zhejiang. Cells were seeded at the concentration of 4 × 103 cells/well in 96-well plates with FBS-reduced media (0.5% FBS). After adherence, the cells were stimulated with 5-FU (0–50 μg/ml ) in different concentrations for 48 h. At the end of the exposure, 10 μl of CCK-8 was added to each well and further incubated at 37°C for 3 h. The absorbance of CCK-8 was detected at 450 nm by a microplate reader (Thermo). Three independent experiments were carried out. The IC50 represented the drug concentration resulting in 50% growth inhibition. Cell viability was expressed as a percentage of the control cell culture value. A control was performed in parallel to monitor the influence of DMEM medium on the assays. The viable cell number was proportional to the absorbance. All assays were performed in triplicate. The cell viability was calculated as follows:
Follow-up
None of the patients suffered major perioperative complications, and all were discharged from the hospital. The follow up was performed through the outpatient tracing reexamination and telephone counseling, began from the surgery of CRC radical resection, and the closing date of follow-up was January 31st, 2015. All patients were followed up for 5 years, and the median follow-up time was 35.24 months (range 5.62–58.00). If the follow-up was incomplete, patients or their families were contacted by telephone thereafter. DFS is defined as the date of palindromia or the date of death because of disease progressing since surgery, and OS is defined as the date of last follow-up since surgery or the date of death induced by any causes.
Statistical analysis
The measurement data were represented as the mean ± SD or median (interquartile range). The differences between groups were analyzed using one-way ANOVA test. Associations among categorical variables were assessed using Fisher's exact probability test or the χ2 test. OS and DFS were measured by the Kaplan-Meier method. The prognostic value of the 8 variables was tested by univariate analysis using the log-rank test. Multivariate Cox proportional hazard models were used to define the potential prognostic significance of individual parameter. For all comparisons, a P-value < 0.05 was considered to be statistically significant. All statistical analyses were performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism software version 5.0 (GraphPad, USA) for Windows.
Results
Analysis of tmTNF-α expression of CRC tissues by IHCand western blotting
In the IHC assay, the anti-tmTNF-α McAb (homemade) was used to detect tmTNF-α protein in cancer tissues and the adjacent tissues obtained from 98 CRC and 43–GC patients, respectively. Positive staining of tmTNF-α in tissues were showed on the surface membranes of cells, and we found that the expression is high (++ or +++) in CRC and GC, weak (+) in benign sections, and negative (-) in adjacent sections (Fig. 1A, B). The positive ratio was noted in 77 (78.6%) of 98 CRC tissues, and 30 (70.0%) of 43 GC tissues, while negative staining was noted in the remaining cases: 9 (10.6%) of 85 CRC adjacent tissues, and 7 (20.0%) of 35 GC adjacent tissues, indicating that the expression of tmTNF-α in cancerous section is significantly higher than the one in adjacent control sections (P < 0.05).
Figure 1.
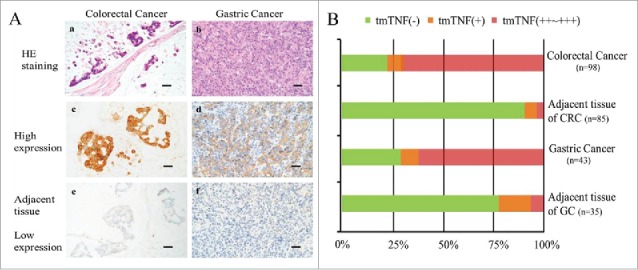
Different levels of tmTNF-α expression (brown) in human CRC and GC tissues and relevant adjacent mass by IHC assay. (A-a, b): the H&E staining for human CRC and GC tissues. (A-c, e): the high and low expression of tmTNF-α in CRC and adjacent tissues. (A d), f: the high and low expression of tmTNF-α in GC and adjacent tissues (parallel control). Bar indicates 50 μm (magnification, × 40). (B) the negative or positive (from + to ++∼+++) rate of tmTNF-α expression was analyzed for each case. The number of samples for each case was indicated in B.
To validate our finding, total proteins were extracted from 98 CRC and adjacent normal tissues, and Western blotting was performed to measure the levels of tmTNF-α proteins. TmTNF-α protein was significantly elevated in CRC tissues of stageII (3.15-fold increased) and stage III (5.6-fold increased) compared with adjacent control tissues (p < 0.001, Fig. 2A , 2B). Western blot analysis also revealed overexpression of tmTNF-α with 52 (53.1% ± 1.5%) of 98 CRC cases and 11 (12.9% ± 2.6%) in 85 control cases, respectively (Fig. 2C), and the trend of tmTNF-α protein expression is consistent with that in IHC assay (78.6% for CRC and 10.6% for control), which indicated that CRC tend to exhibit a higher level of tmTNF-α expression than normal tissue (P < 0.001).
Figure 2.
