Abstract
En1 is a homeobox-containing transcription factor expressed during development in diverse tissues, including the embryonic midbrain and anterior hindbrain. To facilitate investigation of genetic and developmental heterogeneity among cells with a history of En1 expression, we have generated En1Dre, a knock-in allele expressing Dre recombinase. En1Dre can be used with existing Cre and Flp recombinase lines for genetic intersectional labeling, fate mapping, and functional manipulation of subpopulations of cells characterized by transient expression of En1. To avoid disrupting En1 function, the Dre cDNA is inserted at the 3′ end of the En1 coding sequence, together with a viral 2A peptide to mediate translation of separate EN1 and Dre proteins. Consequently, viable and fertile En1Dre homozygotes can be used to increase the proportion of useful genotypes produced in complex crosses. The pattern of Dre expression from En1Dre is indistinguishable from wild-type En1 expression in mid-gestation mouse embryos, and En1Dre controls Dre-responsive indicator alleles by efficiently recombining rox sites in vivo. Through the application of genetic tools that allow manipulation of cells based on combinatorial expression of multiple distinct recombinases, En1Dre will significantly extend the ability to target important subpopulations of neurons and other cells within the broader En1 expression domain.
Keywords: Dre/rox, mouse, midbrain, hindbrain, recombination
INTRODUCTION
The homeobox-containing transcription factor Engrailed 1 (En1) is expressed during development of a variety of structures, including the limb bud, somites, and central nervous system (Davidson et al., 1988; Davis and Joyner, 1988). In the embryonic mouse brain, En1 expression is observed at ~E8.5–E9.5 in a region encompassing the midbrain and rhombomere 1 of the anterior hindbrain (Davidson et al., 1988; Davis and Joyner, 1988; McMahon et al., 1992). This complex domain gives rise to anatomically and functionally diverse cell types in the adult, including important subpopulations of dopaminergic, serotonergic, and noradrenergic neurons (Jensen et al., 2008; Robertson et al., 2013; Simon et al., 2001; Zervas et al., 2004). Although En1 expression persists to adulthood in dopaminergic neurons of the substantia nigra and ventral tegmental area (Simon et al., 2001), expression in most midbrain/hindbrain neurons is transient. Therefore, gaining experimental access to these neurons in the mature brain requires the use of recombinase-based strategies (reviewed in Jensen and Dymecki, 2014) to permanently label cells on the basis of transient En1 expression. A key to this approach is the generation and characterization of transgenic or knock-in alleles which express a site specific recombinase under control of the En1 promoter, and to date, all such alleles have utilized Cre recombinase (Kimmel et al., 2000; Sapir et al., 2004; Sgaier et al., 2005). Access to some of the distinct neuronal subtypes within the broad En1 expression domain has been achieved through the use of genetic intersectional strategies (Awatramani et al., 2003), in which subpopulations of cells were defined by overlapping expression of En1Cre and a second recombinase controlled by a different gene promoter (Jensen et al., 2008; Robertson et al., 2013). Because the vast majority of all characterized recombinase driver lines also express Cre, however, applying intersectional strategies to the midbrain and hindbrain requires the generation of new recombinase drivers, to be used in conjunction with En1Cre, for each neuronal subtype of interest. Generation of an En1 allele expressing a recombinase other than Cre would permit the use of existing Cre drivers for intersectional targeting, reducing the need for laborious characterization of new recombinase driver lines and opening new avenues of investigation in the midbrain and hindbrain.
