Abstract
Obstructive sleep apnea (OSA) is characterized by recurrent upper airway blockage, with continued diaphragmatic efforts to breathe during sleep. Brain structural changes in OSA appear in various regions, including white matter sites that mediate autonomic, mood, cognitive, and respiratory control. However, the relationships between brain white matter changes and disease severity in OSA are unclear. Our aim was to examine associations between an index of tissue integrity, magnetization transfer (MT) ratio values, which show MT between free and proton pools associated with tissue membranes and macromolecules, and disease severity [apnea-hypopnea index (AHI)] in OSA subjects. We collected whole-brain MT imaging data from 19 newly-diagnosed, treatment-naïve OSA subjects [age, 50.4±8.6 years; 13 males; AHI, 39.7±24.3 events/hour], using a 3.0-Tesla MRI scanner. Using these data, whole-brain MT ratio maps were calculated, normalized to common space, smoothed, and correlated with AHI scores using partial correlation analyses (covariates; age, gender; p<0.005). Multiple brain sites in OSA subjects, including superior and inferior frontal regions, ventral medial pre-frontal cortex and nearby white matter, mid-frontal white matter, insula, cingulate and cingulum bundle, internal and external capsules, caudate nuclei and putamen, basal forebrain, hypothalamus, carpus callosum, and temporal regions showed principally-lateralized negative correlations (p<0.005). These regions showed significant correlations even with correction for multiple comparisons (cluster-level, family wise error, p<0.05), except for a few superior frontal areas. Predominately negative correlations emerged between local MT values and OSA disease severity, indicating potential usefulness of MT imaging to examine the OSA condition. The findings indicate that OSA severity plays a significant role in white matter injury.
Keywords: Apnea-hypopnea index, Hypoxia, Magnetization transfer imaging, Insula, Frontal white matter
Graphical Abstract
The magnetization transfer imaging procedure shows white matter injury increases with disease severity (apnea-hypopnea index) in obstructive sleep apnea (OSA) subjects, suggesting a role of disease severity in white matter changes. The findings also indicate that method can be used in OSA and other neurological conditions to monitor disease progression.
INTRODUCTION
Obstructive sleep apnea (OSA) is a condition characterized by partial or complete obstruction of the upper airway, with continued diaphragmatic efforts to breathe during sleep, leading to episodic O2 desaturations accompanied by significant blood pressure changes (Strollo and Rogers 1996). Brain structural injury appears in various nuclear sites, as well as in white matter regions (Kumar et al. 2012; Kumar et al. 2014; Macey et al. 2008; Tummala et al. 2015), and those structural changes are supplemented by functional deficits in autonomic, cognitive, affective, and breathing functions affected in the condition (Henderson et al. 2003; Zhang et al. 2013). However, the relationships between regional brain tissue integrity and disease severity in recently-diagnosed, treatment-naïve OSA subjects are unclear.
The disease severity of OSA is typically assessed with the apnea-hypopnea index (AHI), which is derived from overnight polysomnography (1999). In OSA, both apnea and hypopnea occur, representing complete and partial pauses to breathe, respectively. The AHI is an average of the combined number of apneas and hypopneas per hour of sleep, with larger AHI values indicating greater OSA severity.
Multiple non-invasive magnetic resonance imaging (MRI) procedures, including magnetization transfer (MT) imaging, can examine brain white matter integrity. In MRI procedures, signals emerge predominantly from the free water proton pool, which derives from extracellular/axonal water. However, the free water proton pool is influenced by the bound water proton pool (i.e., water protons associated with macromolecules, membranes, lipids, and myelinated axons) through dipole-dipole biophysical interactions, and the ratio of MT levels between the two pools can be quantified by MT imaging. In MT imaging, two images (MT pulse on and MT pulse off) are acquired, with and with-out off-resonance saturation pulses, and a quantitative measure “MT ratio (MTR),” a marker of white matter integrity that varies with the number of macromolecules in the tissue, is computed (Grossman et al. 1994; Wolff and Balaban 1989). The MTR value decreases in compromised white matter/myelin, since MT transfer is reduced between bound and free water proton pools.
Multiple studies have employed MT imaging for diagnosis and monitoring of white matter integrity in various disease conditions in humans and animal models. These conditions include examination of myocardial infarction (Scholz et al. 1995), liver cirrhosis (Rovira et al. 2001), multiple sclerosis (Pike et al. 2000), Alzheimer’s disease (Mascalchi et al. 2013; Ridha et al. 2007; Ropele et al. 2012), Parkinson’s disease (Anik et al. 2007; Tambasco et al. 2011), Huntington disease (van den Bogaard et al. 2013), stroke (Sibon et al. 2015), and gliomas (Tozer et al. 2011). However, based on our knowledge, MT procedures have not been used to study the OSA condition, and this description would be first investigation in this condition. The unique insights provided by MT imaging-based procedures should be valuable in examining relationships between regional white matter integrity and disease severity in OSA.
