Graphical abstract

Introduction
The imaging of prostate cancer is emerging as an important component of the staging and management of prostate cancer patients, and methods for biologic characterisation of prostate tumors is an area of increased investigation.1–6
Assessment of androgen receptor status in prostate cancer is potentially important for assessing patients most likely to respond to anti-androgen receptor therapy. Due to the heterogeneous nature of tumors, biopsy samples may not accurately measure androgen receptor expression. Nuclear medicine imaging techniques such as positron emission tomography (PET) have the potential to noninvasively quantify androgen receptor density of the entire tumor, and identify if there are differences in androgen receptor expression in multiple metastatic lesions.
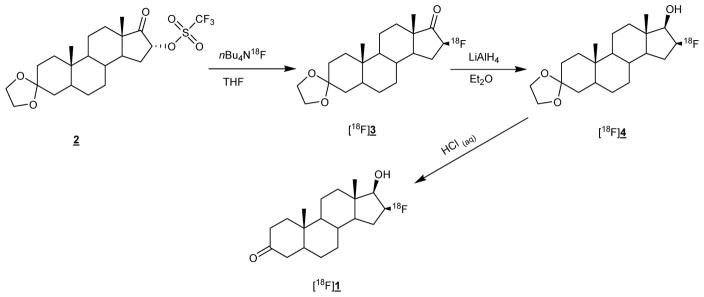
Among the PET imaging agents developed, 16β-[18F]fluoro-5α-dihydrotestosterone [18F]FDHT (1) has proven to be the most promising tracer so far.7–10 Figure 1 shows the synthesis of [18F]FDHT first reported by Liu et al.11
Figure 1.
Synthesis of [18F]FDHT ([18F]1)
Although the [18F]FDHT precursor (2) is now commercially available, the complex synthesis procedure has so far prevented its widespread use in clinical applications. The development of a fully automated synthesis procedure would therefore make this exciting imaging agent more widely available.
A recently published automated synthesis using the ELIXYS synthesiser uses in-house made drying cartridges and the rotary evaporation technique for the reformulation of [18F]FDHT.12
Two other manuscripts on [18F]FDHT synthesis have reported the use of NaBH4 as an alternative to LiAlH4 reduction but have not fully automated the entire process.13,14
Our aim was to develop a fully automated synthesis of [18F]FDHT using only commercially available components and using the SPE technique for radiotracer reformulation.
We chose to develop the production of [18F]FDHT for clinical use in our centre using the iPHASE technologies FlexLab module since it is ideally suited to the automation of complex radiosynthesis procedures.15,16
Materials and methods
Solvents were purchased from MERCK and used without further purification. [19F]FDHT standard and [18F]FDHT precursor were synthesised at the Memorial Sloan Kettering Cancer Centre Organic synthesis core. All other reagents were purchased from Sigma-Aldrich and used without further purification. Chromafix PS-HCO3 cartridges were purchased from ABX, Radeberg. Sep-Pak® tC18 Plus short cartridges (part no. WAT036810) were purchased from Waters Corporation, Milford, MA., preconditioned with 2 mL of ethanol and washed with 10 mL of water before use. A Millipore Millex 0.22 μm GV Filter unit (Ref. SLGV033RS) was used for sterile filtration of the final formulation. Latex- and silicone-free B|Braun Injekt® Luer Solo syringes were used for the handling of all solvents and for the preparation of all reagent solutions.
No-carrier-added [18F]fluoride was produced by the 18O(p,n)18F nuclear reaction with an 18 MeV proton beam generated by the IBA Cyclone 18/9 cyclotron in a niobium target using [18O]H2O at Austin Health, Centre for PET. Typical irradiation parameters were 38 μA for 20 min, which resulted in 25.9–28.6 GBq (0.7–0.8 Ci) of [18F]fluoride being transferred into the synthesis module.