Here, we describe a new En1 knock-in allele (En1Dre) that expresses Dre recombinase (Anastassiadis et al., 2009; Sauer and McDermott, 2004) instead of Cre. Several features of this new allele will make it particularly useful for gaining experimental access to neuronal subpopulations within the complex midbrain/hindbrain En1 expression domain. En1Dre is compatible with a recently described fluorescent indicator allele (Plummer et al., 2015) that is responsive to three different recombinases (Dre, Flp, and Cre), rendering it useful for investigation of genetic and developmental heterogeneity among En1-expressing cell populations that were previously defined using single- or dual-recombinase strategies (Jensen et al., 2008; Robertson et al., 2013; Simon et al., 2001; Zervas et al., 2004). Unlike the existing En1Cre knock-in alleles (Kimmel et al., 2000; Sapir et al., 2004; Sgaier et al., 2005), the Dre insertion in En1Dre does not disrupt En1 expression. Therefore, En1Dre mice can be bred to homozygosity, increasing the percentage of experimentally useful offspring in the complex crosses required for intersectional analyses. We have confirmed that Dre expression copies endogenous En1 expression in mid-gestation embryos, when En1 expression is most widespread. Furthermore, Dre levels in En1Dre heterozygotes are sufficient to efficiently recombine rox target sites in Dre-responsive indicator alleles. Thus, En1Dre will be a valuable tool for analysis of neurons in the midbrain/hindbrain and En1-expressing cells elsewhere in the embryo.
RESULTS AND DISCUSSION
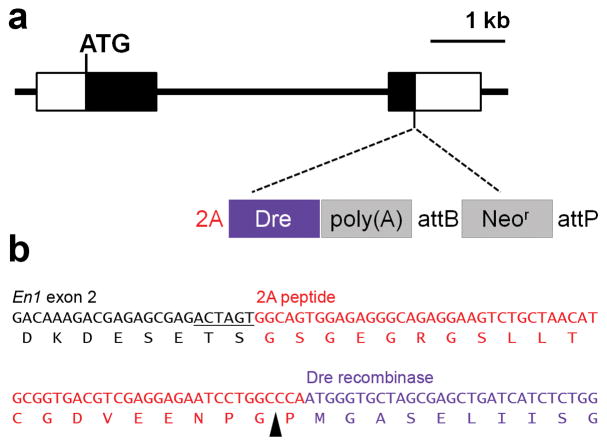
To express Dre recombinase under control of the En1 promoter without disrupting En1 transcription or translation, we used a 2A peptide derived from Thosea asigna virus, which permits expression of two separate polypeptides from a single mRNA (Donnelly et al., 2001; Trichas et al., 2008). A construct consisting of the 2A peptide sequence, mammalian codon-optimized Dre cDNA (Anastassiadis et al., 2009), and SV40 poly(A) cassette was targeted to exon 2 of the mouse En1 gene by homologous recombination in embryonic stem cells. This construct replaces the stop codon and 41 bp of the 3′ UTR without altering the coding sequence of En1 (Fig. 1). Mice homozygous for the new En1Dre allele are viable and fertile, without the perinatal lethality and developmental defects associated with mutation of En1 (Wurst et al., 1994), indicating that the 2A-Dre-poly(A) insertion does not disrupt En1 function.
Figure 1. Structure of the En1Dre allele.
(a) Schematic diagram of the En1 locus showing insertion site of 2A peptide, Dre cDNA, and Neomycin resistance gene (Neor). Black rectangles represent En1 coding sequence. Unfilled rectangles represent 5′ and 3′ untranslated regions. In En1Dre mice, Neor has been excised by ϕC31-mediated recombination of the attB and attP sites. (b) Sequence from the En1Dre allele showing the junction between En1 exon 2, 2A peptide, and Dre cDNA. An exogenous SpeI restriction site (underlined) links En1 exon 2 to the 2A peptide. The black arrowhead indicates the cleavage site within the 2A peptide.
