Abstract
Background and aims
The aim of this study was to investigate the value of serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA 19-9) correlated with some tissue molecules as predictive markers for recurrence in colon cancer.
Methods
A total of 30 patients diagnosed with colon cancer stage II or III who underwent optimal surgery were enrolled in study. Tumor markers CEA and CA 19-9 were determined before surgery. Tumor samples were prepared using tissue microarray kit (TMA) then stained for different cellular markers (Ki 67, HER2, BCL2, CD56, CD4, CD8) and analyzed using Inforatio programme for quantitative determination. All patients received standard adjuvant treatment, which consisted of eight cycles chemotherapy type XELOX. The patients were followed up for 3 years.
Results
Upon 3 years follow-up, 67% of patients developed tumor relapse, the most common site of metastasis being the liver. No correlations were observed between either serum or tissue tumor markers and the risk of tumor relapse.
Conclusion
Over 50% of patients with colon cancer who had optimal treatment developed metastasis. No statistically significant predictive value for investigated molecules was found. Future studies are needed to confirm the use of molecular markers in monitoring patients with colorectal cancer
Keywords: colon cancer, chemotherapy, predictive markers
Introduction
Colorectal cancer represents a major public health problem, being the second cause of death in the world [1]. Depending on histology, tumor size and tumor invasion, The American Joint Committee on Cancer classify colon tumors as follows: stage I which consist of tumor with T1-2 size, N0, M0; stage II with T3-T4, N0, M0; stage III with T1-4, N1-2, M0 and stage IV with any T, any N, M1 [2]. The treatment of stages I, II, III is surgery with or without any adjuvant chemotherapy whereas stage IV is, usually, treated by chemotherapy alone. Even if the survival of patients with colon cancer has improved in the last years with the introduction of the adjuvant treatment, over of 50% of patients will develop metastasis within the next 5 years [3].
There are no specific molecules to predict the risk of tumor relapse after curative surgery. Clinico-pathological features do not provide powerful prognostic information regarding the aggressiveness of the tumor. The microsatellite instability and the effect of chromosome 18q21 loss of heterozygosity (LOH) were studied in adjuvant setting, being correlated with tumor response to chemotherapy and survival [4,5]. In addition several germline polymorphisms had been associated with different degrees of risk of tumor relapse. Using modern biological molecular techniques such as microarray, a large number of genes have been found to be associated with recurrence risk of the tumor [6,7]. The major limits of these approaches remain to be standardized and applied on large populations.
The present study attempts to identify specific molecules that can be used in monitoring relapses in patients with colorectal cancer stage II and III who underwent surgery of primary tumor, followed by standard chemotherapy.
Patients and methods
Patient population
This retrospective study was performed on a total of 30 patients who had curative surgery for colon cancer stage II and III at “Prof. dr. Ion Chiricuta” Oncology Institute in Cluj-Napoca between 2013 and 2014. All the patients enrolled in this study were confirmed with the diagnosis of colorectal carcinoma through proctoscopy or colonoscopy with biopsy. The stage of disease was established using imaging evaluations (computer tomography) before surgery.
Methods
Tumor markers CEA and CA 19-9 were measured in the serum of patients before surgery using a commercially available immunometric assay kit. The upper limit of normal for CEA was 4 U/mL and 37 U/mL for CA19-9.
Tumor samples were prepared using tissue microarray kit (TMA) then stained for different cellular markers (Ki 67, HER2, BCL2, CD56, CD4, CD8) and analyzed using Inforatio programme.
Protocol: all tissues were fixed in neutral buffered 10% formalin. The slides were examined by the pathologist to mark areas of interest. Each region was assigned a number, so it can be identified given the case number and block designation. Using a hollow needle 0.6 mm diameter tissue cores were removed from the marked regions of the donor block. Then they were inserted in a recipient paraffin block in a coordinate recorded in Microsoft Excel. Finally the recipient block was baked in 42 °C for 40 minutes with sectioning the area facing down on a glass slide. Then the block was placed on a cooling plate for 10 minutes. Using a microtome, 4 μm thick sections were cut to place the slides in a holder for drying at room temperature (RT) overnight.