Isolation of the [18F]fluoride ion from [18O]H2O was achieved by trapping on a Chromafix PS-HCO3 ion exchange column. The column was eluted with a solution containing KHCO3 (2 mg) and kryptofix 2.2.2 (11 mg) in a mixture of 1 mL of methanol and 200 μL of water. Evaporation to dryness with 1 mL of acetonitrile gave the anhydrous [18F]fluoride used in the labelling experiments.
The HPLC system used for semi-preparative workup of [18F]FDHT was a Knauer Smartline 1050 pump with a Smartline Manager 5050 for quaternary gradient capability. The Knauer UV detector 2520 (254 nm) and a CsI(Tl) crystal PIN diode radioactivity detector were used for the detection of compounds.
The quality control and specific activity measurement of [18F]FDHT was performed using a Shimadzu Prominence HPLC system equipped with a 20 μL injection loop, a SPD-20A UV-Vis detector and an LC-20AT solvent pump. The stationary phase was a Phenomenex Gemini C-18, 5μ RP column, 150×4.6 mm. An isocratic mixture of 55% acetonitrile and 45% water at a flow rate of 1 mL/min was used as the mobile phase. The Raytest Gabi Star NaI (Tl) detector was used for the detection of radioactive compounds. Specific activity was measured with a cold mass standard curve of known concentrations of [19F]FDHT at 208 nm.
Radiochemistry
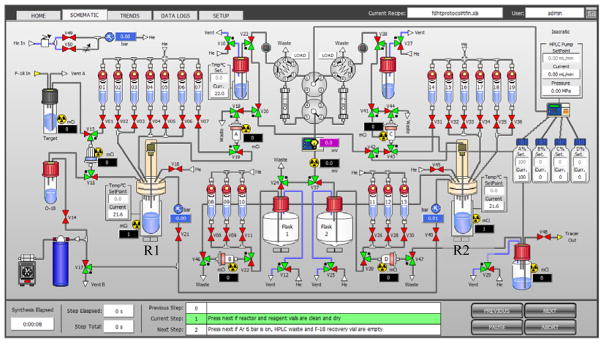
Figure 2 shows the schematic of the FlexLab module used for the preparation of [18F]FDHT. Before the start of synthesis, all reagents were loaded into reagent vials (Table 1) and 5 mg of solid NaBH4 added to reactor 2 (R2). [18F]Fluoride was then transferred from the cyclotron, trapped on the Chromafix ion exchange cartridge and eluted into reactor 1 (R1) using eluent from vial 1. Dry acetonitrile (1 mL) was added from vial 2 and the solution was dried at 90 °C for 7 min under vacuum with argon flow. After drying, the reactor was cooled to 40 °C and the precursor (2) added from vial 3. Radiolabelling was achieved by heating at 40 °C for 15 min in a closed reactor. Water (10 mL) was then added from vial 6 and the solution passed through the Sep-Pak cartridge in position A for the trapping of fluorinated products. Water (4 mL) was then added to reactor 1 from vial 5 and the residual contents of reactor 1 passed through the Sep-Pak cartridge in position A. The Sep-Pak cartridge was then eluted with 1.2 mL of ethanol from vial 7 and the eluate transferred into reactor 2, which contained the NaBH4. Reduction of the intermediate [18F]3 was performed at 20 °C for 7 min. HCl (1 M, 1.5 mL) was then added to reactor 2 and the mixture heated to 85 °C for 7 min to destroy excess NaBH4 and remove the protecting group. NaOH (0.33 M, 3 mL) was then added and the crude [18F]FDHT solution injected into the semi-preparative HPLC using a Cosmosil 5C18-PAQ, 10×250 mm as the stationary phase. Separation of [18F]FDHT from impurities was achieved via a gradient elution technique using acetonitrile (A) and water (B) with 0.1% ammonium formate as the mobile phase. The gradient used to purify [18F]FDHT was 0–30 min: 35–50% A at a flow rate of 4 mL/min. The peak at 20.5 min was collected in HPLC vial 2 and reformulated using the SPE technique by trapping on the C-18 Sep-Pak cartridge located in position D. Sterile filtration of the final formulation was achieved by passing through a Millex GV filter unit.