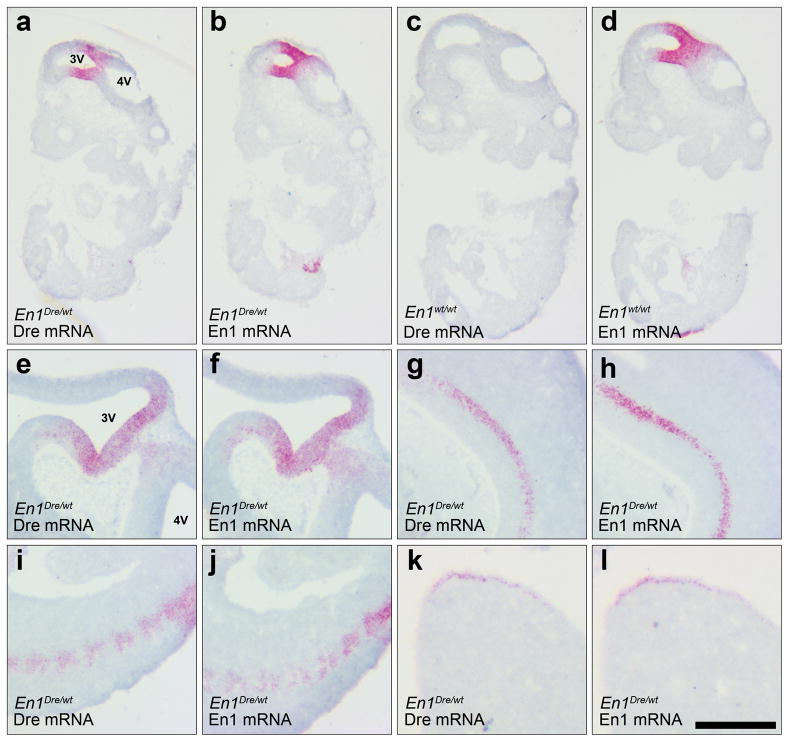
In the mouse embryo, En1 expression has been detected in the neural plate at the 1-somite stage (~E8.0) and by E8.5/E9.0 is observed in the midbrain and anterior hindbrain (Davis and Joyner, 1988; McMahon et al., 1992). By E12, expression can be observed in a variety of other tissues, including spinal cord, somites, and limb bud (Davidson et al., 1988; Davis and Joyner, 1988). To confirm that Dre expression recapitulates the wild-type expression pattern of En1, we used in situ hybridization to detect En1 and Dre mRNA in sections from En1Dre heterozygotes and littermate control embryos at approximately E9.5 and E11.0. As expected, we observed Dre expression spanning the midbrain/hindbrain junction, and in spinal cord, somites, and limb buds (Fig. 2). The distribution of Dre labeling was indistinguishable from that of En1.
Figure 2. Expression of Dre matches En1 in mid-gestation embryos.
Sagittal sections of E9.5 (a–d) and E11 (e–l) embryos labeled by RNA in-situ hybridization using Dre or En1 riboprobes. In an En1Dre heterozygous embryo at E9.5, Dre expression at the midbrain/hindbrain junction (a) matches expression of En1 (b). In a wild-type embryo (c–d), only En1 expression is observed, confirming specificity of the Dre riboprobe. At E11, Dre and En1 expression patterns are indistinguishable at the midbrain/hindbrain junction (e–f) and in spinal cord (g–h), somites (i–j), and hindlimb bud (k–l). 3V, third ventricle; 4V, fourth ventricle. Scalebar: 645 μm (a–d), 500 μm (e–j), or 250 μm (k–l).
