Abstract
BACKGROUND
Optical coherence tomography (OCT) is capable of providing a non-invasive real-time cross-sectional image of the skin through the use of light-based interferometry– a method sometimes described as a “light-based ultrasound.” One key application of OCT in dermatology is the visualization of dermal collagen during processes such as chronological aging, photoaging, or photodamage. These skin conditions are typically managed by the practitioner’s subjective assessment of severity and response to therapy.
METHODS & MATERIALS
We searched Medline, PubMed, EMBASE, Web of Science, Google Scholar, and Cochrane databases for published literature on the imaging of skin collagen by OCT using the following search terms: “optical coherence tomography,” “OCT,” “skin,” “collagen,” “photoaging,” “wrinkles,” and “photodamage.”
RESULTS
Our search resulted in 23 articles investigating OCT skin collagen imaging meeting our search criteria.
CONCLUSION
We anticipate tremendous growth in the field of OCT skin imaging that will parallel the development ultrasound technology has experienced over the past 30 years. We foresee that OCT imaging to evaluate skin aging will not only help identify pathological changes earlier, but will also assist evaluation of response-to-therapy longitudinally without biopsy.
Keywords: skin imaging, OCT, optical coherence tomography, chronological aging, photoaging, photodamage, collagen
INTRODUCTION
Optical coherence tomography (OCT) is an imaging modality that is altering the way clinicians visualize and evaluate the skin. OCT images the skin through the use of light-based interferometry and provides a digital image of skin.1-6 OCT was first adopted clinically for measuring eye length and then evolved into a clinical tool to evaluate a number of ophthalmic diseases.7 Since then, OCT has expanded to a number of other medical fields, including dermatology, where it is currently used in the clinical assessment and research of skin diseases.2,7-13 OCT allows real-time visualization of the epidermis, superficial to mid-dermis, skin appendages, and blood vessels.2 This real-time imaging is non-invasive and holds tremendous potential to become a future standard to diagnose skin diseases, evaluate their progress, and measure response-to-therapy while foregoing biopsies.
A major application of OCT in dermatology is the visualization of dermal collagen. Collagen proteins are the primary extracellular matrix proteins in the skin.14 The location, orientation, density, and reflective properties of collagen fibrils render the dermis birefringent and allow visualization on OCT imaging.15 Additionally, the amount and organization of skin collagen is altered during a number of normal and pathological processes that include: chronological aging, photoaging, and photodamage.
Skin aging is a complex biological process that occurs intrinsically during chronological aging, or from long-term sun exposure in photoaging.16,17 In chronological aging, reactive oxygen species (ROS) are generated throughout a person’s lifetime affecting various cell functions and integrity of extracellular collagen and elastin.18 In photoaging, ultraviolet irradiation from sunlight leads to ROS generation and subsequent degeneration and disorganization of collagen and other dermal components.16 These photo-induced alterations cause wrinkling, coarseness, pigment irregularities, telangiectasia, and are associated with skin neoplasms.19 Therefore, the utility of OCT as a rapid, non-invasive method to characterize and quantify photodamage is highly valuable to clinicians, and specifically dermatologists.
A number of other non-invasive imaging modalities have been used to evaluate skin. One challenge facing all non-invasive skin imaging modalities is the inherent trade-off between resolution and depth of penetration (Figure 1).5 Table 1 compares the penetration depths and resolutions of different imaging techniques used to image the skin. For instance, high-frequency ultrasound (US) has a penetration depth of approximately 15 mm and with a resolution of approximately 300 μm that limits its ability to evaluate small variations in tissue properties.20 Similarly, computed tomography (CT) and magnetic resonance imaging (MRI) have excellent depth of penetration, but possess a restricted resolution of 100 μm that limits their utility in distinguishing fine variations in skin.20,21 In contrast, confocal laser microscopy possess a high resolution that allows distinction of fine skin features, however, its shallow penetration depth of 0.2 mm limits imaging of dermal structure and collagen status.1,5 Therefore, OCT may provide an ideal balance through combining a penetration depth of 2 mm with a resolution of 4-10 μm. This allows OCT to image deeper skin structures while maintaining the resolution necessary to evaluate fine variations in skin properties.22
Fig. 1.

Trade-off between penetration depth and resolution. Computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound (US) have high penetration depths but at the expense of reduced resolutions. Conversely, confocal microscopy (CM) has high resolution at a much lower penetration depth. Optical coherence tomography (OCT) can image deeper tissues than confocal microscopy while maintaining resolution exceeding those of CT, MRI and HFUS.
Table 1.
Comparison of penetration depths and resolution of imaging techniques applied to skin imaging
| Imaging Modality | Best Penetration Depth |
Maximum Resolution |
|---|---|---|
| Confocal microscopy |
0.2 mm 1 | 0.5 – 1 μm 1 |
| Gabor-Domain Optical Coherence Microscopy |
0.7 mm 47 | 2 μm 47 |
| Optical coherence tomography |
2 mm 1 | 4-10 μm 1 |
| High-frequency ultrasound |
15 mm 20 | 30 μm 20 |
| Computed tomography |
Total body penetration |
100 μm 1 |
| Magnetic resonance imaging |
Total body penetration |
100 μm 1 |
Imaging principles between OCT and ultrasound imaging are similar. However, instead of using sound waves, OCT uses light and captures the light reflectance to generate an image. OCT images are either 2- or 3-dimensional images that provide a cross-sectional image of the superficial skin resembling histological images.23 Therefore, OCT is sometimes referred to as an “optical biopsy,” as it aims to provide histologic information non-invasively. OCT imaging systems contain an interferometer illuminated by light. OCT-emitted light is split into two fractions, one fraction is directed to a reference mirror and the other is directed to the tissue.22-26 Light reflected back from the mirror and the tissue are recombined and guided to a detector that collects the interference signal and transmits to a computer to generate the image.23,24
Different types of OCT exist to provide structural, functional, and quantitative information on skin collagen content. The swept-source (SS)-OCT modality can provide structural information on skin collagen that is directly related to the degree of close packing and density of collagen bundles.5,27 Polarization-sensitive (PS)-OCT can collect 3-dimensional collagen imaging by measuring alterations in the polarization state of reflected light.5,27 Tissues with increased levels of collagen content, such as skin scarring and fibrosis, rapidly change the polarization state of light and have high phase retardation rates; tissues with decreased collagen content, such as photoaged or photodamaged skin, slowly change the polarization state of light and have low phase retardation rates.28
OCT has several unique benefits that clinicians and researchers can take advantage of. Specifically, unlike traditional skin biopsies, OCT can conduct a non-invasive and time-efficient survey of multiple skin sites without causing any patient discomfort.29 Studies demonstrate that OCT has excellent intra-observer and inter-observer reliability, and that OCT imaging may facilitate standardized clinical scoring of diseases.29 Finally, OCT requires minimal training to operate and allows clinicians to save, store, and transmit images for later reading.29 This workflow facilitates accurate and blinded data analysis in clinical trials.29 We foresee OCT playing an important role in the management and research of various epidermal and dermal cutaneous diseases.