Figure 2.
Schematic of the FlexLab module
Table 1.
Preparation of the FlexLab module for the production of [18F]FDHT
| Entry | Position | Reagents or Materials | Quantities |
|---|---|---|---|
| 1 | Vial 1 | KHCO3/kryptofix 2.2.2 | 2 mg KHCO3 and 11 mg kryptofix 2.2.2 in 1 mL of methanol and 200 μL water |
| 2 | Vial 2 | Dry acetonitrile | 1 mL |
| 3 | Vial 3 | FDHT precursor (2) | 4 mg in 500 μL dry acetonitrile |
| 4 | Vial 4 | empty | |
| 5 | Vial 5 | water | 4 mL |
| 6 | Vial 6 | water | 10 mL |
| 7 | Vial 7 | ethanol | 1.2 mL |
| 8 | Vials 8–10 | empty | |
| 9 | Vial 11 | water | 10 mL |
| 10 | Vial 12 | ethanol | 1 mL |
| 11 | Vial 13 | Saline (0.9%) | 9 mL |
| 12 | Vial 14 | empty | |
| 13 | Vial 15 | 0.33 M NaOH | 3 mL |
| 14 | Vial 16 | 1 M HCl | 1.5 mL |
| 15 | Vials 17–19 | empty | |
| 16 | Reactor 2 | NaBH4 (dry solid) | 5 mg |
| 17 | HPLC vial 2 | water | 60 mL |
| 18 | Cartridge A | Sep-Pak® tC18 Plus short | 1 |
| 19 | Cartridge D | Sep-Pak® tC18 Plus short | 1 |
| 20 | F-18 trapping Cartridge | Chromafix PS-HCO3 | 1 |
Results and Discussion
Our general strategy for the complete automation of the [18F]FDHT synthesis process using the FlexLab module was to perform the radioabelling step in reactor 1, then purify [18F]3 by diluting the crude reaction mixture with water, trapping [18F]3 on an SPE cartridge in position B and transferring [18F]3 into reactor 2 for the reduction and hydrolysis steps.
We initially used a solution containing 3.5 mg of anhydrous K2CO3 (0.025 mmol) and 20 mg of kryptofix 2.2.2 (0.053 mmol) in 0.4 mL of acetonitrile plus 0.2 mL of water as eluent for the F-18 recovery. However, this resulted in an unsatisfactory decay corrected radiochemical yield of 5–7% (n=5) of [18F]FDHT. When using KHCO3/kryptofix for the elution of fluorine-18 from the chromafix cartridge we obtained [18F]FDHT in 25–33% yields. This eluent system requires a 1.2 mL solution of methanol/water and can only recover ~90% of the fluorine-18 trapped on the cartridge.17 Due to the large volume of eluent used, the drying time required was 7 min rather than 6 min for the K2CO3/kryptofix eluent.
We found that performing the radiolabelling at 40 °C resulted in high selectivity for the desired stereoisomer, thus simplifying the HPLC purification of the final product.
We chose to use NaBH4 rather than LiAlH4 for the reduction of the ketone since using LiAlH4 either requires the reaction vessel to be kept at −70 ºC or the speed of the addition of the LiAlH4 solution needs to be carefully controlled if the reduction is performed at RT.
In order to avoid line or valve blockages, we decided to add the NaBH4 as a solid to reactor 2 rather than dissolving the reducing agent in ethanol and subsequently adding the solution to reactor 2 via one of the reagent vials.
