Abstract
Background and aims
Despite the fact that implants are sterilized, antiseptic techniques are applied and systemic antibiotics are routinely administered prior to and after craniofacial surgery, infection rates between 3% and 40% are still reported for alloplastic implants, urging for implant removal. The present study focuses on the development of a fiber-reinforced composite (FRC) implant for craniofacial reconstruction with antimicrobial properties.
Methods
A new fiber-reinforced composite coated with gentamicin was developed and tested for bacterial adherence and antibacterial efficiency, using two of the most involved bacterial strains in the postoperative infections: Staphylococcus aureus and Pseudomonas aeruginosa.
Results
Bacteria were efficiently inactivated in direct contact with gentamicin coatings (p<0.05). The inhibition zone for Staphylococcus aureus ranged from 17.21 mm to 20.13 mm and for Pseudomonas aeruginosa ranged from 12.93 mm to 15.33 mm. Although no significant statistical results were found for bacterial adhesion and gentamicin concentration, (Staphylococcus aureus: β= −0.974; p=0.144>0.05 and Pseudomonas aeruginosa: β = −0.921; p=0.255>0.05), a negative relation was observed, indicating the reversed relation between the antibiotic dosage and the bacterial adherence.
Conclusion
The results of the two applied microbiological protocols used in the study suggested that gentamicin eluting coating inhibited not only the bacterial growth, but also led to a lower initial bacterial adhesion to the surface of the implant. Thus, antibiotic coating of craniofacial implants may reduce the infection rate related to reconstructive surgery.
Keywords: bacterial adhesion, anti-bacterial agents, cranial implantation
Background and aims
The morphological and functional deficits produced by the alteration of the cranial vault and facial bones impair an individual’s life quality. In spite of the developments in the field of microsurgery and tissue engineering, alloplastic materials for cranio-facial reconstruction are still widely used. Being seen as a lower cost alternative readily available on the market, their benefits include resistance to functional and external forces, no resorption, easy handling during surgery and favorable cosmetic results due to the possibility of being prefabricated as custom-made implants using additive manufacturing techniques [1–3].
Infection rates between 3% and 40% are still reported for alloplastic implants based on titanium alloys, porous polyethylene, polymethylmetacrylate (PMMA), carbon fiber-reinforced polymer (CFRP) or hydroxyapatite placed during reconstructive surgery, requiring implant removal [4–7].
Despite the fact that implants are sterilized, antiseptic surgical techniques are applied and systemic antibiotics are routinely administered prior to and after surgery, infection rates do not decrease significantly. As a result, research was conducted on local antimicrobial strategies in order to prevent implant-associated infections and to reduce the need for revision surgeries. One prophylactic strategy refers to “the race for the surface”, a concept described about 30 years ago [8]. The fate of an alloplastic implant was considered a race between bacterial adhesion and biofilm formation on an implant surface versus tissue integration, ensured by bone ingrowth or soft-tissue ongrowth. In order to exhibit antimicrobial behavior, the surface of the implant should inhibit biofilm formation and satisfy the following requests: anti-adhesive properties, biocide activity and antimicrobial actions after contact. Generally two of these strategies are combined to overcome limitations of having just one property [9].
In relation to this, the present study focuses on the development of a fiber-reinforced composite (FRC) implant for craniofacial reconstruction with antimicrobial properties, by incorporating gentamicin as an external layer of the implant.
Materials and method
Samples, on which surfaces gentamicin was deposited, were made of monomer mixture (Bis-GMA: UEDMA: TEGDMA=1:6:3) and chopped fiber glass (organic phase 35% and inorganic phase 65%). They were achieved from teflon moulds and presented a diameter of 6 mm and a height of 1 mm. Each sample was weighted using an analytical balance and placed in a divided box. In this manner, a sample confusing bias was avoided.
The same samples without gentamicin coating were used as controls.