The slides were all incubated at RT, then rinsed in wash buffer and incubated with Ultra V Block 5 minutes. Afterwards, they were rinsed in wash buffer 2 times and incubated with the antibodies used for the IHC staining for 30 minutes. Then rinsed in wash buffer 3 times, followed by 30 minutes incubation with peroxidase-polymer and rinsed in wash buffer 2 times. Developing in Diaminobenzidine solution for 5 minutes was next, along with rinse in distilled water. The next step, counterstaining in hematoxylin 5 minutes was followed by rinse in tap water, rinse in lithium carbonate water diluted 1:5 from standard solution for 1 minute and rinse in tap water for 5 minutes. The slides were dehydrated in graded ethanol coverslipped and analyzed using Inforatio programme for quantitative determination.
Study assessment
All patients had the same regiment of adjuvant chemotherapy (8 cycles Xelox) with 3 years follow-up for relapse. Each cycle comprised a two-hour infusion of 85 mg/m2 of oxaliplatin (Eloxatin, Sanofi-Synthelabo) on day 1 followed by Capecitabine 2500 mg/mp days 1–14. The patients were follow-up according to standard recommendation using imaging methods and serum amounts of antigen markers CEA and CA19-9 for 3 years [8].
The values of both serum antigen markers measured before surgery and tissue molecules were studied to predict the risk of tumor relapse.
Statistical methods
For each study patient correlations were established between serum tumor markers, tissue molecules and tumor relapse confirmed by imaging evaluation with or without increase of tumor antigen serum value. These correlations were assessed using Student’s t-test and Fisher’s exact test. Data were analyzed using the statistical software program Stata 10.0.
Results
Patients
30 patients were included in this study, 53% females and 47% males, aged between 30 to 80 years. Average age was 56 years.
Most frequently the tumor was localized in the colon (transverse plus ascendant), rather than in the recto-sigmoidian junction. The stage of tumor was II in 55% of cases and 46% of patients had stage III. The most common differential tumor grade (grading of tumor) was II. Baseline patient and tumor characteristics are summarized in Table I. Regarding the serum values of tumor markers before surgery – CEA and CA19-9 were elevated at 63% respectively 47% of patients (Table II). The presence of tissue markers on the tumor samples – the tumor labeling index for different molecules ranged from 10 to 85% positivity (Table III).
Table I.
Characteristics of patients.
| Parameter | Cases | |
|---|---|---|
| Gender | Male | 14 (47%) |
| Female | 16 (53%) | |
| Age (years) | 30–40 | 1(3%) |
| 41–50 | 6 (20%) | |
| 51–60 | 13 (44%) | |
| 61–70 | 7 (23%) | |
| 71–80 | 3 (10%) | |
| Primary site | Colon | 16 (53,4%) |
| Rectum | 8 (26,6%) | |
| Rectosigmoidian junction. | 6 (20%) | |
| Grading | G1 | 6 (20%) |
| G2 | 17(56,7%) | |
| G3 | 7 (23,3%) | |
| Stage | II B | 13 (41%) |
| IIC | 4 (14%) | |
| III B | 10 (35%) | |
| III C | 3 (10%) |
Table II.
Serum values of tumor markers.
| Serum marker | High (% of patients) | Low |
|---|---|---|
| CEA | 63% | 37% |
| CA19-9 | 47% | 53% |
Table III.