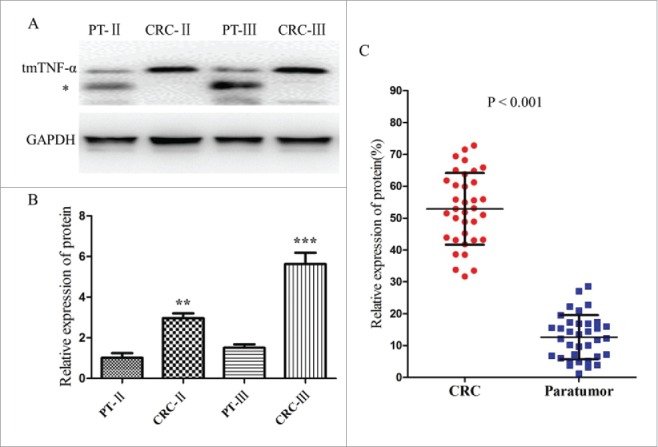
Different levels of tmTNF-α expression in human CRC and Paratumor (PT) tissues by Western blotting assay. (A) the electrophoresis on 15% PAGE of tmTNF-α from the paired CRC and PT tissues of stageIIand III, GAPDH was used as an internal control. The bands below may be the subunit sheared from tmTNF-α (*). (B) the relative ratio of tmTNF-α expression in CRC of stageIIor III was 3.15 or 5.6-fold than that of the PT tissue, respectively, and the difference were statistically significant (**, P < 0.05; ***, P < 0.001). (C) extracting tissue total protein of 35 cases of CRC and corresponding PT tissues selected randomly, Western blotting was performed to analysis the tmTNF-α expression, and the relative amount (%) of tmTNF-α of CRC tissues was obviously higher than that of the corresponding PT. The relative values of all results were determined and expressed as mean ± SD of 3 experiments induplicate (P < 0.001).
Correlation between tmTNF-α expression and clinicopathological and molecular characteristics in CRC
The demographics and histopathological characteristics were summarized in Table 1. The 98 patients with 50 men and 48 women (22–81 y of age, the median age of 57.1 years) were pathologically confirmed stage II (n = 65) and stage III (n = 33) primary CRC. In the degree of differentiation, 23 cases of poor differentiation, and 75 cases of moderate and well differentiation. Of the 98 patients, 77/98 (78.6%) were with high tmTNF-α expression, and the 21/98 (21.4%) were with low tmTNF-α expression. For the MSI testing, there were 24 (24.5%) cases of MSI-H, 59 cases of MSI-L, 15 cases of MSS. Among them, BAT25 was 34.7% (34/98), BAT26 was 42.9% (42/98), D2S123 was 18.4% (18/98), D5S346 was 20.4% (20/98), and D17S250 was 16.3% (16/98). In the all, there was no significant correlation between tmTNF-α protein expression and patients' age (P = 0.617), gender (P = 0.625), tumor location (P = 0.138), perforation/obstruction (P = 1.000), depth of invasion (P = 0.327), and MSI status (P = 0.150), but was closely correlated with tumor differentiation (P = 0.019), TNM stage (P = 0.039), lymph nodes metastasis (P = 0.024) and lymphovascular invasion (P = 0.027).
Table 1.
Correlation between tmTNF-α expression and clinicopathological and molecular characteristics in CRC.
| Cases | tmTNF-α expression |
|||
|---|---|---|---|---|
| Variable | (n = 98) | Low (n = 21) | High (n = 77) | P |
| Age (years) | 0.617 | |||
| Mean (SD) | 57.1 | 58.8 | 53.5 | |
| Median (range) | 22–81 | 23–80 | 21–79 | |
| <65 | 59(60.2%) | 14(66.7%) | 45(58.4%) | |
| ≥65 | 39(39.8%) | 7(33.3%) | 32(41.6%) | |
| Gender | 0.625 | |||
| Male | 50(51.0%) | 12(57.1%) | 38(49.4%) | |
| Female | 48(49.0%) | 9(42.9% | 39(50.6%) | |
| Tumor location | 0.138 | |||
| Proximal | 45(45.9%) | 13(61.9%) | 32(41.6%) | |
| Distal | 53(54.1%) | 8(38.1%) | 45(58.4%) | |
| Differentiation | 0.019 | |||
| Poor | 23(23.5%) | 9(42.9%) | 13(17.1%) | |
| Well/ intermediate | 75(76.5%) | 12(57.1%) | 63(82.9%) | |
| Perforation/Obstruction | 1.000 | |||
| No | 92(93.9%) | 20(95.2%) | 72(93.5%) | |
| Yes | 6(6.1%) | 1(4.8%) | 5(6.5%) | |
| TNM Stage | 0.039 | |||
| II | 65(66.3%) | 18(85.7%) | 47(61.0%) | |
| III | 33(33.7%) | 3(14.3%) | 30(39.0%) | |
| Depth of invasion | 0.327 | |||
| T1/T2 | 55(56.1%) | 14(66.7%) | 41(53.2%) | |
| T3/T4 | 43(43.9%) | 7(33.3%) | 36(46.8%) | |
| Lymph nodes metastasis | 0.024 | |||
| Negative | 42(42.9%) | 14(66.7%) | 28(36.4%) | |
| Positive | 56(57.1%) | 7(33.3%) | 49(63.6%) | |
| Lymphovascular invasion | 0.027 | |||
| Negative | 48(49.0%) | 15(71.4%) | 33(42.9%) | |
| Positive | 50(28.6%) | 6(28.6%) | 44(57.1%) | |
| MSI status | 0.150 | |||
| MSI-H | 24(24.5%) | 8(38.1%) | 16(20.8%) | |
| MSI-L/MSS | 74(75.5%) | 13(61.9%) | 61(79.2%) | |
High tmTNF-α expression predicted poor prognosis of CRC patients
To investigate the prognostic value of tmTNF-α, we used Kaplan-Meier 5-year survival curve to show different clinical outcomes between low and high tmTNF-α expression of CRC patients. During the follow-up period, 25 patients had relapse and metastasis, and 19 patients died. Among the 98 cases, there were 21 cases with low tmTNF-α expression, 1 case relapsed, and none died; 77 cases with high tmTNF-α expression, 24 cases relapsed, and 19 cases died (P = 0.012 for Recurrence, P = 0.01 for Death, Table 2). As shown in Fig. 3, high levels of tmTNF-α expression were significantly associated with poor OS and DFS. The 5-year OS and DFS were 77.2% and 78.3%, respectively for low tmTNF-α expression patients, while the high tmTNF-α patients had 5-year OS and DFS of 66.5% and 64.1%, respectively (P = 0.0163 for OS, and P = 0.0209 for DFS). Hence tmTNF-α expression seemed to be a useful predictive factor for prognosis of CRC patients.