Hydrolysis of excess NaBH4 and removal of the protecting group was achieved by adding 1 M HCl and heating to 85 °C for 7 min. We did not observe any decomposition or unwanted side-products by not quenching any excess NaBH4 with acetone/THF.14
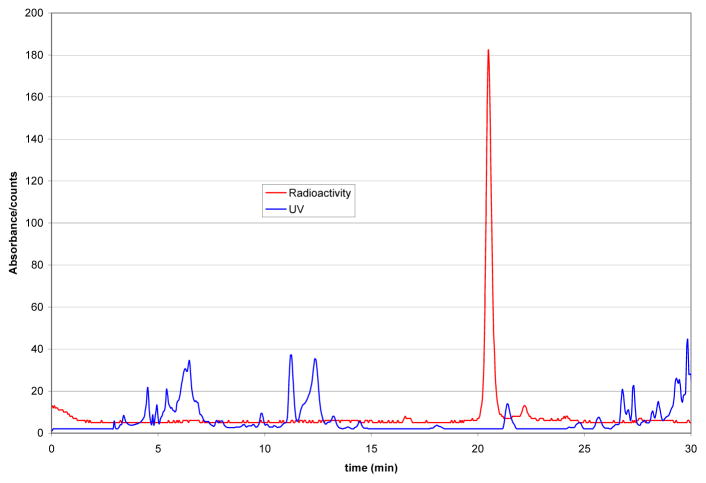
Figure 3 shows a HPLC trace of the semi-preparative purification of the crude [18F]FDHT solution.
Figure 3.
semi-preparative HPLC chromatogram of [18F]FDHT workup
Apart from [18F]FDHT, no other significant radioactive peaks were observed and good separation between [18F]FDHT and other impurities was achieved. After reformulation and sterile filtration, decay corrected radiochemical yields were 25–33% (n=11). Only ~4% of [18F]FDHT remained trapped on the sterile filter. Total synthesis time including radiotracer reformulation was 90 min. Although synthesis time and yields are very similar to the results reported by others, it is important to note that these results were achieved using only 4 mg of [18F]FDHT precursor.12
Quality control
All batches of [18F]FDHT prepared by our method passed the pre-release and retrospective quality control tests shown in table 2.
Table 2.
Quality control assessment of [18F]FDHT
| Pre-release tests | ||
|---|---|---|
| Test | Acceptance Criteria | Result |
| Vial integrity | Vial is intact | Pass |
| Vial contents | Clear, colourless, particulate free | Pass |
| Label inspection | Correctly labelled “FDHT” | Pass |
| pH | 4.5 to 8.5 | 7.5 |
| Radiochemical Identity (HPLC) | RT within ± 10% of standard | Pass |
| Radiochemical purity | ≥ 90% [18F]FDHT | 99.9% |
| Specific activity | > 18.5 GBq/μmol | Pass |
| Retrospective tests | ||
|---|---|---|
| Test | Acceptance Criteria | Result |
| Radionuclide identity | Half-life 105–115 min | 111.4 |
| Radionuclide identity | 511 keV (± 10 keV) | 512.4 |
| Kryptofix content | ≤ 50 μg/mL (≤ 50 ppm) | Pass |
| Acetonitrile content | ≤ 0.4 mg/mL (≤ 0.04%) solvent | Pass |
| Membrane filter integrity | Bubble point ≥ 50 PSI | Pass |
| Sterility test | Meets requirements | Pass |
| Bacterial endotoxins | < 17.5 EU/mL | < 2.5 EU/mL |
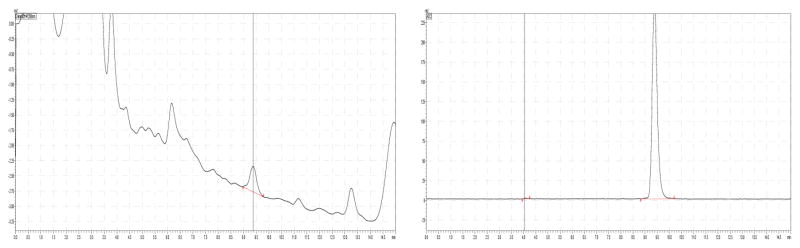
Radiochemical purity and specific activity were measured using reversed phase HPLC. We found that the use of latex- and silicone-free B|Braun Injekt® Luer Solo syringes improved the accuracy of the specific activity measurement of [18F]FDHT since no interference from lubricants was observed. Figure 4 shows a HPLC chromatogram of [18F]FDHT. Radiochemical purity of the final formulation was > 99% and specific activity was > 18.5 GBq/μmol for all batches.