Our aim was to examine associations between regional brain white matter integrity and disease severity in newly-diagnosed, treatment-naïve OSA subjects. We hypothesized that regional MTR values of multiple brain regions, including surrounding white matter areas of the insula, basal ganglia, frontal, and limbic sites, which are involved in autonomic, cognitive, and mood control, would show predominantly negative correlations with AHI in OSA subjects, indicating altered white matter integrity in those regions.
MATERIALS AND METHODS
Study design
We conducted a cross-sectional study design to examine associations between regional brain white matter integrity and disease severity in recently-diagnosed, treatment-naïve OSA subjects.
Subjects
We recruited 19 OSA subjects from an accredited Sleep Disorders Laboratory at the University of California at Los Angeles (UCLA) Medical Center. A subset of OSA subjects used here were part of our previously-published manuscripts based on entirely different research questions (Park B 2016a; Park B 2016b; Tummala et al. 2015). All OSA subjects were newly-diagnosed via overnight polysomnography (PSG), with at least moderate severity (AHI ≥ 15), and were without any treatment for the breathing condition. All subjects underwent an MRI study within 7-30 days after diagnosis with OSA. Subjects were not taking any cardiovascular-altering medications (e.g., β-blockers, α-agonists, angiotension-converting enzyme inhibitors, and vasodilators), or mood altering drugs (e.g., serotonin reuptake inhibitors). All OSA subjects with a history of diagnosed brain conditions, other than OSA, metallic implants, stroke, or heart failure, were excluded. All data acquisition procedures were conducted in accordance with approval from the Institutional Review Board at UCLA, and written informed consent was obtained from all subjects prior to the study.
Overnight Polysomnography
All PSG data were evaluated by a board-certified sleep physician at the UCLA Medical Center. AHI scores, defined as the ratio of the total number of apnea and hypopneas to the total sleep time in hours, were calculated. OSA subjects were categorized with mild OSA, if AHI values were between 5-14 events/hour, with moderate OSA, if AHI values were between 15-30 events/hour, and with severe OSA, for AHI values greater than 30 events/hour (1999).
Assessment of Sleep Quality and Daytime Sleepiness
We employed two self-administered questionnaires to investigate sleep quality and daytime sleepiness in OSA subjects (Knutson et al. 2006). Sleep quality was evaluated with the Pittsburgh sleep quality index (PSQI), and daytime sleepiness was assessed with the Epworth sleepiness scale (ESS). For PSQI, a score >5, and for ESS, a value >9, was considered as an abnormal score.
Mood Evaluation
Both anxiety and depressive symptoms in OSA subjects were assessed using the Beck anxiety inventory (BAI) and Beck depression inventory II (BDI-II) (Beck et al. 1988; Beck et al. 1996). These tests are self-administered questionnaires (21 questions; scores for each question ranged from 0-3), with each total score ranging from 0-63 based on symptom severity. For both BAI and BDI-II, values >9 were used to classify OSA subjects with anxious and depressive symptoms, respectively.
Cognition Assessment
We used the Montreal Cognitive Assessment (MoCA) test for cognition assessment in OSA subjects. This test is designed for rapid examination of various cognitive abilities, including attention and concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation (Nasreddine et al. 2005). A MoCA score <26 was considered abnormal. We also used the Trail making A & B tests to examine the cognitive aspects. These tests are used for screening of dementia, including visual search, mental flexibility, executive function, scanning, and speed of processing. We considered a value >31 s for Trial A, and >63 s, for Trial B, to classify OSA subjects as abnormal (Tombaugh 2004).
Magnetic Resonance Imaging
All brain images were collected using a 3.0-Tesla MRI scanner (Magnetom Tim-Trio; Siemens, Erlangen, Germany). High resolution T1-weighted images were collected with magnetization-prepared rapid gradient-echo pulse sequence in the sagittal plane, based on the following parameters: [repetition time (TR) = 2200 ms; echo time (TE) = 2.34 ms; inversion-time = 900 ms; flip-angle (FA) = 9°; matrix size = 320×320; field of view (FOV) = 230×230 mm2; slice thickness = 0.9 mm). Proton-density and T2-weighted images were obtained in the axial plane using a duel-echo turbo spin-echo pulse sequence: (TR = 10,000 ms; TE1, 2 = 17, 134 ms; FA = 130°; matrix size = 256×256, FOV = 230×230 mm2; slice thickness = 3.5 mm). Magnetization transfer imaging data were collected using a spin-echo pulse sequence in the axial plane: (TR = 3100 ms; TE = 12 ms; FA = 90°; bandwidth = 130 Hz/pixel; matrix size = 192×256; FOV = 230×230 mm2; slice thickness = 4 mm; 40 slices; no interslice-gap; saturation pulse band-width = 187 Hz; frequency offset = 1.2 kHz; MT pulse angle = 500°).