The purpose of this review is to present the available evidence on the ability of OCT to image normal, chronologically aged, photoaged or photodamaged skin in human subjects. Herein, we present an overview of current data on the utility of OCT imaging and clinical applications in the management and treatment of normal, chronologically aged, photoaged, and photodamaged skin.
MATERIALS AND METHODS
We performed a review of published literature from January 1990 to December 2014 on the imaging of skin collagen using OCT (Figure 2). We searched Medline, PubMed, EMBASE, Web of Science, Google Scholar, and Cochrane databases using the following search terms: “optical coherence tomography,” “OCT,” “skin,” “collagen,” “photoaging,” “wrinkles,” and “photodamage.” The relevant articles that met the following criteria were selected for inclusion: research studies evaluating OCT imaging of collagen in normal, chronologically aged, photoaged or photodamaged skin. Literature published in languages other than English were excluded. Articles were assigned a level of evidence (LOE) and afterward graded according to the Oxford Center for Evidence-based Medicine Levels of Evidence Grades of Recommendation (GOR) (Tables 2 and 3).30
FIGURE 2.
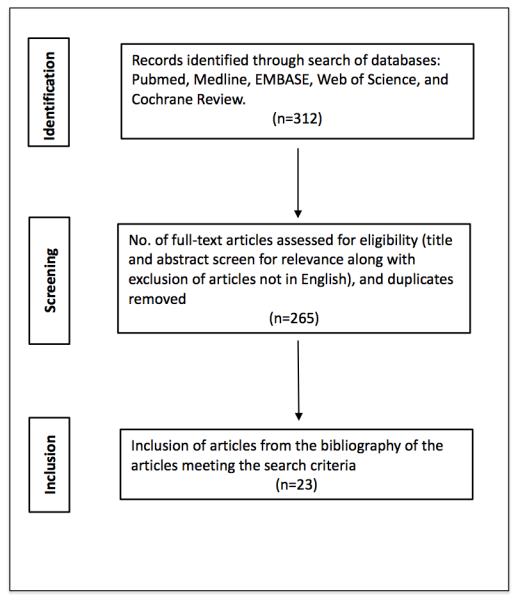
Search Strategy Results.
Table 2.
Level of Evidence (LOE)
| 1a. Systematic review of RCTs |
| 1b. Individual RCT |
| 2a. Systematic review of cohort studies |
| 2b. Individual cohort study (including low-quality RCT) |
| 3a. Systematic review of case-control studies |
| 3b. Individual case-control study |
| 4b. Case series |
| 5. Case reports, expert opinion, bench research |
RCT, Randomized controlled trial.
Data from Oxford Center for Evidence-based Medicine Levels of Evidence 30
Table 3.
Grades of Recommendation (GOR)
| A. Studies with consistent LOE 1a and/or 1b |
| B. Studies with consistent LOE 2a, 2b, 3a, or 3b; or extrapolations from studies with LOE 1a or 1b |
| C. Studies with LOE 4 or extrapolations from studies with LOE 2a, 2b, 3a, or 3b |
| D. Studies with LOE 5 or troubling inconsistent or inconclusive studies of any level |
LOE, Level of evidence.
Data from Oxford Center for Evidence-based Medicine Levels of Evidence 30
RESULTS
Our search resulted in 312 articles (Figure 2). After duplicates were removed, a total of 265 unique articles were considered and screened. Of the remaining 265 articles, 23 articles investigating OCT skin collagen imaging met inclusion criteria and are included in this review. A summary of the studies identified is presented in Table 4.
Table 4.
Research studies evaluating OCT imaging of collagen in normal, chronologically-aged, photoaged or photodamaged skin
| Authors | LOE | Study Aim | Population Characteristics |
Findings | Limitations | Year |
|---|---|---|---|---|---|---|
| Normal Skin (Grade of Recommendation C) | ||||||
| Alex et al. 33 | 4 | To compare images of human skin in vivo from different locations at three different wavelength regions |
Healthy volunteers; (n = 3); examined skin of the PIP joint of the middle finger and dorsal forearm |
The epidermis and dermal- epidermal junction was best imaged using OCT at 800 nm. Images of deeper dermal layers were best imaged using OCT at 1300 nm. |
Small sample size |
2010 |
| Lee et al. 47 | 4 | To investigate morphological differences in 3-D images with cellular resolution between nonmelanoma skin cancer and normal skin using GD-OCM |
1 patient with BCC of the nose, and 2 patients with SCC of the ear and cheek. Normal skin sample of the nose, ear, and cheek of 3 healthy patients. |
GD-OCM excellent resolution images. Skin appenages such as hair follicles, ducts of sebaceous glands, sweat glands, blood vessels, and extracellular matrix of connective tissue were visualized. GD-OCM showed the disruption of normal skin layers and disruption of the organized array of keratinocytes in BCC and SCC compared to normal skin. |
Small sample size |
2012 |
| Mogensen et al. 4 |
4 | To describe normal skin collagen morphology using PS-OCT imaging |
Healthy volunteers aged 0.5-59 years; (n = 20); examined forehead, ear lobe, nose, cheek, chin, back of the neck, chest, hands, arms and calf |
PS-OCT images showed characteristic structures due to the birefringence differences between epidermis, papillary, and reticular dermis |
Small proportion of children relative to adults in the study |
2008 |
| Pierce et al.