Table 2.
Univariate Cox Regression Analysis of clinicopathologic features and tmTNF-α status in CRC.
| Recurrence/Metastasis |
Death |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Total NO. | Cases | χ2 | P | Cases | χ2 | P |
| Age | |||||||
| <65 | 59 | 9(15.3%) | 8.206 | 0.008 | 7(11.9%) | 5.369 | 0.035 |
| ≥ 65 | 39 | 16(41.0%) | 12(30.8%) | ||||
| Gender | |||||||
| Male | 50 | 14(28.0%) | 0.333 | 0.646 | 8(16.0%) | 0.750 | 0.449 |
| Female | 48 | 11(22.9%) | 11(22.9%) | ||||
| Tumor location | |||||||
| Proximal | 45 | 9(16.4%) | 2.9 | 0.112 | 8 17.8% | 0.138 | 0.801 |
| Distal | 53 | 16(30.2%) | 11 20.8% | ||||
| Differentiation | |||||||
| Poor | 23 | 10(43.5%) | 5.106 | 0.031 | 9 39.1% | 7.495 | 0.013 |
| Well/ intermediate | 75 | 15(20.0%) | 10 13.3% | ||||
| Perforation/Obstruction | |||||||
| No | 92 | 24(26.1%) | 0.263 | 1.000 | 19(20.7%) | 1.537 | 0.593 |
| Yes | 6 | 1(16.7%) | 0(0.0%) | ||||
| TNM Stage | |||||||
| II | 65 | 12(18.5%) | 5.047 | 0.03 | 8(12.3%) | 6.191 | 0.017 |
| III | 33 | 13(39.4%) | 11(33.3%) | ||||
| Depth of invasion | |||||||
| T1/T2 | 55 | 11(20.0%) | 2.003 | 0.170 | 9(16.4%) | 2.367 | 0.180 |
| T3/T4 | 43 | 14(32.6%) | 10(30.3%) | ||||
| Lymph nodes metastasis | |||||||
| Negative | 42 | 7(16.7%) | 3.025 | 0.103 | 5(11.9%) | 2.633 | 0.127 |
| Positive | 56 | 18(32.1%) | 14(25.0%) | ||||
| Lymphovascular invasion | |||||||
| Negative | 48 | 7(14.6%) | 5.911 | 0.020 | 5(10.4%) | 4.845 | 0.040 |
| Positive | 50 | 18(36.0%) | 14(28.0%) | ||||
| tmTNF-α expression | |||||||
| High | 77 | 24(31.2%) | 6.055 | 0.012 | 19(24.7%) | 6.428 | 0.01 |
| Low or deficiency | 21 | 1(4.8%) | 0(0%) | ||||
| MSI status | |||||||
| MSI-H | 24 | 2(8.3%) | 4.935 | 0.031 | 1(4.2%) | 4.712 | 0.036 |
| MSI-L/MSS | 74 | 23(31.1%) | 18(24.3%) | ||||
Figure 3.
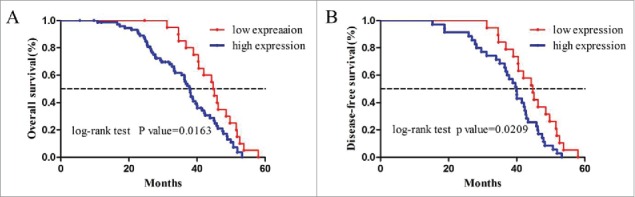
Kaplan-Meier survival curves of 98 patients with CRC. (A) OS for high and low tmTNF-α expression patients; (B) DFS for high and low tmTNF-α expression patients. Patients with high tmTNF-α expression have more favorable OS and DFS (P = 0.0163 for OS and P = 0.0209 for DFS).