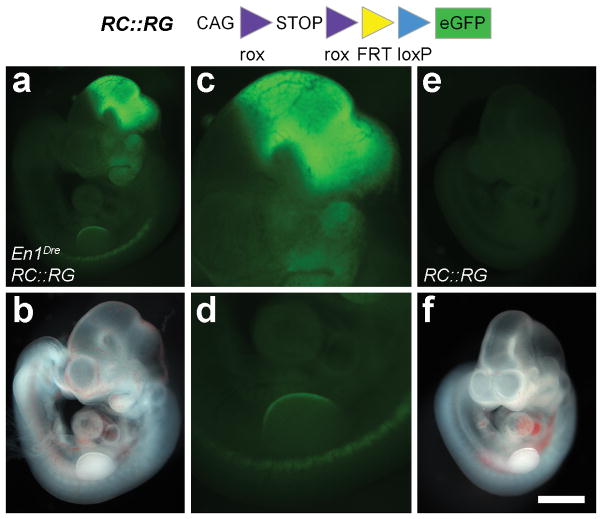
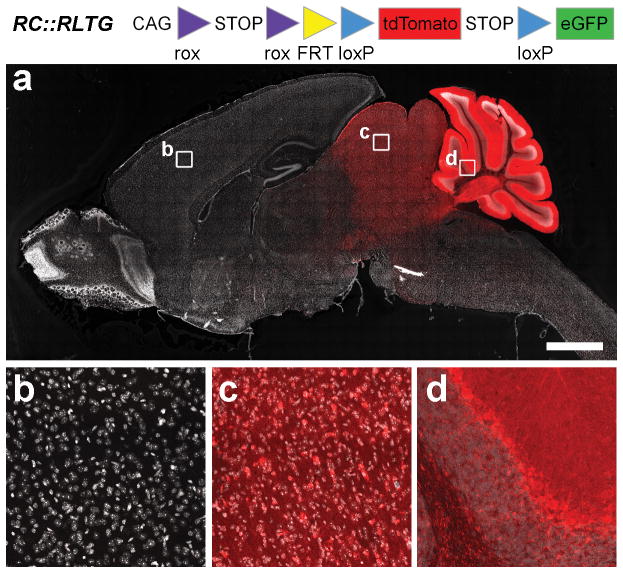
The usefulness of En1Dre as a recombinase driver allele depends on its ability to recombine rox sites, the targets of Dre recombinase that are analogous to the loxP target sites of Cre (Sauer and McDermott, 2004). We tested this capability in vivo using two Dre-responsive fluorescent indicator alleles, RC::RG and RC::RLTG (Plummer et al., 2015), which express eGFP or tdTomato, respectively, following Dre-mediated excision of a rox-flanked transcriptional stop cassette. In whole-mount En1Dre; RC::RG double heterozygous embryos at E10.5 (Fig. 3a–d), we observed eGFP fluorescence in the midbrain/hindbrain, first pharyngeal arch, limb buds, and somites. The pattern of recombination-dependent fluorescence is consistent with the cumulative expression of En1 by this developmental stage and is very similar to the labeling observed at E10.5 when a Cre-dependent LacZ indicator is recombined by En1Cre (http://www.informatics.jax.org/recombinase/specificity?id=MGI:2446434&system=head). No fluorescence was observed in the absence of En1Dre (Fig. 3e–f). In sagittal sections of adult En1Dre; RC::RLTG double heterozygous brain, tdTomato was observed in midbrain and anterior hindbrain, including the entire cerebellum (Fig. 4a). At higher magnification, recombination appeared complete within the En1 expression domain, with no unlabeled cells that would indicate mosaic expression or insufficient recombinase activity (Fig. 4b–d). It has been reported that Dre recombinase can interact with loxP sites in some circumstances (Fenno et al., 2014), potentially complicating the use of En1Dre for intersectional analyses. To test this phenomenon, we used the RC::RLTG allele, which expresses eGFP following excision of the rox-flanked stop cassette and the loxP-flanked tdTomato-stop cassette (Fig. 4). Thus, eGFP will be expressed in En1Dre; RC::RLTG brain only if Dre recombines the loxP sites as well as the rox sites. We observed no eGFP labeled cells (n=4 brains), consistent with prior observations that Dre and loxP sites are generally incompatible in transgenic systems (Chuang et al., 2015; Park and Leach, 2013; Plummer et al., 2015), with significant cross-reactivity only observed in the context of viral vector-driven overexpression (Fenno et al., 2014). These results confirm that En1Dre is suitable for use in intersectional genetic analyses together with Cre driver alleles.