34 |
4 | To measure anatomic variations in birefringence using PS-OCT |
Healthy volunteers aged 24-35 years; (n = 5); examined lower back, temple, and hand |
Mean phase retardation highest for skin of the lower back and lowest for skin of the temple |
Study limited to male subjects; small sample size |
2004 |
| Pircher et
al. 31 |
4 | To use phase resolved PS-OCT to investigate polarization properties of different regions of human skin in vivo |
Healthy volunteers; (n not specified); examined fingertip and hand |
3-D PS-OCT has potential to increase contrast and quantify retardation and orientation of birefringent structures in skin |
Selected skin regions examined |
2004 |
| Yasuno et
al. 32 |
4 | To investigate normal skin birefringence using PS-OCT |
Healthy volunteers | PS-OCT successfully revealed the birefringent nature of human skin tissue |
Limited information on volunteers |
2002 |
| Chronological Aging (Grade of Recommendation C) | ||||||
| Florence et
al. 40 |
4 | To investigate the functional and structural alterations that occur in scalp skin with aging using OCT |
Healthy volunteers; (n = 15, mean age 30; n = 15, mean age 62); examined scalp skin and mid-forehead skin as control |
OCT demonstrated total skin (epidermis + dermis) thickness increased with age on both scalp and forehead. The thickness of scalp epidermis decreased with age but no significant changes on the forehead. |
Study limited to Caucasian female subjects |
2013 |
| Gambichler et al. 37 |
4 | To investigate the influence of age, gender, skin type, and anatomic site on the mean epidermal thickness (ET) using OCT in vivo |
Healthy volunteers; (n = 83); examined skin of forehead, pectoral, scapular, forearm, buttock, and calf |
OCT was precise in terms of repeatability and reproducibility. A significant decrease of ET with age in all anatomic sites. ET did not significantly differ between Caucasian and ethnic individuals |
Limited skin regions examined |
2006 |
| Hara et al. 41 | 4 | To calculate Young’s modulus of the stratum corneum using OCT |
Healthy volunteers aged 20-68; (n = 78); examined cheek |
OCT aided in the calculation of the mean Young’s modulus of the stratum corneum (1.993 MPa) and dermis (0.066 MPa). There was a weak relationship between Young’s modulus of the stratum corneum and age (r = 0.337) |
Selected ethnic population |
2013 |
| Kunzi-Rapp et al 42 |
4 | Use OCT to evaluate new collagen synthesis after scar treatment with the Er:YAG laser |
Post-traumatic and acne scar patients aged 12-39 years; (n = 12); examined face and extremities |
OCT demonstrated the production of new collagen bundles after scar treatment with the Er:YAG laser |
N/A – OCT was not the main goal of study but rather used in assessment |
2006 |
| Neerken et
al. 39 |
4 | To compare age- related changes in human skin in vivo using OCT and CLSM |
Healthy volunteers; (n = 15, mean age 22.5; n = 15, mean age 55.3); examined volar aspect of forearm and temple |
Both OCT and CSLM allowed in
vivo characterization of epidermal and dermal layers. The second bright reflecting layer on OCT corresponds to the deep bright fibrous layer visible in CLSM. |
Limited skin regions examined |
2004 |
| Shlivko et
al. 35 |
4 | To study the age- specific morphofunctional features of skin using OCT |
Healthy volunteers aged 4-74; (n = 43); examined 18 different anatomic areas |
OCT revealed areas near external actions showed statistically significant correlations between age and thickness of the epidermis, pigmentation level, and elasticity. Areas exposed to external actions had significant correlations between age and hydration and elasticity. |
Limited information on sample ethnicity and sex |
2013 |
| Shlivko et
al. 48 |
4 | To investigate the influence of topical corticosteroid therapy and tacrolimus on morphological indices of different skin phototypes and to optimize therapy using OCT |
Healthy volunteers aged 20 to 30; (n = 20); examined flexor surface of forearm |
OCT demonstrated morphological manifestations of skin atrophy occurred earlier with clobetasol propionate than with hydrocortisone 17- butyrate, with changes occurring faster in phototype V and VI. |
Small sample size and limited selection of steroid therapy |
2014 |
| Tsugita et
al. 36 |
4 | To investigate the positional differences and aging changes using OCT |
Healthy volunteers; (n = 116); examined 11 different anatomic sites |
OCT revealed that with advancing age, the epidermal thickness becomes less at some site but no change at other sites. A thinning trend of epidermal thickness was observed up to age of 30. |
Sample population limited to Japanese female subjects |
2013 |
| Photoaged or photodamaged (Grade of Recommendation C) | ||||||
| Barton et al.