Gentamicin submission
The samples obtained were placed in the moulds, separated by 0.5 mm thick teflon dividers. A mixture comprising of polymethylmethacrylate (88.25%), gentamicin sulphate (3.75%) and zirconium oxide (10%) was made. The resulting powder was sieved in order to be homogenized and an amount of methylmethacrylat was placed over it (MMA: powder mixture in a proportion of 1:19). The resulting paste was introduced in a 1 ml syringe. Afterwards, one drop of the paste was placed on each sample. The moulds were then covered and pressured with a glass slide that weighted 100 grams. After half an hour, the samples were removed, trimmed and placed with the coating on the lower part of the mould. The 0.5 mm thick teflon divider was removed and replaced with a 1 mm thick one, thus the final sample thickness became 2 mm. The same procedures described above were repeated for the other part of the sample.
Bacterial strains and culture conditions
Two strains were used in this study: Staphylococcus aureus ATCC-25923 and Pseudomonas aeruginosa ATCC-27853. The cultures were maintained on Mueller Hinton agar (bioMérieux, Marcy l’Etoile, France). The bacteria were cultured overnight in 5 ml Mueller Hinton broth (bioMérieux, Marcy l’Etoile, France) in a shaker incubator (Heidolph Inkubator 1000 coupled with Heidolph Unimax 1010, Schawbach, Germany) at 37°C, 150 rpm until the culture was formed. An inoculation loop was first sterilized by passing it through a flame. When the loop was cool, it was dipped into the Mueller Hinton broth. The inoculation loop was then dragged across the surface of Petri dishes containing the agar (Muller Hinton agar) using the three-phase streaking pattern, known as the T-Streak. The technique was used to isolate a pure strain from a single species of microorganism. The dishes were incubated at 37°C for 18 h. A sufficient number of colonies were removed and placed into 9 ml of sterile saline. The bacterial concentration corresponding to 107CFU ml−1 was established using the Nanodrop Spectrophotometer ND-1000 (Delaware, USA). Successive dilutions up to 105CFU ml−1 were obtained.
Antibacterial efficiency tests
For each of the bacterial strain studied, discs of 6 mm diameter, with different concentration of gentamicin, were used. The Muller Hinton agar was inoculated with 100 μl bacterial suspension with 105CFU ml−1 dilution. The bacterial suspension was uniformly distributed on the surface of the Petri dish using a Drigalski loop. Two discs of FRC were exposed on the surface of each agar dish. The dishes were incubated 24 h at 37°C and the inhibition diameters measured afterwards.
Bacterial adherence
The bacterial adherence was tested to simulate the affinity of microorganisms to the implant surface, directly after implantation. A protocol adapted from Tanner and Nganga [10] was applied for the microorganisms (Staphylococcus aureus ATCC-25923 and Pseudomonas aeruginosa ATCC-27853) on uncoated and coated specimens.
The bacteria were precultured from a frozen glycerol preparation and inoculated in 45 mL Tryptic Soy Broth (Liofilchem, Roseto Degli Abruzzi, Italy) of 16 h at 37°C. After harvesting the bacteria by centrifugation (4 000 rpm, + 4°C, 10 min, Centrifuge 5804R, Eppendorf, Hamburg, Germany), they were washed once with physiological sterile saline. Then cells were resuspended in physiological saline at a concentration of ~ 0.035 at A550, which corresponded to ~ 1×107 colony-forming units (CFU) (Nanodrop Spectrophotometer ND 1000, Delaware, USA). The suspension was gently sonicated and vortexed to homogenize the solution. Then the specimens were placed in 15 ml test tubes with 5 ml of bacterial suspension. After shaking (Heidolph Unimax 1010, Schwabach, Germany) at room temperature for 30 min, the specimens were washed three times in abundant physiological saline and gently dried without touching the surface. Thereafter, the bacterial samples from the specimen surfaces were collected for analysis of viability (three replicates).
The bacteria attached to the surface of the specimens were collected with micro brushes into 2 ml microtubes containing 900 μL of Tryptic Soy Broth (Liofilchem, Roseto Degli Abruzzi, Italy) with 10% glycerol. Thereafter, the bacteria were homogenized (Eppendorf 5804 R, Hamburg, Germany), serially diluted in physiological saline (10 μL of 1:10, 1:100, and 1:1000) and cultured on Muller Hinton agar plates. CFU measurements were done after 24 h of culturing at 37°C.
Data analysis
Data analysis was performed with IBM SPSS 20. Descriptive statistics and linear regression were applied in order to investigate the effects of antibiotic dosage on the bacterial adhesion. ANOVA test was applied to investigate the statistical significance of the data between test groups.