In addition, to assess whether tmTNF-α could be used as an independent prognostic factor or not, the Cox proportional hazards model analysis was performed (Table 2 and 3). Firstly, the univariate analyses indicated that the extent of differentiation (P = 0.031), age (P = 0.008), TNM stage (P = 0.030), and lymphovascular invasion (P = 0.034), MSI status (P = 0.028) and tmTNF-α expression (P = 0.012) were risk factors for Recurrence/Metastasis in CRC, and also the same for Survival: the extent of differentiation (P = 0.013), age (P = 0.035), TNM stage (P = 0.017), and lymphovascular invasion (P = 0.017), MSI status (P = 0.015) and tmTNF-α expression (P = 0.010), as shown in Table 2.
Table 3.
Multivariate Cox Regression Analysis of Recurrence/Metastasis and Survival in CRC.
| Recurrence/Metastasis |
Survival |
|||||
|---|---|---|---|---|---|---|
| Variable | sig. | HR | 95%CI | sig. | HR | 95%CI |
| Age | 0 | 1.021 | 1.010–1.031 | 0.009 | 1.014 | 1.003–1.024 |
| TNM Stage | 0.002 | 1.582 | 1.182–2.118 | 0.002 | 0.507 | 0.331–0.775 |
| Lymphovascular invasion | 0.002 | 1.303 | 1.098–1.545 | 0.015 | 0.774 | 0.630–0.951 |
| tmTNF-α expression | 0.001 | 2.189 | 1.537–3.119 | 0.002 | 1.681 | 1.208–2.341 |
| MSI status | 0.031 | 2.208 | 1.031–4.094 | 0.027 | 0.996 | 0.536–2.185 |
| Differentiation | 0.104 | 1.309 | 0.946–1.810 | 0.130 | 1.186 | 0.892–1.547 |
Further Multivariate analysis was performed for all of the significant variables in the Univariate analysis. The results showed that the expression extent of tmTNF-α (P = 0.001, P = 0.002), age (P = 0.000, P = 0.009), TNM stage (P = 0.002, P = 0.002), and lymphovascular invasion (P = 0.002, P = 0.015), and MSI status (P = 0.031, P = 0.027), but not differentiation (P = 0.104, P = 0.130), were significantly correlated with Recurrence/Metastasis and Survival, respectively. The results documented that tmTNF-α may be a prognostic factor for Recurrence/Metastasis and Survival in CRC patients of stage II and III (Table 3).
TmTNF-α expression influence the chemotherapeutic effect of 5-FU
To analyze the influence of tmTNF-α expression on the chemotherapeutic effect of 5-FU in vitro, the tmTNF-α expression on DLD-1, HT-29, LOVO, and SW480 cells were firstly detected by FACS assays. As shown in Fig. 4(A, B), the positive expression ratio of tmTNF-α was 13.1% for DLD-1, 5.3% for HT-29, 50.5% for LOVO, and 30.1% for SW480, respectively. It indicated that the highest expression of tmTNF-α is in LOVO cells, and the lowest in HT-29 cells, which providing the basis for the CCK-8 experiment. Therefore, LOVO and HT-29 cells were chose for following cytotoxic experiments.
Figure 4.
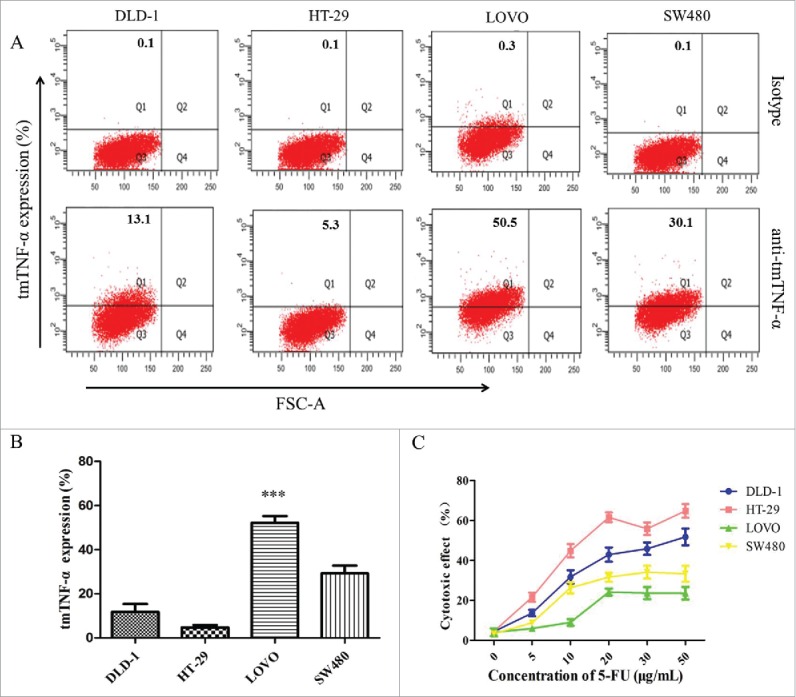
TmTNF-α expression and the cytotoxic effect of 5-FU to human colon cancer cell lines. (A) the tmTNF-α McAb was used in FACS to detect tmTNF-α expression in different colon cancer cell lines (DLD-1, HT-29, LOVO, SW480), and a mouse anti-human IgG McAb served as the isotype. (B) the ratio of tmTNF-α expression was analyzed for each cell line. (C) the cytotoxic effect of chemotherapy drugs 5-FU on human colon cancer cell lines with different doses for 48 hours, and the cell viability was detected by CCK-8 assay. The data represent the mean ± SD of 3 independent experiments. ***, p < 0.001, versus control group.