43 |
4 | To investigate the appearance of sun-protected and sun-damaged skin using OCT |
Volunteers with sun damaged skin; (n = 20); examined forearm (sun- exposed)and upper inner arm (sun- protected) |
OCT images of sun-damaged skin revealed increased signal in the epidermis and rapid attenuation of light. |
Small sample size |
2003 |
| Gambichler et al. 44 |
4 | To investigate changes of epidermal thickness following UVA1- and UVB- irradiation using OCT |
Healthy volunteers; (n = 12); examined upper back |
OCT demonstrated UVA and UVB exposed skin showed significant increase of epidermal thickness of 11% and 25%, respectively. Epidermal thickness between UVA and UVB exposed skin differed significantly. |
Small sample size and limited skin regions examined |
2005 |
| Gambichler et al. 45 |
4 | To evaluate the kinetics of acute UVB- and UVA1- induced skin alterations by OCT |
Healthy volunteers; (n = 20); examined upper back |
OCT imaging revealed markedly higher values of epidermal thickness on UVB-irradiated skin and slightly increased epidermal thickening on UVA1-irraidated skin. After confirmation with histology, UVB-irradiated skin showed disruption of entrance signal that corresponds to hyperkeratosis and parakeratosis. |
Small sample size and limited skin regions examined |
2005 |
| Korde et al. 49 |
4 | To evaluate skin sun damage in a large population with histology and OCT |
Healthy volunteers; (n = 112); examined forearm (sun- exposed) and upper inner arm (sun- protected) |
OCT images revealed statically significant difference between the average attenuation values of skin with minimal and severe solar elastosis. OCT images distinguished actinic keratosis from normal skin with 86% sensitivity and 83% specificity. |
Limited skin regions examined |
2007 |
| Querleux et
al. 50 |
4 | To investigate skin structural features in different ethnic groups using OCT |
Healthy volunteers (African Americans, Mexicans, Caucasians, and Chinese) aged 18- 78; (n = 388); examined ventral forearm (sun- protected) and dorsal forearm and cheek (sun-exposed) |
OCT revealed skin thickness is higher on the cheek compared to dorsal and ventral forearm with no relationship to ethnicity or age. OCT images revealed thickness of the dermal- epidermal junction decreased with age and was higher in African Americans than Caucasians. |
Limited skin regions examined |
2009 |
| Sakai et al.
16 |
4 | Use PS-OCT to assess intrinsic age-related and photo-age-related differences in 3-D dermal birefringence |
Healthy volunteers; (n = 2); examined cheek (sun-exposed) and inner upper arm (sun- protected) |
PS-OCT revealed significantly smaller dermal birefringence of the cheek in the old group compared to the young group. The inner upper arm showed no significant difference. |
Small sample size and limited skin regions examined |
2008 |
| Sakai et al. 51 |
4 | To investigate dermal birefringence and elasticity and skin morphology using PS-SD-OCT |
Healthy volunteers; (n = 19); examined outer corner of the eyes |
OCT demonstrated the averaged upper dermal birefringence showed significant depth- dependent correlation with skin roughness. |
Small sample size |
2009 |
| Sakai et al.
52 |
4 | To investigate anisotropic changes in the dermal birefringence of mechanically deformed human skin using PS- OCT |
Healthy volunteers aged 50 to 58; (n = 22); examined center of forehead |
PS-OCT revealed papillary- dermal birefringence of the forehead increased significantly when skin was shrunk parallel to body axis, and decreased significantly when skin was shrunk perpendicular to body axis. Skin shrinking in both instances promoted formation of macro rope-like birefringent domains |
Small sample size and limited skin regions examined |
2011 |
| Vasquez – Pinto et al. 46 |
4 | To evaluate changes in skin topography during tests of a wrinkle- reduction product using OCT |
Healthy volunteers aged 41 to 53; (n = 30); examined periorbital region |
OCT imaging demonstrated 10% reduction in skin roughness and a reduction in occurrence of wrinkles deeper than 170 μm after treatment. |
Small sample size |
2014 |
DISCUSSION
OCT Imaging of Normal Skin Collagen
OCT has been studied frequently in the imaging of normal skin collagen.4,31,32 One focus of normal skin imaging research is to optimize the depth and resolution parameters to continue to improve OCT technology allowing better resolution when imaging deeper dermal layers.33 Studies on normal skin suggest that integrating multiple wavelengths of light may allow OCT imaging into deeper layers of the dermis. Specifically, due to the inherent penetrance properties of 800-nm wavelength light, 800-nm OCT is able to provide high axial resolution in superficial skin layers, which allowed detailed visualization of skin structures located in the epidermis or superficial dermis. However, 1300-nm OCT provides an image of deeper dermal regions with a trade-off in resolution compared to 800-nm. Future OCT devices that allow the user to use 800-nm and 1300-nm simultaneously may aid researchers and clinicians in quickly evaluating the skin at the optimal resolution for each layer.
In addition to image optimization, OCT has been used to study collage profiles of different normal skin at anatomic sites.34 One study demonstrated this principle by collecting PS-OCT images of the lower back, temple, and hand of healthy volunteers.34 In these images, the mean phase retardation rate, a measure of collagen content, varied by anatomical location; the lower back skin had the highest values, while the temple skin had the lowest values.34 Importantly, these measures were reproducible and supported the idea that OCT is sensitive enough to measure small basal variations in skin collagen contents. Furthermore, the reproducibility of these measures also suggests that OCT imaging can be used to track progression and response-to-therapy in skin conditions characterized by altered collagen content.
Grade of Recommendation
Grade C for OCT imaging of normal skin collagen (6 studies with LOE 4). We anticipate that continued research on OCT imaging of normal skin will foster a better understanding of the diagnostic and management capabilities of OCT imaging.18 In the future, we believe OCT may become a good alternative to skin biopsy to quantify normal skin collagen.
Chronological Aged Skin
OCT has the ability to measure the characteristic skin changes that result from chronological aging, such as alterations in epidermal thickness and elasticity.35,36 For instance, one study demonstrated that OCT is capable of measuring age-correlated decreases in epidermal thickness of the forehead, pectoral, scapular, forearm, buttock, and calf.37 A number of additional studies have confirmed that OCT is capable of detecting decreases in epidermal thickness and increases in tissue attenuation characteristic chronological aging of skin.38-40 One study utilized OCT, along with skin elasticity measures, to evaluate the age-related changes in skin elasticity and found that OCT was capable of identifying a statistically significant relationship between skin and age.41 This study suggests that OCT may have a role alongside other instruments to assess skin elasticity.