Figure 3. En1Dre efficiently recombines a Dre-responsive indicator allele in mouse embryos.
Fluorescence (a, c–e) or brightfield (b, f) images of a whole-mount En1Dre; RC::RG double heterozygous embryo (a–d) and a Dre-negative littermate control (e–f) at E10.5. eGFP fluorescence, indicating recombination of the rox-flanked transcriptional stop cassette, is visible in midbrain/hindbrain (a, c), first pharyngeal arch (a), somites (a, d), and forelimb bud (a, d) of the double heterozygote. No fluorescence is observed in the control embryo (e). Images show native fluorescence. Scalebar: 1000 μm (a–b, e–f); 458 μm (c–d).
Figure 4. En1Dre can be used to trace cells with a history of embryonic En1 expression into the adult brain.
Sagittal section of adult brain from an En1Dre; RC:RLTG double heterozygote. tdTomato expression, indicating recombination of the rox-flanked stop cassette, is restricted to cells derived from embryonic midbrain and rhombomere 1 of the anterior hindbrain. Nuclear DAPI staining (false-colored gray) reveals tissue that does not express tdTomato (a, b). At higher magnification, recombination appears complete, with no unlabeled cells observed in superior colliculus (c) and cerebellum (d). Images show immunofluorescence. Scalebar: 2000 μm (a) or 97 μm (b–d).
This characterization demonstrates that En1Dre faithfully recapitulates the wild-type expression pattern of En1 and efficiently recombines rox sites in vivo. Therefore, En1Dre is compatible with the diverse Dre-responsive genetic tools and strategies that have been developed in the past several years, including fluorescent indicator alleles responsive to Dre and one or more additional site-specific recombinases (Madisen et al., 2015; Plummer et al., 2015). Because Dre and Cre have different target sites, they can function independently in the same animal. For example, recombination of an indicator allele by En1Dre could be used to trace specific cell populations in the context of Cre-dependent conditional mutagenesis of a different gene. Alternatively, Dre-dependent Cre alleles (Hermann et al., 2014; Sajgo et al., 2014) offer the possibility of intersectional control of conditional mutagenesis and Cre-dependent effector alleles. These strategies will provide experimental access to cells with a history of En1Dre expression, including those that express En1 transiently during development. To specifically target cells that express En1 in the adult, without interference from embryonic En1 domains, En1Dre can be used in conjunction with injection of Dre-responsive and Dre/Cre-responsive viral constructs encoding indicators and effectors (Chuang et al., 2015; Fenno et al., 2014; Madisen et al., 2015). Several of these recently described constructs utilize engineered mutant rox target sites to permit control of indicator or effector gene expression by Dre-dependent inversion and excision similar to Cre-dependent FLEx/DIO constructs (Chuang et al., 2015; Fenno et al., 2014). Given these possibilities, we expect that En1Dre will become an important part of the genetic toolbox for analysis of neurons in the mammalian midbrain and hindbrain.