Next, we explored whether tmTNF-α expression influenced the chemotherapy effect of 5-FU on colon cancer cell lines. As shown in Fig. 4C, a 2-day treatment with 5-FU (0–50 μg/ml) led to a maximal killing effect in 48 h. Cytotoxic effect of the drug was observed in these cells with 0% assigned for the control cells. The increasing drug concentration had increased cell death from 0% to 61.6%. Notably, the cytotoxic effect of 5-FU showed the maximum at the concentration of 20 μg/ml: 61.6% for HT-29 cells, 42.9% for DLD-1 cells, 31.6% for SW480 cells and 24.1% for LOVO cells, respectively. Our study documented that the chemotherapeutic effect of 5-FU was poor when tmTNF-α expression increased, on the contrary, the chemotherapeutic effect of 5-FU was good when tmTNF-α expression decreased, indicating that tmTNF-α expression might influence the chemotherapeutic effect of 5-FU. The mechanism analysis for these observations warrants further study.
Discussion
CRC is a common malignancy in China. In recent years, the incidence of CRC has been steadily increasing. Surgery is still the only cure treatment of CRC,26 while postoperative adjuvant chemotherapy based on 5-FU can significantly help suppressing the symptoms, preventing tumor metastasis and prolonging the survival of patients at stage II and III CRC.27 In addition, the combination of oxaliplatin and others with 5-FU has effectively improved lifetime of patients, and is considered a standard chemotherapy for with metastatic CRC.28 Despite all these efforts, local recurrence or distant metastasis occurred in more than half of CRC patients with advanced stages, and the overall 5-year survival rate is still low.
Statistical survey indicates that the clinic-pathological indictors such as lymphovascular invasion, advanced TNM stage, lymph node metastasis, chemotherapy resistance and status of molecular markers may obviously related with early Recurrence/ Metastasis after CRC surgery.29-31 In our study, there was a statistically difference significant between tmTNF-α expression and Differentiation, TNM stage, Lymph nodes metastasis and Lymphovascular invasion, but there was not significantly difference with Age, Gender, Tumor location, Perforation/Obstruction, Depth of invasion, and MSI status. Furthermore, results showed that not differentiation and tumor invasion, but the tmTNF-α expression, age, TNM stage, lymphovascular invasion, and MSI status were significantly correlated with Recurrence/Metastasis and Survival, suggesting that tmTNF-α may be an independent prognostic factor in CRC patients of stage II and III. Some studies showed that the depth of tumor invasion was a significant prognostic factor of postoperative Recurrence/ Metastasis and Survival in CRC patients after curative resection.32,33 In our study, Depth of invasion was not correlated with postoperative relapse and survival in CRC. For MSI status, MSI-H was found in approximately 24.5% (24/98) cases in our CRC patients, which was slightly higher than the reported (15%-20%) in some researches.34,35 These data was contrary to some studies. The retrospective nature of their analysis, differences in inclusion criteria and different number of patients between studies may explain the differences.
In addition, the prognosis analysis declared the high levels of tmTNF-α expression were significantly associated with poor OS and DFS, but the OS survival was lower than the DFS in both the high and low tmTNF-α patients. There were several reasons. It had retrospective analysis and all the potential cases might be not captured. As this report only included patients having resections, it is possible that some of the patients not included died prior to resection or had metastatic disease that precluded resections. Otherwise, patients died of no tumor (other combined diseases or accident) were included and the outcome of the different histological typing of tissues was also not analyzed. And yet, other factors such as the prospective nature of their analysis and different number of patients were likewise contributed to the discrepancy between studies.36,37
At present, clinical diagnosis for high-risk CRC patients is based mainly on the pathological features and molecular markers with prognostic value. Research shows that MSI status is a molecular marker of favorable prognosis and may be used for outcome prediction, which was recommended to use for the patients with CRC treated with a single drug 5-FU.35 Although MSI status can guide current adjuvant chemotherapy, but the predictive value of MSI needs further studies to confirm.38,39 However, our study was a small sample study with not absolute randomness, and the patients with the minimal other diseases combined, 5-FU as adjuvant chemotherapy because of physical condition or other factors were enrolled. Moreover, the research is still in the analysis stage of clinical data, the regulation mechanism included had not been explored. In addition, the factors of the older population (57.1 y for mean), the insufficient evaluation indexes and so on may influence the objectivity of the conclusions. Consequently, to further expand the population and to sufficiently assess physiological function, life expectancy, social factors and risks and benefits related treatment of patients is still needed, which will be good for evaluating the effect of tmTNF-α on the prognosis of stage II and III CRC and improving the prognosis value of tmTNF-α.