OCT may also be helpful for monitoring efficacy of cosmetic and medical therapies targeting chronological skin aging.41 For instance, assessment of clinical response to ablative and non-ablative laser is often subjective. A recent study demonstrated that OCT is a good modality to objectively assess the results of Er:YAG laser treatment for improvement of rhytids.42 Specifically, OCT was used to visualize new collagen fiber formation that correlated with improved elasticity at four weeks post-treatment.42 OCT measures of skin elasticity will allow clinicians and researchers to personalize laser settings based on individual response-to-therapy that may lead to better patient outcomes. Skin aging is an area of active research within our group and we envision that OCT imaging will be useful in gaining a deeper understanding of the chronological aging process and evaluation of both old and new methods to treat and prevent chronological skin aging.
Grade of Recommendation
Grade C for OCT imaging of chronological aging skin (8 studies with LOE 4). We believe OCT has the capabilities of identifying chronological aging skin and is a promising tool for monitoring response to treatment. OCT has the potential to personalize therapies targeting chronological skin aging based on objective imaging of individual response at regular time-intervals.
Photoaged or Photodamaged Skin
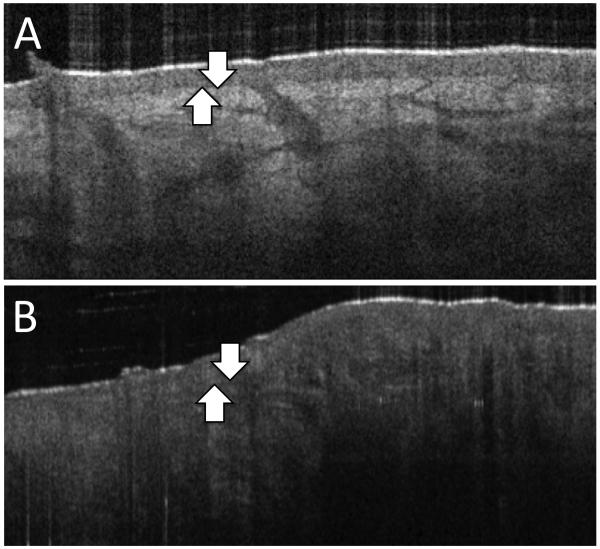
OCT imaging has the ability to identify photoaging skin and monitor the skin’s response to therapy. Characteristic findings on OCT imaging of photoaged skin include: epidermal atrophy, loss of papillary dermis, and dermal mottled texture (Figure 3). OCT imaging also demonstrates uneven epidermal surface, thickening of the stratum corneum, and a greater attenuation of light in sun-damaged skin compared to sun-protected skin.43 Several studies have demonstrated OCT is capable of reliably identifying the features inherent to photoaged skin.43
FIGURE 3. A.
OCT image of normal healthy skin demonstrating a clear change in OCT contrast between epidermis and papillary dermis (arrows). B. OCT image of photodamaged skin. Image depicts undefined dermal-epidermal junction (arrows), epidermal atrophy, loss of papillary dermis, and dermal mottled texture loss due to collagen solar elastosis. (Images courtesy of Michelson Diagnostics)
One such study utilized OCT to evaluate photoaged skin in human volunteers.16 Researchers measured dermal collagen of patient cheek skin (a sun-exposed site, representative of photoaging) and compared to interior upper arm skin (a sun-protected site, representative of chronological aging).16 Results demonstrated that dermal birefringence differed by location and photoaged skin had significant decrease in collagen and birefringence.16 A second study from the same group reported additional OCT indicators of photoaging skin, in which OCT was able to reliably assess photoaging skin by evaluating upper dermis degeneration, skin roughness, and wrinkle formation. Furthermore, recent clinical studies demonstrate that OCT is not only capable of imaging chronic changes, but also acute changes that results from UVA1 and UVB exposure and that these findings correlate well with histological features.44,45 Further research needs to be done to evaluate the sensitivity and specificity of epidermal attenuation, thickness, dermal degeneration, and wrinkle measures when clinically evaluating chronologically and photoaged skin and response to therapy.
OCT may one day be a mainstay in clinical trials evaluating topical anti-aging products. One clinical trial utilized OCT to measure wrinkle-depth improvement after application of a topical agent to treat photoaging.46 After application of the topical study agent, “Natura Chronos Flavonóides de Passiflora 45+ FPS15,” twice daily for 28 days, OCT imaging was used to measure a 10% reduction in skin roughness and a significantly reduced number of wrinkles deeper than 170 μm which corresponded well to clinical improvement.46 This clinical study is an example of the utility of OCT in assessing the efficacy of topical agents aimed at preventing or reversing UV-induced photodamage; a feat that was previously not possible without invasive methods.
Grade of Recommendation
Grade C for OCT imaging of photoaged or photodamaged skin (9 studies with LOE 4). We believe OCT can provide information equivalent to or in addition to skin biopsies for assessing photoaged or photodamaged skin. In the future, we envision OCT will be a mainstay in clinical trials evaluating novel therapies aimed at treating or preventing skin photoaging.
Current Limitations and Future Direction of OCT
OCT has some technical limitations that would further enhance clinical utility if improved upon. Penetration depth is a significant limitation of OCT that may be improved through concurrent use of other acousto-optic imaging systems. These combination systems may provide clinicians with a more detailed picture and better understanding of skin status, with greater penetration depth to image deeper structures of skin.