Results
Antimicrobial efficacy test
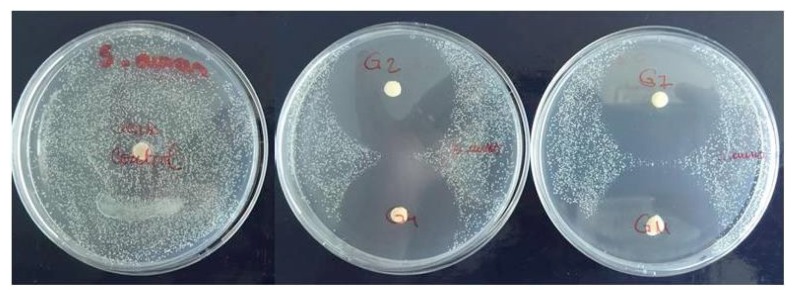
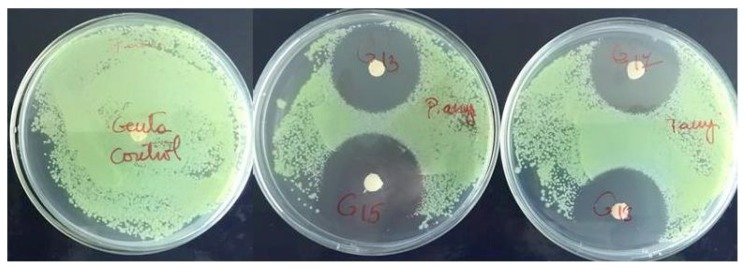
In the test for antimicrobial activity the coatings were highly effective against both bacteria strains (Figure 1, Figure 2).
Figure 1.
Antimicrobial efficacy test for Staphylococcus aureus showing inhibition zone around gentamicin coated FRC discs.
Figure 2.
Antimicrobial efficacy test for Pseudomonas aeruginosa showing inhibition zone around gentamicin coated FRC discs.
Bacteria were efficiently inactivated with direct contact to gentamicin coatings. The results showed a diameter of inhibition zone for Staphylococcus aureus ranging from 17.21 mm to 20.13 mm (Table I). Regarding the diameter of inhibition zone for Pseudomonas aeruginosa, the results showed values ranging from 12.93 mm to 15.33 mm (Table II).
Table I.
Antimicrobial efficacy test: diameters of inhibition zone (mean and standard deviation) for Staphylococcus aureus ATCC-25923 around gentamicin coated fiber reinforced composite (FRC-G) discs.
| Group | Count | Inhibition zone (mm) | |
|---|---|---|---|
| Mean | Standard deviation (+/−) | ||
| Control | 6 | 0.13 | 0.015 |
| FRC-G 1 | 6 | 20.13 | 0.019 |
| FRC-G 2 | 6 | 17.21 | 0.022 |
| FRC-G 3 | 6 | 19.73 | 0.027 |
| FRC-G 4 | 6 | 19.11 | 0.035 |
| FRC-G 5 | 6 | 18.31 | 0.021 |
| FRC-G 6 | 6 | 17.47 | 0.022 |
| FRC-G 7 | 6 | 18.05 | 0.025 |
Table II.
Antimicrobial efficacy test: diameters of inhibition zone (mean and standard deviation) for Pseudomonas aeruginosa ATCC-27853 around gentamicin coated fiber reinforced composite (FRC-G) discs.
| Group | Count | Inhibition zone (mm) | |
|---|---|---|---|
| Mean | Standard deviation (+/−) | ||
| Control | 6 | 0.904 | 0.048 |
| FRC-G 8 | 6 | 13.97 | 0.015 |
| FRC-G 9 | 6 | 13.85 | 0.022 |
| FRC-G 10 | 6 | 14.18 | 0.025 |
| FRC-G 11 | 6 | 13.426 | 0.024 |
| FRC-G 12 | 6 | 15.33 | 0.022 |
| FRC-G 13 | 6 | 12.93 | 0.017 |
| FRC-G 14 | 6 | 12.98 | 0.015 |
All the test groups showed a statistically significant difference when compared to the control group (p<0.05)
Bacterial adherence
In the adherence test, the initial adhesion, which is the interaction between the cell surface and the material surface, was measured.