The presence of tissue markers on the tumor samples.
| Molecular marker | Positive | Negative |
|---|---|---|
| CD4 | 60% | 40% |
| CD8 | 73% | 27% |
| CD56 | 0% | 100% |
| Bcl2 | 20% | 80% |
| Her2 | 7% | 93% |
| Ki67 | 73% | 27% |
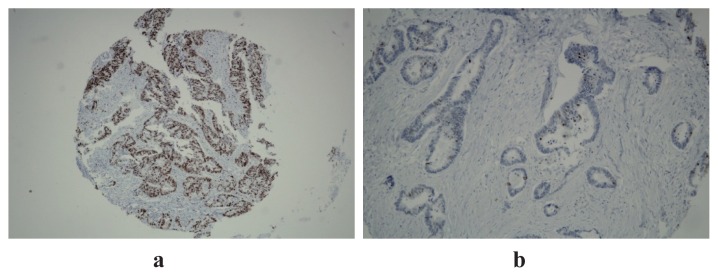
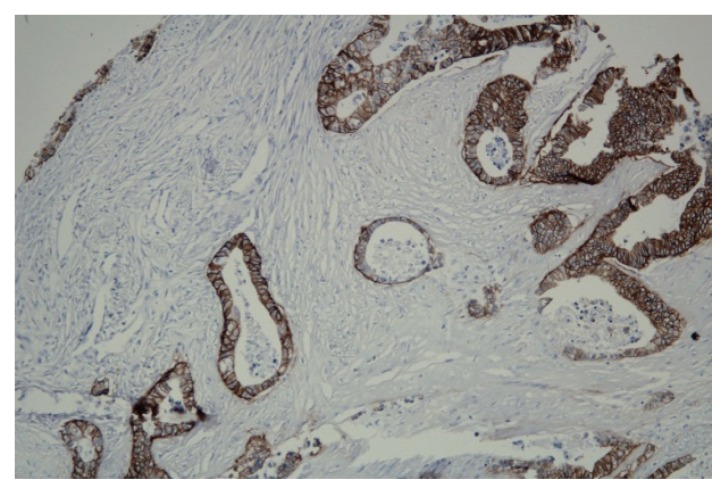
The immunohistochemical expression of Ki 67 is shown below. Most of the patients had high expression (Figure 1a) of the proliferation index, Ki67, explaining the aggressiveness of tumors, compared with the low expression observed in 27% of cases (Figure 1b). Figure 2 illustrates Her2 expression on tissue samples, rarely over-expressed.
Figure 1.
High (a) and low (b) expression of Ki67 20x.
Figure 2.
High expression of Her2 20x.
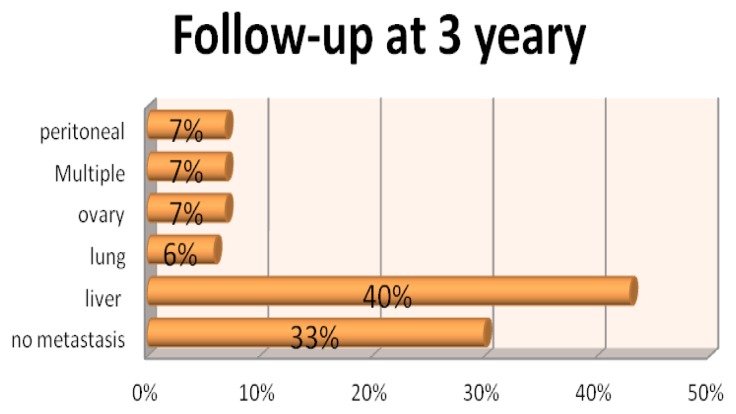
Upon 3 years follow-up, 67% of patients developed tumor relapse, the most common site of metastasis being the liver (Figure 3). No significant association was found between clinical-pathological characteristics (age, sex, etc) and tumor relapse. No correlations were observed between either serum (CEA and CA19-9) or tissue (CD4,CD8, CD56, Bcl2, Her2, Ki67) tumor markers in the tumor microenvironment and the risk of tumor relapse; none of the calculated P values showed statistical significance (Table IV).
Figure 3.
The site of metastasis.
Table IV.