Using OCT, clinicians can obtain a timely visual assessment of skin collagen; however, current OCT systems are limited in their ability to rapidly quantify collagen content. Replacing the current third party applications and algorithms used in collagen data analysis with built-in visual overlays and quantification tools may improve clinical utility of collagen quantification. For instance, a high-resolution OCT imaging system that combines color mapping with OCT imaging to evaluate collagen content may provide more clinicians the ability to rapidly quantify a patient’s collagen status without taking any skin biopsies. Furthermore, OCT’s ability to quickly provide visual and quantitative analyses of skin collagen content holds tremendous potential as a platform to assist in overcoming teledermatology’s tactile limitations when examining patients.28
However, OCT technology is not without limitations. Unfortunately, the current cost and size of OCT systems are two major obstacles to widespread adoption. As costs decline and technology trends toward hand-held designs, we anticipate that adoption will increase among both clinicians and researchers. Future innovations and cost reductions in the telecommunication sector will likely trickle down to reductions in size and cost of OCT machines. Another limitation with OCT is that the majority of studies for dermatologic applications of OCT are limited by small sample sizes. OCT technology is still early on its development and in spite of these limitations, we anticipate tremendous growth in the field of OCT skin imaging that will parallel the development ultrasound technology has experienced over the past 30 years.28
In conclusion, OCT generates 2- or 3-dimensional skin images with sufficient depth and resolution to characterize a number of normal and pathological skin processes. OCT imaging provides a non-invasive and rapid method to diagnose and evaluate many dermatologic conditions while maintaining patient comfort. Skin disorders with decreased collagen content, such as photoaged and photodamaged skin have been successfully imaged using OCT technology. We believe that OCT is well suited for monitoring chronological aged and photoaged skin progression and the effects of dermatologic anti-aging interventions. The rapid and reproducible images generated by OCT have the potential to transform dermatologic imaging and provide new insights into the physiology, pathology, management, and research of numerous cutaneous conditions. We foresee that OCT imaging to evaluate skin aging will not only help identify pathological changes earlier, but will also assist in evaluating response to therapy longitudinally without biopsy. In turn, OCT will facilitate the development and implementation of new products or methods to treat skin aging and will lead to earlier treatment of skin aging with improved patient outcomes.
Acknowledgments
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award TL1 TR000133.
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002 and linked award KL2 TR000134.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CSLM
Confocal Scanning Laser Microscopy
- CT
Computerized tomography
- Er:YAG
Erbium:YAG laser
- FD-OCT
Frequency domain optical coherence tomography
- MRI
Magnetic resonance imaging
- OCT
Optical coherence tomography
- PS-OCT
Polarization-sensitive optical coherence tomography
- RCSM
Reflectance confocal scanning microscopy
- SD-OCT
Spectral domain optical coherence tomography
- SS-OCT
Swept-source optical coherence tomography
- US
Ultrasound
Footnotes
Conflict of Interest: The authors declare that they have no disclosures or conflicts of interest.
REFERENCES
- 1.Dalimier E, Salomon D. Full-field optical coherence tomography: a new technology for 3D high-resolution skin imaging. Dermatology. 2012;224(1):84–92. doi: 10.1159/000337423. [DOI] [PubMed] [Google Scholar]
- 2.Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Archives of dermatological research. 2011 Sep;303(7):457–473. doi: 10.1007/s00403-011-1152-x. [DOI] [PubMed] [Google Scholar]
- 3.Matcher SJ. Practical aspects of OCT imaging in tissue engineering. Methods in molecular biology. 2011;695:261–280. doi: 10.1007/978-1-60761-984-0_17. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen M, Morsy HA, Thrane L, Jemec GB. Morphology and epidermal thickness of normal skin imaged by optical coherence tomography. Dermatology. 2008;217(1):14–20. doi: 10.1159/000118508. [DOI] [PubMed] [Google Scholar]
- 5.Sattler E, Kastle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18(6):061224. doi: 10.1117/1.JBO.18.6.061224. [DOI] [PubMed] [Google Scholar]
- 6.Welzel J. Optical coherence tomography in dermatology: a review. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2001 Feb;7(1):1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nature biotechnology. 2003 Nov;21(11):1361–1367. doi: 10.1038/nbt892. [DOI] [PubMed] [Google Scholar]
- 8.Cahill RA, Mortensen NJ. Intraoperative augmented reality for laparoscopic colorectal surgery by intraoperative near-infrared fluorescence imaging and optical coherence tomography. Minerva chirurgica. 2010 Aug;65(4):451–462. [PubMed] [Google Scholar]
- 9.Chu CR, Izzo NJ, Irrgang JJ, Ferretti M, Studer RK. Clinical diagnosis of potentially treatable early articular cartilage degeneration using optical coherence tomography. Journal of biomedical optics. 2007 Sep-Oct;12(5):051703. doi: 10.1117/1.2789674. [DOI] [PubMed] [Google Scholar]
- 10.Unterhuber A, Povazay B, Bizheva K, et al. Advances in broad bandwidth light sources for ultrahigh resolution optical coherence tomography. Physics in medicine and biology. 2004 Apr 7;49(7):1235–1246. doi: 10.1088/0031-9155/49/7/011. [DOI] [PubMed] [Google Scholar]