Studies have certified that tumor tissues of breast cancer, ovarian cancer, liver cancer, lung cancer, gastric cancer, acute lymphoblastic leukemia, and lymphoma, but not adjacent tissues, strongly express tmTNF-α. Furthermore, stem cells from leukemia (such as the acute myeloid leukemia), but not normal peripheral blood cells, exhibit rich tmTNF-α expression as well.12,18,40-43 Recent studies have found the expression of tmTNF-α in 105 cases of invasive ductal carcinoma was about 75.24%, and it was significantly associated with tumor size and lymph node metastasis (p < 0.05); the positive rate of tmTNF-α expression in 24 cases (Her-ER-PR-) of breast cancer was 83.3%. It was further demonstrated that the cells of breast cancer and B lymphoma with high tmTNF-α could persistently activate NF- kappa B, and then promote tumor growth and metastasis through the reverse signal.12,18 The above experimental evidence indicated that tmTNF-α would contribute to tumor growth and development. Our study found that tmTNF-α expression was significantly increased in CRC and GC tissues compared with adjacent tissues, which supported that cancer tissues high expressed tmTNF-α protein.
We had observed that TmTNF-α expression might influence the chemotherapeutic effect of 5-FU by the cytotoxicity assay. Yet, the role of tmTNF-α in solid tumors has not been fully understood and our finding only partially unveil tmTNF-α may use as a molecular marker in CRC prognosis. In the future, we plan to prepare the tmTNF-α monoclonal antibody, and analyze the targeted therapy focused on the high tmTNF-α tumor. Hence, future studies and bigger study participants are required to validate our findings and elucidate the role of tmTNF-α in CRC.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Mingxia Yu for supporting the study.
Authors contributions
Conception and design: Xiaogai Li, Ming LiangDevelopment of methodology: Xiaogai Li, Shihai WangAcquisition of data (provided cell lines, acquired and managed patients, provided facilities, etc.): HuiJun Ren, Junfen Ma, Nan LiAnalysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Xiaogai Li, HuiJun Ren, Nan LiWriting, review, and /or revision of the manuscript: Xiaogai Li, HuiJun RenAdministrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Xiaogai Li, Shihai Wang, Kaida Huang, Min XuStudy supervision: Xiaogai Li, Ming Liang
Funding
This work was supported by the Hospital Fund of The First Affiliated Hospital of Zhengzhou University.
References
- 1.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64:1637-49; PMID:26041752; http://dx.doi.org/10.1136/gutjnl-2014-309086 (2015) [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014; 383(9927):1490-502; PMID:24225001; http://dx.doi.org/ 10.1016/S0140-6736(13)61649-9 [DOI] [PubMed] [Google Scholar]
- 3.Park SH, Song CW, Kim YB, Kim YS, Chun HR, Lee JH, Seol WJ, Yoon HS, Lee MK, Lee JH, et al.. Clinicopathological characteristics of colon cancer diagnosed at primary health care institutions. Intest Res 2014. April; 12(2):131-8; PMID:25349580; http://dx.doi.org/ 10.5217/ir.2014.12.2.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pectasides D, Karavasilis V, Papaxoinis G, Gourgioti G, Makatsoris T, Raptou G, Vrettou E, Sgouros J, Samantas E, Basdanis G, et al.. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer 2015; 15(1):384; PMID:25956750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res 2015; 75(2):245-9; PMID:25593032; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2240 [DOI] [PubMed] [Google Scholar]
- 6.Zhen YH, Liu XH, Yang Y, Li B, Tang JL, Zeng QX, Hu J, Zeng XN, Zhang L, Wang ZJ, et al.. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother 2015; 64:1083-93; PMID:25990075; http://dx.doi.org/10.1007/s00262-015-1715-3 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 2006; 25(3):409-16; http://dx.doi.org/10.1007/s10555-006-9005-3 (2006) [DOI] [PubMed] [Google Scholar]
- 8.Bertazza L, Mocellin S. The dual role of tumor necrosis factor (TNF) in cancer biology. Curr Med Chem 2010; 17(29):3337-52; PMID:20712570; http://dx.doi.org/ 10.2174/092986710793176339 [DOI] [PubMed] [Google Scholar]
- 9.Techasen A1, Namwat N, Loilome W, Bungkanjana P, Khuntikeo N, Puapairoj A, Jearanaikoon P, Saya H, Yongvanit P. Tumor necrosis factor-α (TNF-α) stimulates the epithelial-mesenchymal transition regulator Snail in cholangiocarcinoma. Med Oncol 2012; 29(5):3083-91; PMID:22903530; http://dx.doi.org/10.1007/s12032-012-0305-x (2012) [DOI] [PubMed] [Google Scholar]