Statistical correlations.
| CEA | P=0.37 |
| CA19-9 | P=0.64 |
| CD4 | P=0.46 |
| CD8 | P=0.99 |
| CD56 | - |
| Bcl2 | P=0.99 |
| Her2 | P=0.99 |
| Ki67 | P=0.99 |
Discussions
Currently, colon cancer, a leading cause of cancer-related deaths worldwide represents a serious health problem due to the growing incidence and high mortality rate.
The survival of patients with colon cancer has improved in the past 15 years with the introduction of the adjuvant treatment. The study published by Moertel et al in 1990 showed that surgery alone cured 55% of patients with colon cancer stages II and III whereas the addition of 6 months chemotherapy improved 3.5-year survival up to 71%. These results were statistically significant for patients with stage III, recurrence-free survival being 47% for surgery alone and 63% with 5FU-levamisol chemotherapy (p<0.0001) [9].
Various regimens of chemotherapy have been evaluated in adjuvant setting including 5 FU/LV plus Oxaliplatin (known as FOLFOX4), 5FU/LV plus Irinotecan (FOLFIRI), UFT, Capecitabine, Capecitabine plus Oxaliplatin (CapOX) [10,11,12,13]. Three therapy which consists in addition of Oxaliplatin or Irinotecan to 5FU/LV, was more efficacy in metastatic colorectal cancer. In adjuvant setting, the addition of Irinotecan did not improve the results whereas the combination of Oxaliplatin with 5FU/LV in 2246 patients who had surgery for colon cancer stages II and III improved disease free survival of 6.6% at 4 years (76.4 versus 69.8%) [14,15]. Based on these results, FOLFOX4 regimens were approved by FDA as adjuvant treatment of colon cancer. The side effects of chemotherapy are important, especially peripheral persistent neuropathy which affects the quality of life in patients cured of disease. Several studies confirmed these results showing a small benefit for stage II colon cancer with an improvement in disease free survival (DFS) between 2.6–5% with addition of adjuvant chemotherapy, not statistically significant [16,17,18]. Keeping in mind these side-effects and the lack of efficacy for stage II, where the addition of chemotherapy to surgery improved disease free survival with less than 5%, FOLFOX4 regimen might be preferred for high- risk patients. These data show the need for molecular markers in order to avoid administration of chemotherapy to patients who do not benefit from this treatment.
Our results showed, with 3 years follow-up, that 67% of patients developed metastasis, the most common site of being the liver, which is in concordance with the data from literature. The high rate of metastasis development justifies the importance of finding molecular markers for predicting the risk of tumor relapse.
The identification of prognostic molecules able to predict the risk of tumor relapse and to indicate aggressive adjuvant chemotherapy represents a challenge for medical practice.
Carbohydrate antigen 19-9 (CA 19-9) was originally identified on human colorectal cancer cell line as a mucin like product [19]. Many studies suggested the importance of CA19-9 serum elevation as useful marker in the diagnosis of adenocarcinoma of the upper gastrointestinal tract and in monitoring tumor of colon, even if it is a tumour associated, not a tumor specific antigen, being synthesized by normal human pancreatic, biliary cells, gastric, colonic, endometrial and salivary epithelia [20,21]. Carcinoembryonic antigen (CEA) is a glycoprotein presented in normal mucosal cells but elevated amounts can be seen in a wide variety of malignant and benign conditions including colorectal cancer, lung cancer, breast, stomach cancer and also in inflammatory bowel disease, respiratory disease, smoking [22,23]. CEA is considered a tumour marker useful in assessing prognosis, detecting recurrence and monitoring treatment of patients with colorectal cancer. Sensitivity and specificity are low, so CEA is more effective for monitoring than for screening or diagnosis of colon cancer [24,25].
Baseline elevated tumors markers CEA, CA 19.9 may indicate an increased risk of recurrent disease in patients with gastrointestinal tract tumors [26]. The recommendations regarding the follow-up of patients with colon cancer include the imaging evaluation in addition with serum antigen values, when tumor markers are initially elevated, for detection of recurrence or surveillance of progressive disease, even if these tumors antigens are elevated in many malignancies [27,28]. Our results showed an elevation of serum value of CA19-9 in 47%, while CEA was high in 63% of patients, without no correlations between these values and the risk of tumor relapse. These observations confirmed the results of other studies which reported low specificity and sensitivity of CEA and CA19-9 in diagnosis of colon cancer [29].