- 11.Lamirel C, Newman N, Biousse V. The use of optical coherence tomography in neurology. Reviews in neurological diseases. 2009;6(4):E105–120. Fall. [PubMed] [Google Scholar]
- 12.Tearney GJ, Brezinski ME, Bouma BE, et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997 Jun 27;276(5321):2037–2039. doi: 10.1126/science.276.5321.2037. [DOI] [PubMed] [Google Scholar]
- 13.Welzel J, Lankenau E, Birngruber R, Engelhardt R. Optical coherence tomography of the human skin. Journal of the American Academy of Dermatology. 1997 Dec;37(6):958–963. doi: 10.1016/s0190-9622(97)70072-0. [DOI] [PubMed] [Google Scholar]
- 14.Krieg T, Aumailley M, Koch M, Chu M, Uitto J. Fitzpatrick's Dermatology in General Medicine. 8th McGraw-Hill; New York: 2012. Collagens, Elastic Fibers, and Other Extracellular Matrix Proteins of the Dermis. [Google Scholar]
- 15.Liu B, Vercollone C, Brezinski ME. Towards improved collagen assessment: polarization-sensitive optical coherence tomography with tailored reference arm polarization. International journal of biomedical imaging. 2012;2012:892680. doi: 10.1155/2012/892680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai S, Yamanari M, Miyazawa A, et al. In vivo three-dimensional birefringence analysis shows collagen differences between young and old photo-aged human skin. J Invest Dermatol. 2008 Jul;128(7):1641–1647. doi: 10.1038/jid.2008.8. [DOI] [PubMed] [Google Scholar]
- 17.Ferraq Y, Black DR, Theunis J, Mordon S. Superficial wounding model for epidermal barrier repair studies: comparison of Erbium:YAG laser and the suction blister method. Lasers in surgery and medicine. 2012 Sep;44(7):525–532. doi: 10.1002/lsm.22054. [DOI] [PubMed] [Google Scholar]
- 18.Hensley K, Floyd RA. Reactive oxygen species and protein oxidation in aging: a look back, a look ahead. Arch Biochem Biophys. 2002 Jan;15397(2):377–383. doi: 10.1006/abbi.2001.2630. [DOI] [PubMed] [Google Scholar]
- 19.Baillie L, Askew D, Douglas N, Soyer HP. Strategies for assessing the degree of photodamage to skin: a systematic review of the literature. The British journal of dermatology. 2011 Oct;165(4):735–742. doi: 10.1111/j.1365-2133.2011.10416.x. [DOI] [PubMed] [Google Scholar]
- 20.Crisan M, Crisan D, Sannino G, Lupsor M, Badea R, Amzica F. Ultrasonographic staging of cutaneous malignant tumors: an ultrasonographic depth index. Archives of dermatological research. 2013 May;305(4):305–313. doi: 10.1007/s00403-013-1321-1. [DOI] [PubMed] [Google Scholar]
- 21.Han JH, Kang JU, Song CG. Polarization sensitive subcutaneous and muscular imaging based on common path optical coherence tomography using near infrared source. Journal of medical systems. 2011 Aug;35(4):521–526. doi: 10.1007/s10916-009-9388-0. [DOI] [PubMed] [Google Scholar]
- 22.Mogensen M, Thrane L, Joergensen TM, Andersen PE, Jemec GB. Optical coherence tomography for imaging of skin and skin diseases. Seminars in cutaneous medicine and surgery. 2009 Sep;28(3):196–202. doi: 10.1016/j.sder.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Wessels R, De Bruin DM, Faber DJ, Van Leeuwen TG, Van Beurden M, Ruers TJ. Optical biopsy of epithelial cancers by optical coherence tomography (OCT) Lasers in medical science. 2013 Mar;16 doi: 10.1007/s10103-013-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce MC, Strasswimmer J, Park BH, Cense B, de Boer JF. Advances in optical coherence tomography imaging for dermatology. J Invest Dermatol. 2004 Sep;123(3):458–463. doi: 10.1111/j.0022-202X.2004.23404.x. [DOI] [PubMed] [Google Scholar]
- 25.Gladkova ND, Petrova GA, Nikulin NK, et al. In vivo optical coherence tomography imaging of human skin: norm and pathology. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2000 Feb;6(1):6–16. doi: 10.1034/j.1600-0846.2000.006001006.x. [DOI] [PubMed] [Google Scholar]
- 26.Tadrous PJ. Methods for imaging the structure and function of living tissues and cells: 3. Confocal microscopy and micro-radiology. The Journal of pathology. 2000 Aug;191(4):345–354. doi: 10.1002/1096-9896(200008)191:4<345::AID-PATH696>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Phillips KG, Wang Y, Levitz D, et al. Dermal reflectivity determined by optical coherence tomography is an indicator of epidermal hyperplasia and dermal edema within inflamed skin. Journal of biomedical optics. 2011 Apr;16(4):040503. doi: 10.1117/1.3567082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babalola O, Mamalis A, Lev-Tov H, Jagdeo J. Optical coherence tomography (OCT) of collagen in normal skin and skin fibrosis. Archives of dermatological research. 2014 Jan;306(1):1–9. doi: 10.1007/s00403-013-1417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abignano G, Aydin SZ, Castillo-Gallego C, et al. Virtual skin biopsy by optical coherence tomography: the first quantitative imaging biomarker for scleroderma. Annals of the rheumatic diseases. 2013 Feb 20; doi: 10.1136/annrheumdis-2012-202682. [DOI] [PubMed] [Google Scholar]
- 30.Ho D, Jagdeo J. Biological properties of a new volumizing hyaluronic Acid filler: a systematic review. Journal of drugs in dermatology : JDD. 2015 Jan 1;14(1):50–54. [PubMed] [Google Scholar]
- 31.Pircher M, Goetzinger E, Leitgeb R, Hitzenberger C. Three dimensional polarization sensitive OCT of human skin in vivo. Optics express. 2004 Jul 12;12(14):3236–3244. doi: 10.1364/opex.12.003236. [DOI] [PubMed] [Google Scholar]
- 32.Yasuno Y, Makita S, Sutoh Y, Itoh M, Yatagai T. Birefringence imaging of human skin by polarization-sensitive spectral interferometric optical coherence tomography. Optics letters. 2002;27(20):1803–1805. doi: 10.1364/ol.27.001803. [DOI] [PubMed] [Google Scholar]