METHODS
Generation of En1Dre mice
To generate the En1Dre targeting vector, we first used pCAGGS-Dre-IRES-Puro (Anastassiadis et al., 2009) as template to amplify a mammalian codon-optimized Dre cDNA lacking the start codon. We used the primers 5′-CAGTACTAGTGGCAGTGGAGAGGGCAGAGGAAGTCTGCTAACATGCGGTGACGTCGAGGAGAATCCTGGCCCAATGGGTGCTAGCGAGCTGAT, which contains the 2A peptide (underlined), and 5′-CAGTCTGCAGTTATCACACTTTCCTCTTCTTCTTAGGACCG. The PCR product was cloned into pGEM-5Z (Clontech Laboratories, Mountain View, CA), using the SpeI and PstI sites included in the primers, to generate pGEM-2ADre. The SV40 poly(A) cassette was amplified from pBS302 (Addgene plasmid #11925) (Sauer, 1993) using primers 5′-CAGTCTGCAGGATCATAATCAGCCATACCA and 5′-CAGTGTCGACGATCCAGACATGATAAGATA and cloned between the PstI and SalI sites of pGEM-2ADre to generate pGEM-2ADre-stop. A 469 bp fragment of En1 intron 1 and exon 2 ending immediately before the stop codon was amplified using primers 5′-CAGTGGGCCCAGCAGCTGGCCTCTACAATC and 5′-CAGTACTAGTCTCGCTCTCGTCTTTGTCCT and cloned between the ApaI and SpeI sites upstream of the 2A peptide in pGEM-2ADre-stop. Primers 5′-CAGTGTCGACGGTGCCAGGGCGTGCCCTTGGGCTCCCCGGGCGCGTTAGGTCTGAAGAGGAGTTT and 5′-CAGTCATATGCAGTCCTAGGCCCCCAACTGAGAGAACTCAAAGGTTACCCCAGTTGGGGATTAAGGGTTCCGCAAGCTCT containing attB35 and attP39B sites, respectively (underlined) (Groth et al., 2000) were used to amplify a neomycin resistance gene with both PGK and Em7 promoters from PL452 (Liu et al., 2003). The attB- attP-flanked neomycin cassette was cloned between the SalI and NdeI sites downstream of the Sv40 poly(A) cassette. 497 bp of En1 3′UTR was amplified with primers 5′-CAGTCCTAGGGTGCCACCTCCAGGCTCCTC and 5′-CAGTCATATGGGTGCAGGAGGTACCCAGAG and cloned using the AvrII and NdeI sites adjacent to the Neo Cassette. After the resulting plasmid was digested with ApaI and NdeI, the two fragments of En1 served as targets for homologous recombination with bacterial artificial chromosome (BAC) RP23-488D12 in E. coli strain DY380 (Lee et al., 2001). To generate the final targeting vector from the recombinant BAC, a 12.5 KB fragment consisting of 4.4 KB 5′ homology arm, 2ADre-Neo cassette, and 4.9 KB 3′ homology arm was retrieved into pL253 (Liu et al., 2003) by homologous recombination in DY380 cells. For linearizing the vector, an AscI site was added at the 3′ end of the 3′ homology arm.
Linearized targeting vector DNA was electroporated into G4 embryonic stem cells (B6129 F1 genetic background). Homologous recombinants were identified by long range PCR using the Expand Long Range dNTP Pack (Roche Applied Science) and primers specific for the 5′and 3′ ends of each recombinant locus. Southern blots probed for the neomycin resistance gene were used to confirm absence of random integrations. The karyotypes of recombinant clones were assessed, and several clones were transfected with pPGKPhiC31obpa (Raymond and Soriano, 2007) to remove the Neo cassette. The clones were then injected into blastocysts of B6(Cg)-Tyrc-2J/J mice to produce chimeric mice, and male chimeras were bred to female C57BL/6J mice to establish the En1Dre mouse line. En1Dre mice will be available to the research community upon publication of this manuscript.
Experimental crosses
En1Dre mice were maintained as heterozygotes by back-crossing to C57BL/6J mice or as homozygotes by intercrossing. For assessment of in vivo recombination, En1Dre heterozygotes were crossed with Gt(ROSA)26Sortm1.2(CAG-tdTomato, -EGFO)Pjen (RC::RLTG) and RC::RG mice (Plummer et al., 2015). All mouse experiments were performed with approval of the National Institute of Environmental Health Sciences (NIEHS) Institutional Animal Care and Use Committee.