It is demonstrated that the immune system is involved in the control of tumor development, and the correlations between the immune cells and patients survival in many malignancies are described. Several studies suggested the important role of immune system in colorectal cancer based on the presence of tumor infiltrating immune cells, particularly T helper lymphocyte (CD4+), cytotoxic T lymphocyte, (CTL, CD8+) or natural killer cell (NK, CD3−/CD56+), [30,31,32].
In our study, we evaluated the prognostic impact of immune cells (CD4+, CD8+ and CD56+) correlated with proliferative or apoptotic markers (Ki67, Her2 respectively Bcl2), in regard to tumor relapse. The presence of tissue markers on the tumor samples – the tumor labeling index for different molecules ranged largely from 10 to 85% positivity. Regarding the question which cell of the immune system plays the most significant role in the antitumor effect, some studies suggest that the activation of cytotoxic T cell is more important than the cells involved in innate immunity (NK cells) [33].
Our results have showed similar results, there were no NK cells in tumor samples whereas the expression of CD4+, CD8+ was high in 60 and respectively 73% of patients. The proliferation marker (Ki67) was elevated in 73% of cases suggesting the aggressiveness of the tumor. Regarding the other tissue markers studied, Bcl2, Her2, they had a low expression on the tumor sample. Even if some tissue markers had showed high expression, no statistically significant correlations were observed between tissue markers (CD4, CD8, CD56, Bcl2, Her2, Ki67) in the tumor microenvironment and the risk of tumor relapse.
The existing literature regarding the prognostic value of the studied molecules in colon cancer is limited. The results of our study showed no statistically significant prognostic value for the molecules studied. A larger study is needed to confirm or deny the determination and use of these markers in monitoring patients with colorectal cancer for tumor recurrence.
Acknowledgments
We gratefully acknowledge the help of the members of Department of Surgery and Chemotherapy from Cancer Institute “I Chiricuta” Cluj-Napoca in collecting the data.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. [Google Scholar]
- 3.Mohammad WM, Balaa FK. Surgical Management of Colorectal Liver Metastases. Clin Colon Rectal Surg. 2009;22:225–232. doi: 10.1055/s-0029-1242462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jen J, Kim H, Piantadosi S, Liu ZF, Levitt RC, Sistonen P, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med. 1994;331:213–221. doi: 10.1056/NEJM199407283310401. [DOI] [PubMed] [Google Scholar]
- 5.Sarli L, Bottarelli L, Bader G, Iusco D, Pizzi S, Costi R, et al. Association between recurrence of sporadic colorectal cancer, high level of microsatellite instability, and loss of heterozygosity at chromosome 18q. Dis Colon Rectum. 2004;47:1467–1482. doi: 10.1007/s10350-004-0628-6. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MA, Gil J, Lu B, Zhang W, Yang D, Yun J, et al. Genomic profiling associated with recurrence in patients with rectal cancer treated with chemoradiation. Pharmacogenomics. 2006;7:67–88. doi: 10.2217/14622416.7.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet. 2015;47:320–329. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 8.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007:CD002200. doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 10.Andre T, Colin P, Louvet C, Gamelin E, Bouche O, Achille E, et al. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol. 2003;21:2896–2903. doi: 10.1200/JCO.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 11.Twelves C, Wong A, Nowacki MP, Abt M, Burris H, 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 12.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 13.Wolmark N, Wieand S, Lembersky B, Colangelo L, Smith R, Pazdur R. A phase III trial comparing oral UFT to FULV in stage II and III carcinoma of the colon: results of NSABP Protocol C-06. J Clin Oncol. 2004;22:3508. [Google Scholar]