Less Staphylococcus aureus and Pseudomonas aeruginosa were found attached to the gentamicin coated specimens than to the control discs (Table III and Table IV). Although, no significant statistical results were found for bacterial adhesion and gentamicin concentration, (Staphylococcus aureus: β= −0.974; p=0.144>0.05) and Pseudomonas aeruginosa: β= −0.921; p=0.255>0.050), a negative relation was observed, indicating the reversed relation between the antibiotic dosage and the bacterial adherence.
Table III.
Adherence of Staphylococcus aureus ATCC-25923 to gentamicin coated fiber reinforced composite (FRC-G) discs.
| Group | Count | Gentamicin dosage (g) | CFU | |
|---|---|---|---|---|
| Mean | Standard deviation (+/−) | |||
| Control | 3 | 0 | 21×103 | 707 |
| FRC-G 15 | 3 | 0.0026 | 10×103 | 707 |
| FRC-G 16 | 3 | 0.0019 | 11×103 | 100 |
| FRC-G 17 | 3 | 0.0016 | 12×103 | 707 |
Table IV.
Adherence of Pseudomonas aeruginosa ATCC-27853 to gentamicin coated fiber reinforced composite (FRC-G) discs.
| Group | Count | Gentamicin dosage (g) | CFU | |
|---|---|---|---|---|
| Mean | Standard deviation (+/−) | |||
| Control | 3 | 0 | 11×105 | 100 |
| FRC-G 18 | 3 | 0.0016 | 25×103 | 707 |
| FRC-G 19 | 3 | 0.0031 | 5×103 | 707 |
| FRC-G 20 | 3 | 0.0018 | 15×103 | 707 |
Discussion
Biomaterial-associated infection is a feared complication of reconstructive surgery, often leading to prolonged patient hospitalization, functional and morphological impairment. Perioperative antibiotic prophylaxis showed a lower rate of implant-associated infections but did not eradicate it [11]. Furthermore, implantation of a biomaterial impairs the innate local host response and may raise the risk of infection [12,13]. As a result, there is a strong need for an antibacterial implant surface that can overcome implant-induced defects in the local immune response.
Antibiotic eluting coatings represent one of the numerous approaches that had been proposed and tested for antibacterial features. Common antibiotics locally released from implant surfaces against potential bacterial infection are gentamicin, tobramycin, vancomycin, rifampicin, cefuroxime and ciprofloxacin. Due to the changes in oral and maxillofacial flora and bacterial resistance to antibiotics, nowadays, recommended therapies are based on ciprofloxacin and gentamicin [14]. Gentamicin has already proved to be an optimal choice due to its activity against both Gram-positive and Gram-negative bacteria. It is known to exhibit a low rate of allergy, free solubility in water and a rare resistance to it [15].
Two aspects are critical when designing an antimicrobial coating: obtaining anti-adhesive surface for the bacteria and inducing bacterial inhibition.
In reconstructive surgery, a strong anti-adhesive layer on implants cannot be used because it could also prevent osseointegration and can lead to early mechanical failure. The solution lies in a coating that retains required host cell interactions while selectively inhibiting bacterial adhesion.
In our study, the results of bacterial adherence test were not statistically significant. This may be related not only to the extent of the sample group, but also to the incubation medium which may affect the affinity of the bacteria to test surfaces. In pure growth medium (TSB), Barton and co-workers [16] reported the amount of adhering bacteria to be halved compared to adherent bacteria cultured in PBS. In the present adhesion test, gentamicin dissolution led to a reduction of its concentration on the sample surface with consecutive reduction of contact bactericidal activity. Compared to the clinical situation, the liquid volume and flow are overrated. Thus the material antimicrobial efficacy is underestimated.
The antimicrobial efficacy test showed good results on both bacteria strains, being related to the elution of antibiotic from the implant coating. The material efficacy may still be slightly overestimated as body fluids, at the implant site, are exchanged continuously [17].
The coating obtained in this study succeeds in inhibiting bacterial adhesion and growth, while gentamicin is one of the well-tolerated antibiotics in terms of cytotoxicity on human osteoblasts [18], fibroblasts and keratinocytes [15,19]. This aspect is critical, taking into account that antibiotic elution from the implant is time-limited and by the moment its antimicrobial properties decrease, the implant should be integrated in the surrounding tissues.