- 33.Alex A, Povazay B, Hofer B, et al. Multispectral in vivo three-dimensional optical coherence tomography of human skin. J Biomed Opt. 2010 Mar-Apr;15(2):026025. doi: 10.1117/1.3400665. [DOI] [PubMed] [Google Scholar]
- 34.Pierce MC, Strasswimmer J, Hyle Park B, Cense B, De Boer JF. Birefringence measurements in human skin using polarization-sensitive optical coherence tomography. Journal of biomedical optics. 2004 Mar-Apr;9(2):287–291. doi: 10.1117/1.1645797. [DOI] [PubMed] [Google Scholar]
- 35.Shlivko IL, Petrova GA, Zor'kina MV, et al. Complex assessment of age-specific morphofunctional features of skin of different anatomic localizations. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2013 Feb;19(1):e85–92. doi: 10.1111/j.1600-0846.2012.00613.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsugita T, Nishijima T, Kitahara T, Takema Y. Positional differences and aging changes in Japanese woman epidermal thickness and corneous thickness determined by OCT (optical coherence tomography) Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2013 Aug;19(3):242–250. doi: 10.1111/srt.12021. [DOI] [PubMed] [Google Scholar]
- 37.Gambichler T, Matip R, Moussa G, Altmeyer P, Hoffmann K. In vivo data of epidermal thickness evaluated by optical coherence tomography: effects of age, gender, skin type, and anatomic site. J Dermatol Sci. 2006 Dec;44(3):145–152. doi: 10.1016/j.jdermsci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Wu S, Li H, Zhang X, Li Z. Optical features for chronological aging and photoaging skin by optical coherence tomography. Lasers Med Sci. 2013 Feb;28(2):445–450. doi: 10.1007/s10103-012-1069-4. [DOI] [PubMed] [Google Scholar]
- 39.Neerken S, Lucassen GW, Bisschop MA, Lenderink E, Nuijs TA. Characterization of age-related effects in human skin: A comparative study that applies confocal laser scanning microscopy and optical coherence tomography. J Biomed Opt. 2004 Mar-Apr;9(2):274–281. doi: 10.1117/1.1645795. [DOI] [PubMed] [Google Scholar]
- 40.Florence P, Cornillon C, D'Arras MF, et al. Functional and structural age-related changes in the scalp skin of Caucasian women. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2013 Nov;19(4):384–393. doi: 10.1111/srt.12057. [DOI] [PubMed] [Google Scholar]
- 41.Hara Y, Masuda Y, Hirao T, Yoshikawa N. The relationship between the Young's modulus of the stratum corneum and age: a pilot study. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2013 Aug;19(3):339–345. doi: 10.1111/srt.12054. [DOI] [PubMed] [Google Scholar]
- 42.Kunzi-Rapp K, Dierickx CC, Cambier B, Drosner M. Minimally invasive skin rejuvenation with Erbium: YAG laser used in thermal mode. Lasers in surgery and medicine. 2006 Dec;38(10):899–907. doi: 10.1002/lsm.20380. [DOI] [PubMed] [Google Scholar]
- 43.Barton JK, Gossage KW, Xu W, et al. Investigating sun-damaged skin and actinic keratosis with optical coherence tomography: a pilot study. Technol Cancer Res Treat. 2003 Dec;2(6):525–535. doi: 10.1177/153303460300200605. [DOI] [PubMed] [Google Scholar]
- 44.Gambichler T, Kunzlberger B, Paech V, et al. UVA1 and UVB irradiated skin investigated by optical coherence tomography in vivo: a preliminary study. Clin Exp Dermatol. 2005 Jan;30(1):79–82. doi: 10.1111/j.1365-2230.2004.01690.x. [DOI] [PubMed] [Google Scholar]
- 45.Gambichler T, Boms S, Stucker M, et al. Acute skin alterations following ultraviolet radiation investigated by optical coherence tomography and histology. Archives of dermatological research. 2005 Nov;297(5):218–225. doi: 10.1007/s00403-005-0604-6. [DOI] [PubMed] [Google Scholar]
- 46.Vasquez-Pinto LM, Maldonado EP, Raele MP, Amaral MM, de Freitas AZ. Optical coherence tomography applied to tests of skin care products in humans - a case study. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2014 Jul 26; doi: 10.1111/srt.12161. [DOI] [PubMed] [Google Scholar]
- 47.Lee KS, Zhao H, Ibrahim SF, Meemon N, Khoudeir L, Rolland JP. Three-dimensional imaging of normal skin and nonmelanoma skin cancer with cellular resolution using Gabor domain optical coherence microscopy. J Biomed Opt. 2012 Dec;17(12):126006. doi: 10.1117/1.JBO.17.12.126006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shlivko IL, Kamensky VA, Donchenko EV, Agrba P. Morphological changes in skin of different phototypes under the action of topical corticosteroid therapy and tacrolimus. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2014 May;20(2):136–140. doi: 10.1111/srt.12095. [DOI] [PubMed] [Google Scholar]
- 49.Korde VR, Bonnema GT, Xu W, et al. Using optical coherence tomography to evaluate skin sun damage and precancer. Lasers in surgery and medicine. 2007 Oct;39(9):687–695. doi: 10.1002/lsm.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Querleux B, Baldeweck T, Diridollou S, et al. Skin from various ethnic origins and aging: an in vivo cross-sectional multimodality imaging study. Skin research and technology : official journal of International Society for Bioengineering and the Skin. 2009 Aug;15(3):306–313. doi: 10.1111/j.1600-0846.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 51.Sakai S, Nakagawa N, Yamanari M, Miyazawa A, Yasuno Y, Matsumoto M. Relationship between dermal birefringence and the skin surface roughness of photoaged human skin. J Biomed Opt. 2009 Jul-Aug;14(4):044032. doi: 10.1117/1.3207142. [DOI] [PubMed] [Google Scholar]
- 52.Sakai S, Yamanari M, Lim Y, Nakagawa N, Yasuno Y. In vivo evaluation of human skin anisotropy by polarization-sensitive optical coherence tomography. Biomedical optics express. 2011 Sep;12(9):2623–2631. doi: 10.1364/BOE.2.002623. [DOI] [PMC free article] [PubMed] [Google Scholar]