- 10.Zhou JP, Gao ZL, Zhou ML, He MY, Xu XH, Tao DT, Yang CC, Liu LK. Snail interacts with Id2 in the regulation of TNF-α-induced cancer cell invasion and migration in OSCC. Am J Cancer Res 2015; 5(5):1680-91; PMID:26175937 [PMC free article] [PubMed] [Google Scholar]
- 11.Shi W, Li Z, Gong F. Comparison of the cytocidal effect induced by transmembrane and secreted TNF-alpha. Chin J Microbiol Immunol 1998; 18:499-504; http://dx.doi.org/10.1007/s11596-007-0201-3 (2007) [Google Scholar]
- 12.Zhang H, Yan D, Shi X, Liang H, Pang Y, Qin N, Chen H, Wang J, Yin B, Jiang X, et al.. Transmembrane TNF-alpha mediates “forward” and “reverse” signaling, inducing cell death or survival via the NF-kappaB pathway in Raji Burkitt lymphoma cells. J Leukoc Biol 2008; 84:789-97; PMID:18550789; http://dx.doi.org/10.1189/jlb.0208078 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edagawa M, Kawauchi J, Hirata M, Goshima H, Inoue M, Okamoto T, Murakami A, Maehara Y, Kitajima S. Role of activating transcription factor 3 (ATF3) in endoplasmic reticulum (ER) stress-induced sensitization of p53-deficient human colon cancer cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through up-regulation of death receptor 5 (DR5) by zerumbone and celecoxib. J Biol Chem 2014. August 1; 289(31):21544-61; PMID:24939851; http://dx.doi.org/ 10.1074/jbc.M114.558890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonen-Korkmaz C, Sevin G, Gokce G, Arun MZ, Yildirim G, Reel B, Kaymak A, Ogut D. Analysis of tumor necrosis factor α-induced and nuclear factor κB-silenced LNCaP prostate cancer cells by RT-qPCR. Exp Ther Med 2014; 8(6):1695-700; http://dx.doi.org/10.3892/etm.2014.2032 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Li B, Li X, Zhao X, Wan L, Lin G, Yu M, Wang J, Jiang X, Feng W, et al.. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol 2014; 192(3):1320-31; PMID:24379122; http://dx.doi.org/ 10.4049/jimmunol.1203195 [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou W, Gan H, Wang J, Jiang X, Yin B, et al.. Targeting transmembrane TNF-α suppresses breast cancer growth. Cancer Res 2013; 73(13):4061-74; PMID:23794706; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3946 [DOI] [PubMed] [Google Scholar]
- 17.Xiaogai Li, Mingxia Yu, Hongyan Chai, Sanyun Wu, Ji Shen, Xuan Jing, Liang Ming and Jiancheng Tu. Homer2 interrupting tmTNF-α effect mediated by NFAT1 increases the cytotoxicity of breast cancer cell. (in contribution). [Google Scholar]
- 18.Yan D, Qin N, Zhang H, Liu T, Yu M, Jiang X, Feng W, Wang J, Yin B, Zhang T, Zhou M, Li Z. Expression of TNF-alpha leader sequence renders MCF-7 tumor cells resistant to the cytotoxicity of soluble TNF-alpha. Breast Cancer Res Treat 2009; 116:91-102; PMID:18618239; http://dx.doi.org/ 10.1007/s10549-008-0111-5 [DOI] [PubMed] [Google Scholar]
- 19.Ocampo SM, Romero C, Aviñó A, Burgueño J, Gassull MA, Bermúdez J, Eritja R, Fernandez E, Perales JC. Functionally enhanced siRNA targeting TNFα attenuates DSS-induced colitis and TLR-mediated immunostimulation in mice. Mol Ther 2012; 20(2):382-90; PMID:22044934; http://dx.doi.org/ 10.1038/mt.2011.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol 2008; 126(2):121-36; PMID:17916444; http://dx.doi.org/ 10.1016/j.clim.2007.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev 2014; 13(1):54-8; PMID:24035809; http://dx.doi.org/ 10.1016/j.autrev.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 22.Yin Y, Chen X, Shu Y. Gene expression of the invasive phenotype of TNF-alpha-treated MCF-7 cells. Biomed Pharmacother 2009; 63(6):421-28; PMID:19564093; http://dx.doi.org/ 10.1016/j.biopha.2009.04.032 [DOI] [PubMed] [Google Scholar]
- 23.Lech G, Słotwiński R, Słodkowski M, Krasnodębski IW. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol 2016; 22(5):1745-55; PMID:26855534; http://dx.doi.org/ 10.3748/wjg.v22.i5.1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinicrope FA, Sargent DJ. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res 2012; 18:1506-12; PMID:22302899; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasui K, Takatsuka T, Sakamoto R, Su L, Matsushita S, Tsuyama S, Izumo S, Murata F. Improvement of supersensitive immunohistochemistry with an autostainer: a simplified catalysed signal amplification system. Histochem J 2002; 34(5):215-22; PMID:12587998; http://dx.doi.org/ 10.1023/A:1021785328984 [DOI] [PubMed] [Google Scholar]
- 26.Tejani MA, ter Veer A, Milne D, Ottesen R, Bekaii-Saab T, Benson AB 3rd, Schrag D, Shibata S, Skibber J, Weiser M, et al.. Systemic therapy for advanced appendiceal adenocarcinoma: an analysis from the NCCN Oncology Outcomes Database for colorectal cancer. J Natl Compr Canc Netw 2014; 12(8):1123-30; PMID:25099444 [DOI] [PubMed] [Google Scholar]