Figure 4.
Quality control of [18F]FDHT. UV (left) and radioactivity (right)
Applying the quality control methods in table 2, this [18F]FDHT synthesis method has now been validated at our centre and is used to prepare patient doses.
In comparison to other published methods, the procedure presented here further simplifies the synthesis of [18F]FDHT since it eliminates the need for in-house made drying cartridges, uses the SPE technique for easy radiotracer reformulation and does not require special conditions for the reduction step. It also uses 50% less [18F]FDHT precursor than other methods. Decay corrected radiochemical yields, specific activity and overall synthesis time are comparable to the method published by Lazari, et al.12 We believe that these modifications can easily be adapted to suit other automated synthesis modules.
Conclusion
A fully automated synthesis of [18F]FDHT was developed on the iPhase FlexLab module. This method only uses commercially available components and the SPE technique for radiotracer reformulation. The synthesis procedure is also highly reliable and reproducible. Synthesis time and yields of [18F]FDHT are comparable with previously published methods, however, only 4 mg of [18F]FDHT precursor are required. The final formulation of [18F]FDHT passed all quality control requirements for injection into humans. The synthesis has now been validated at Austin Health and is currently used for [18F]FDHT studies in patients.
We believe that this method can easily adapted by other modules to further widen the availability of [18F]FDHT.
Acknowledgments
The authors acknowledge financial support from the Movember Foundation and NIH grant P30 CA08748, which supported, in part, the Organic Synthesis Core and the Radiochemistry & Molecular Imaging Probe Core at Memorial Sloan Kettering Cancer Center for the FDHT precursor and standard.
References
- 1.Scher HI, Heller G. Urology. 2000;55:323–7. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Halabi S, Tannock I, et al. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas MA, Nagarajan R, Huda A, et al. NMR in Biomedicine. 2014;27:53–66. doi: 10.1002/nbm.2991. [DOI] [PubMed] [Google Scholar]
- 4.Jadvar H. J Nucl Med. 2011;52:81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee ST, Lawrentschuk N, Scott AM. Semin Nucl Med. 2012;42:231–46. doi: 10.1053/j.semnuclmed.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Chang J, Lim-Joon D, Lee S, et al. European Radiology. 2013;23:1–8. [Google Scholar]
- 7.Larson SM, Morris M, Gunther I, et al. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 8.Dehdashti F, Picus J, Michalski J, et al. Eur J Nucl Med Mol Imaging. 2005;32:344–350. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 9.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. J Nucl Med. 2010;51:183–192. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vargas HA, Wassberg C, Fox JJ, et al. Radiology. 2014;271:220–229. doi: 10.1148/radiol.13130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu A, Dence CS, Welch MJ, et al. J Nucl Med. 1992;33:724–734. [PubMed] [Google Scholar]
- 12.Lazari M, Lyashchenko SK, Burnazi EM, et al. Appl Rad Isotopes. 2015;103:9–14. doi: 10.1016/j.apradiso.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori T, Kiyono Y, Asai T, et al. J Nucl Med. 2010;51(Supplement 2):1525. [Google Scholar]
- 14.Zhou D, Lin M, Yasui N, et al. J Label Compd Radiopharm. 2014;57:371–377. doi: 10.1002/jlcr.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ackermann U, Plougastel L, Goh YW, et al. Appl Rad Isotopes. 2014;94:72–76. doi: 10.1016/j.apradiso.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann U, Plougastel L, Wichmann C, et al. J Label Compd Radiopharm. 2014;57:115–120. doi: 10.1002/jlcr.3175. [DOI] [PubMed] [Google Scholar]
- 17.Moon BS, Park JH, Lee HJ, et al. Appl Rad Isotopes. 2010;68:2279–2284. doi: 10.1016/j.apradiso.2010.06.016. [DOI] [PubMed] [Google Scholar]