Tissue collection
Embryos were fixed by immersion overnight at 4 °C in 4% paraformaldehyde (PFA) diluted in 0.01 M phosphate buffered saline (PBS). Adult mice were anesthetized with sodium pentobarbital and perfused transcardially with 4% PFA in PBS. After dissection, brains were post-fixed overnight by immersion in 4% PFA in PBS at 4 °C, rinsed in PBS, and equilibrated in 30% sucrose diluted in PBS at 4 °C for 48 hours. Embryos were equilibrated in 10%, 20%, and finally 30% sucrose diluted in PBS. The cryoprotected samples were then embedded in Tissue Freezing Medium (General Data Company, Cincinnati, OH) and sectioned on a Leica CM3050 S cryostat (Leica Biosystems, Buffalo Grove, IL). After sectioning, 40-μm thick free floating brain sections were stored at −80 °C in a solution of 30% sucrose and 30% ethylene glycol in PBS. For embryos, 14-μm thick sections were mounted on Superfrost Plus microscope slides (Thermo Scientific, Waltham, MA), air dried, and stored at −80 °C.
In situ hybridization
RNA in situ hybridization using RNAscope technology (Wang et al., 2012) was performed on 14-μm thick embryo sections according to the manufacturer’s instructions for the RNAscope 2.5 HD Reagent Kit-Red (Advanced Cell Diagnostics, Hayward, CA). Probes used were En1 (Cat# 442651, Advanced Cell Diagnostics) and a custom Dre probe (Cat# 442641, Advanced Cell Diagnostics) raised against nucleotides 83-999 of the mammalian codon-optimized Dre coding sequence (Anastassiadis et al., 2009). Hybridized RNA was detected with Fast Red dye, and tissue was counterstained with hematoxylin.
Immunohistochemistry
Immunofluorescent labeling was performed on 40-μm free floating sagittal brain sections from adult mice. tdTomato-expressing cells were labeled by rabbit anti-dsRed primary antibody (1:1000; Cat.# 632496, Clontech Laboratories, Mountain View, CA) and Alexa Fluor 568 goat anti-rabbit secondary antibody (1:1000; Cat.# A11036, Life Technologies, Grand Island, NY). To test for possible eGFP expression resulting from Dre-dependent recombination of loxP sites in RC::RLTG, we used chicken anti-GFP primary antibody (1:10,000; Cat.# ab13970, Abcam, Cambridge MA) and Alexa Fluor 488 goat anti-chicken secondary antibody (1:1000; Cat.# A11039, Life Technologies). After immunolabeling, sections were mounted on microscope slides, and coverslips were attached with Vectashield plus DAPI (Vector Laboratories, Burlingame, CA). Every fourth section across the entire brain was stained and examined.
Imaging
Images of embryo sections labeled by in situ hybridization were collected on an Olympus IX70 inverted microscope (Olympus Corporation, Center Valley, PA), and images of whole mount embryos were collected on a Zeiss SterREO Lumar.V12 stereomicroscope (Carl Zeiss Microscopy, Thornwood, NY). Immunolabeled sagittal sections of adult brain were imaged on a Zeiss LSM 780 inverted confocal microscope. Tile images of the entire section were digitally stitched and confocal z-stacks were converted to maximum intensity projections using Zen 2012 Black Software (Carl Zeiss). Images were then imported into Photoshop Software (Adobe Systems, San Jose, CA) and modified only by adjusting the brightness and contrast of the entire image.
Acknowledgments
NIH Support: This research was supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Environmental Health Sciences (ES102805).
We thank D. D’Agostin, G. Jones, and K. Smith for technical assistance. We thank F. Stewart (Technische Universität Dresden) for the Dre cDNA. Valuable support was provided by the NIEHS Comparative Medicine Branch, Knockout Mouse Core, and Fluorescence Microscopy and Imaging Core. This research was supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Environmental Health Sciences (ES102805).