- 27.Chee CE, Meropol NJ. Current status of gene expression profiling to assist decision making in stage II colon cancer. Oncologist 2014; 19(7):704-11; PMID:24869929; http://dx.doi.org/ 10.1634/theoncologist.2013-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducreux M, Adenis A, Pignon JP, François E, Chauffert B, Ichanté JL, Boucher E, Ychou M, Pierga JY, Montoto-Grillot C, et al.. Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur J Cancer 2013; 49(6):1236-45; PMID:23352604; http://dx.doi.org/ 10.1016/j.ejca.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 29.Nodin B, Johannesson H, Wangefjord S, O'Connor DP, Lindquist KE, Uhlén M, Jirström K, Eberhard J. Molecular correlates and prognostic significance of SATB1 expression in colorectal cancer. Diagn Pathol 2012; 7:115; PMID:22935204; http://dx.doi.org/10.1186/1746-1596-7-115 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozkurt O, Inanc M, Turkmen E, Karaca H, Berk V, Duran AO, Ozaslan E, Ucar M, Hacibekiroglu I, Eker B, et al.. Clinicopathological characteristics and prognosis of patients according to recurrence time after curative resection for colorectal cancer. Asian Pac J Cancer Prev 2014; 15(21):9277-81; PMID:25422212; http://dx.doi.org/ 10.7314/APJCP.2014.15.21.9277 [DOI] [PubMed] [Google Scholar]
- 31.Tsai HL, Chu KS, Huang YH, Su YC, Wu JY, Kuo CH, Chen CW, Wang JY. Predictive factors of early relapse in UICC stage I-III colorectal cancer patients after curative resection. J Surg Oncol 2009; 100(8):736-43; PMID:19757443; http://dx.doi.org/ 10.1002/jso.21404 [DOI] [PubMed] [Google Scholar]
- 32.Wu HW, Gao LD, Wei GH. hMSH2 and nm23 expression in sporadic colorectal cancer and its clinical significance. Asian Pac J Cancer Prev 2013; 14(3):1995-8. [DOI] [PubMed] [Google Scholar]
- 33.Setaffy L, Langner C. Microsatellite instability in colorectal cancer: clinicopathological significance. Pol J Pathol 2015; 66(3):203-18. Review; PMID:26619098 [DOI] [PubMed] [Google Scholar]
- 34.Hong SP, Min BS, Kim TI, Cheon JH, Kim NK, Kim H, Kim WH. The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer 2012; 48(8):1235-43; PMID:22071131; http://dx.doi.org/ 10.1016/j.ejca.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 35.Xiang J, Fang L, Luo Y, Yang Z, Liao Y, Cui J, Huang M, Yang Z, Huang Y, Fan X, et al.. Levels of human replication factor C4, a clamp loader, correlate with tumor progression and predict the prognosis for colorectal cancer. J Transl Med 2014; 12:320; PMID:25407051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piccolo E, Tinari N, D'Addario D, Rossi C, Iacobelli V, La Sorda R, Lattanzio R, D'Egidio M, Di Risio A, Piantelli M, et al.. Prognostic relevance of LGALS3BP in human colorectal carcinoma. J Transl Med 2015; 13:248; PMID:26219351; http://dx.doi.org/10.1200/jco.2009.27.1825 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, et al.. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010; 28(20):3219-26; PMID:20498393; http://dx.doi.org/ 10.1200/JCO.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer 2009; 45(10):1890-6; PMID:19427194; http://dx.doi.org/ 10.1016/j.ejca.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 39.Moura AS, Carmo RA, Teixeira AL, Rocha MO. Soluble inflammatory markers as predictors of hepatocellular damage and therapeutic response in chronic hepatitis C. Braz J Infect Dis 2009; 13(5):375-82; PMID:20428640; http://dx.doi.org/ 10.1590/S1413-86702009000500013 [DOI] [PubMed] [Google Scholar]
- 40.Diamantopoulos AP, Larsen AI, Omdal R. Is it safe to use TNF-α blockers for systemic inflammatory disease in patients with heart failure? Importance of dosage and receptor specificity. Int J Cardiol 2013; 167(5):1719-23; PMID:23245690; http://dx.doi.org/ 10.1016/j.ijcard.2012.11.112 [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Li L, Shi W, Jiang X, Xu Y, Gong F, Zhou M, Edwards CK 3rd, Li Z. Mechanism of action differences in the antitumor effects of transmembrane and secretory tumor necrosis factor-alpha in vitro and in vivo. Cancer Immunol Immunother 2006; 55(12):1470-9; PMID:16555058; http://dx.doi.org/ 10.1007/s00262-006-0150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Zhou S, Li B, Li Q, Gao L, Li D, Gong Q, Zhu L, Wang J, Wang N, et al.. Transmembrane TNF-α preferentially expressed by leukemia stem cells and blasts is a potent target for antibody therapy. Blood 2015; 126:1433-42; PMID:26224647; http://dx.doi.org/10.1182/blood-2015-01-624833 (2015) [DOI] [PubMed] [Google Scholar]