- 14.Saltz LB, Niedzwiecki D, Hollis D, Goldberg RM, Hantel A, Thomas JP, et al. Irinotecan plus fluorouracil/leucovorin (IFL) versus fluorouracil/leucovorin alone (FL) in stage III colon cancer (intergroup trial CALGB C89803) J Clin Oncol. 2004;22:3500. [Google Scholar]
- 15.de Gramont A, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Oxaliplatin/5FU/LV in the adjuvant treatment of stage II and stage III colon cancer: efficacy results with a median follow-up of 4 years. Proc Am Soc Clin Oncol. 2005;23:3501. [Google Scholar]
- 16.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–1363. [PubMed] [Google Scholar]
- 17.Gray RG, Barnwell J, Hills R, McConkey C, Williams N, Kerr D. QUASAR: a randomized study of adjuvant chemotherapy (CT) vs observation including 3238 colorectal cancer patients. J Clin Oncol. 2004;22:3501. [Google Scholar]
- 18.Wolmark N, Wieand S, Lembersky B, Colangelo L, Smith R, Pazdur R. A phase III trial comparing oral UFT to FULV in stage II and III carcinoma of the colon: results of NSABP Protocol C-06. J Clin Oncol. 2004;22:3508. [Google Scholar]
- 19.Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- 20.Stienberg W. The clinical utility of the serum CA 19-9 tumour-associated antigen. Am J Gastroenterol. 1990;85:350–355. [PubMed] [Google Scholar]
- 21.Gupta MK, Arciciga R, Bocci L, Tubbs R, Bukowski R, Deodhar SD. Measurement of monoclonal antibody - defined antigen CA 19-9 in the sera of patients with malignant and nonmalignant disease. Cancer. 1985;56:277–283. doi: 10.1002/1097-0142(19850715)56:2<277::aid-cncr2820560213>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 23.Levy M, Visokai V, Lipska L, Topolcan O. Tumor markers in staging and prognosis of colorectal carcinoma. Neoplasma. 2008;55:138–142. [PubMed] [Google Scholar]
- 24.Fakih MG, Padmanabhan A. CEA monitoring in colorectal cancer. What you should know. Oncology (Williston Park) 2006;20:579–587. [PubMed] [Google Scholar]
- 25.Hara M, Kanemitsu Y, Hirai T, Komori K, Kato T. Negative serum carcinoembryonic antigen has insufficient accuracy for excluding recurrence from patients with Dukes C colorectal cancer: analysis with likelihood ratio and posttest probability in a follow-up study. Dis Colon Rectum. 2008;51:1675–1680. doi: 10.1007/s10350-008-9406-1. [DOI] [PubMed] [Google Scholar]
- 26.Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 27.Lee P, Jain S, Bowne WB, Pincus MR, McPHerson RA. Diagnosis and management of cancer using serologic and tissue tumor markers. In: McPherson RA, Pincus MR, editors. Henry’s Clinical Diagnosis and Management by Laboratory Methods 2. 2nd ed. chap 73 Philadelphia, Pa: Elsevier Saunders; 2011. [Google Scholar]
- 28.Sokoll LJ, Chan DW. Biomarkers for cancer diagnostics. In: Abeloff MD, Armitage JO, Niederhuber JE, et al., editors. Abeloff’s Clinical Oncology. 4th ed. chap 20 Philadelphia, Pa: Elsevier Churchill Livingstone; 2008. [Google Scholar]
- 29.Malati T. Tumour markers: An overview. Indian J Clin Biochem. 2007;22:17–31. doi: 10.1007/BF02913308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svennevig JL, Lunde OC, Holter J, Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49:375–377. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Giorgio A, Botti C, Tocchi A, Mingazzini P, Flammia M. The influence of tumor lymphocytic infiltration on long term survival of surgically treated colorectal cancer patients. Int Surg. 1992;77:256–260. [PubMed] [Google Scholar]
- 32.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, et al. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501. doi: 10.1038/labinvest.3700055. [DOI] [PubMed] [Google Scholar]