References
- Anastassiadis K, Fu J, Patsch C, Hu S, Weidlich S, Duerschke K, Buchholz F, Edenhofer F, Stewart AF. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech. 2009;2:508–515. doi: 10.1242/dmm.003087. [DOI] [PubMed] [Google Scholar]
- Awatramani R, Soriano P, Rodriguez C, Mai JJ, Dymecki SM. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat Genet. 2003;35:70–75. doi: 10.1038/ng1228. [DOI] [PubMed] [Google Scholar]
- Chuang K, Nguyen E, Sergeev Y, Badea TC. Novel Heterotypic Rox Sites for Combinatorial Dre Recombination Strategies. G3 (Bethesda) 2015;6:559–571. doi: 10.1534/g3.115.025841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Graham E, Sime C, Hill R. A gene with sequence similarity to Drosophila engrailed is expressed during the development of the neural tube and vertebrae in the mouse. Development. 1988;104:305–316. doi: 10.1242/dev.104.2.305. [DOI] [PubMed] [Google Scholar]
- Davis CA, Joyner AL. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- Donnelly ML, Hughes LE, Luke G, Mendoza H, ten Dam E, Gani D, Ryan MD. The ‘cleavage’ activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring ‘2A-like’ sequences. J Gen Virol. 2001;82:1027–1041. doi: 10.1099/0022-1317-82-5-1027. [DOI] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods. 2014;11:763–772. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci U S A. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann M, Stillhard P, Wildner H, Seruggia D, Kapp V, Sanchez-Iranzo H, Mercader N, Montoliu L, Zeilhofer HU, Pelczar P. Binary recombinase systems for high-resolution conditional mutagenesis. Nucleic Acids Res. 2014;42:3894–3907. doi: 10.1093/nar/gkt1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Dymecki SM. Essentials of recombinase-based genetic fate mapping in mice. Methods Mol Biol. 2014;1092:437–454. doi: 10.1007/978-1-60327-292-6_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, Joyner AL. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, Gu H, Mills M, Cheng A, Tasic B, Nguyen TN, Sunkin SM, Benucci A, Nagy A, Miyawaki A, Helmchen F, Empson RM, Knopfel T, Boyden ES, Reid RC, Carandini M, Zeng H. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Joyner AL, Bradley A, McMahon JA. The midbrain-hindbrain phenotype of Wnt-1−/Wnt-1− mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell. 1992;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- Park JT, Leach SD. TAILOR: transgene activation and inactivation using lox and rox in zebrafish. PLoS One. 2013;8:e85218. doi: 10.1371/journal.pone.0085218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer NW, Evsyukova IY, Robertson SD, de Marchena J, Tucker CJ, Jensen P. Expanding the power of recombinase-based labeling to uncover cellular diversity. Development. 2015;142:4385–4393. doi: 10.1242/dev.129981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One. 2007;2:e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–1023. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajgo S, Ghinia MG, Shi M, Liu P, Dong L, Parmhans N, Popescu O, Badea TC. Dre - Cre sequential recombination provides new tools for retinal ganglion cell labeling and manipulation in mice. PLoS One. 2014;9:e91435. doi: 10.1371/journal.pone.0091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J Neurosci. 2004;24:1255–1264. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Methods Enzymol. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- Sauer B, McDermott J. DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Res. 2004;32:6086–6095. doi: 10.1093/nar/gkh941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgaier SK, Millet S, Villanueva MP, Berenshteyn F, Song C, Joyner AL. Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron. 2005;45:27–40. doi: 10.1016/j.neuron.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Simon HH, Saueressig H, Wurst W, Goulding MD, O’Leary DD. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J Neurosci. 2001;21:3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trichas G, Begbie J, Srinivas S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 2008;6:40. doi: 10.1186/1741-7007-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst W, Auerbach AB, Joyner AL. Multiple developmental defects in Engrailed-1 mutant mice: an early mid-hindbrain deletion and patterning defects in forelimbs and sternum. Development. 1994;120:2065–2075. doi: 10.1242/dev.120.7.2065. [DOI] [PubMed] [Google Scholar]
- Zervas M, Millet S, Ahn S, Joyner AL. Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron. 2004;43:345–357. doi: 10.1016/j.neuron.2004.07.010. [DOI] [PubMed] [Google Scholar]