Conclusions
The results of the two applied microbiological protocols used in the present study suggest that gentamicin eluting coating inhibited not only the bacterial growth, but also led to a lower grade of initial bacterial adhesion to the surface of the fiber-reinforced composite implant. Thus, antibiotic coating of cranio-facial implants may reduce the infection rate related to reconstructive surgery of the skull.
References
- 1.Abdo Filho RC, Oliveira TM, Lourenço Neto N, Gurgel C, Abdo RC. Reconstruction of bony facial contour deficiencies with polymethylmethacrylate implants: case report. J Appl Oral Sci. 2011;19(4):426–430. doi: 10.1590/S1678-77572011000400021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayashankara Rao JK, Malhotra V, Batra RS, Kukreja A. Esthetic correction of depressed frontal bone fracture. Natl J Maxillofac Surg. 2011;2(1):69–72. doi: 10.4103/0975-5950.85858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Gool AV. Preformed polymethylmethacrylate cranioplasties: report of 45 cases. J Maxillofac Surg. 1985;13(1):2–8. doi: 10.1016/s0301-0503(85)80005-9. [DOI] [PubMed] [Google Scholar]
- 4.Yaremchuk MJ. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111(6):1818–1827. doi: 10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- 5.Frederick M, Mittermiller P, Hoffman W. Alloplastic cranioplasty outcomes in previously infected sites. Scientific Program, 90th Annual Meeting of the American Association of Plastic Surgeons; April 9–12, 2011; Available from: http://meeting.aaps1921.org/abstracts/2011/9.cgi. [Google Scholar]
- 6.Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26(6):E10. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 7.Wurm G, Tomancok B, Holl K, Trenkler J. Prospective study on cranioplasty with individual carbon fiber reinforced polymere (CFRP) implants produced by means of stereolithography. Surg Neurol. 2004;62:510–521. doi: 10.1016/j.surneu.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 9.Ho CH, Tobis J, Sprich C, Thomann R, Tiller JC. Nanoseparated polymeric network with multiple antimicrobial properties. Advanced Materials. 2004;16:957–961. [Google Scholar]
- 10.Tanner J, Vallittu PK, Söderling E. Adherence of Streptococcus mutans to an E-glass fiber-reinforced composite and conventional restorative materials used in prosthetic dentistry. J Biomed Mater Res. 2000;49:250–256. doi: 10.1002/(sici)1097-4636(200002)49:2<250::aid-jbm14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15(8):13849–13880. doi: 10.3390/ijms150813849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009;175:161–170. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerli W, Sendi P. Pathogenesis of implant-associated infection: the role of the host. Semin Immunopathol. 2011;33:295–306. doi: 10.1007/s00281-011-0275-7. [DOI] [PubMed] [Google Scholar]
- 14.Orzechowska-Wylęgała B, Wylęgała A, Buliński M, Niedzielska I. Antibiotic therapies in maxillofacial surgery in the context of prophylaxis. Biomed Res Int. 2015;2015:819086. doi: 10.1155/2015/819086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahlig H, Dingeldein E, Bergmann R, Reuss K. The release of gentamicin from polymethylmethacrylate beads. An experimental and pharmacokinetic study. J Bone Joint Surg Br. 1978;60-B(2):270–275. doi: 10.1302/0301-620X.60B2.659478. [DOI] [PubMed] [Google Scholar]
- 16.Barton AJ, Sagers RD, Pitt WG. Bacterial adhesion to orthopedic implant polymers. J Biomed Mater Res. 1996;30:403–410. doi: 10.1002/(SICI)1097-4636(199603)30:3<403::AID-JBM15>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Jee WS. Integrated bone tissue physiology: anatomy and physiology. In: Cowin SC, editor. Bone mechanics handbook. Boca Raton: CRC Press; 2001. [Google Scholar]
- 18.Duewelhenke N, Krut O, Eysel P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob Agents Chemother. 2007;51(1):54–63. doi: 10.1128/AAC.00729-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pyo HC, Kim YK, Whang KU, Park YL, Eun HC. A comparative study of cytotoxicity of topical antimicrobials to cultured human keratinocytes and fibroblasts. Korean J Dermatol. 1995;33(5):895–906. [Google Scholar]