Data Processing and Analysis
We used various software applications for image pre-processing, analysis, and image visualization, which included the statistical parametric mapping package (SPM 12; WellCome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm), MRIcroN (Rorden et al. 2007), and MATLAB (The MathWorks Inc, Natick, MA). High-resolution T1-weighted, PD-, and T2-weighted images of all OSA subjects were examined visually to ensure absence of any serious anatomical defects, including cysts, tumors, or any other lesions before data processing. No OSA subjects included here showed any of these abnormalities on visual examination. Both images, with and without MT contrast, were also assessed visually for image quality, including motion artifacts for further processing.
MTR maps calculation
For all subjects, the MT ratio maps were calculated as follows: MTR = (MToff − MTon)/MToff * 100% (Grossman et al. 1994). Where, MToff represents images without off-resonance pulses applied, and MTon represents images with off-resonance pulses. We used a noise threshold to exclude non-brain areas, and a ceiling threshold to reduce partial volume effects; identical thresholds were applied to all OSA subjects (Kumar et al. 2005).
Segmentation, normalization, and smoothing of MTR maps
The MTon images were partitioned into gray and white matter tissue types using SPM12 software. We extracted the normalization parameters as a 4D image file (3D file for each direction) from the MTon images, based on a modified unified segmentation approach implemented in SPM12. The MTR maps were normalized to Montreal Neurological Institute (MNI) space using normalization information obtained from the MTon images. The normalized MTR maps were smoothed using an isotropic Gaussian filter (kernel size, 10 mm).
We normalized high-resolution T1-weighted images of subject to MNI space, and cerebrospinal fluid and other non-brain regions were removed. The resulted images were used as background for structural identification.
Global brain mask
We normalized both gray and white matter probability maps from all subjects, using the normalization parameters obtained from corresponding MTon images. The normalized white matter probability maps from all subjects were averaged to create a global white matter probability map; similarly, the normalized gray matter probability maps were averaged to obtain a global gray matter probability map. The global brain mask was obtained by combining the thresholded global gray and white matter probability maps (gray matter >0.3, white matter >0.3) (Tummala et al. 2015).
Region-of-Interest (ROI) Analyses
We also performed the ROI analyses to determine regional MTR values and calculate correlations between regional MTR values and AHI scores. We used the ROIs from Neuromorphometrics, Inc. (www.neuromorphometrics.com) to extract the regional MTR values using the corresponding smoothed MTR maps.
Statistical Analysis
We used SPM12 and IBM Statistical Packages for the Social Sciences (SPSS v22) software tools for statistical analyses. We examined significant correlations between regional brain tissue integrity and disease severity in OSA subjects voxel-by-voxel using the smoothed MTR maps and AHI scores with partial correlation procedures, accounting for age and gender (SPM12, uncorrected threshold, p<0.005, extended threshold 5 voxels). We also tested whether regions that show significant correlations based on uncorrected threshold survive after multiple comparisons correction at cluster-level (family wise error, p<0.05). We used the global brain mask to restrict the analysis within gray and white matter regions. We overlaid clusters with significant correlations onto background images for structural identification.
Correlation coefficients were calculated using regional MTR values, determined by ROI analyses, and AHI scores with partial correlation procedures (SPSS software; covariates, age and gender). A p-value of less than 0.05 was used to establish statistical significance.
RESULTS
Demographics and other variables
Demographic, sleep, mood, cognitive, and other clinical variables are summarized in Table 1. Of 19 OSA subjects, 9 subjects were classified as severe and 10 subjects with moderate disease severity. Six OSA subjects showed cognitive deficits, based on the MoCA scores, and 6 and 9 subjects showed cognitive disabilities, based on Trial A & B tests, respectively. Thirteen OSA subjects showed poor sleep quality, assessed by the PSQI, and 6 subjects exhibited daytime sleepiness, based on ESS scores. Also, of 19 OSA subjects, 5 subjects showed elevated depressive, and 4 subjects showed enhanced anxiety symptoms.
Table 1.
Demographic, physiologic, cognitive, mood, and sleep data from OSA subjects.
| Variables | OSA (mean ± SD) n = 19 |
|---|---|
| Age (years) | 50.4±8.6 |
| Gender | 13 male |
| BMI (kg/m2) | 32.0±7.7 |
| Education (years) | 17.5±3.5 |
| Heart Rate (beats/min) | 79.6±12.5 |
| Systolic BP (mm of Hg) | 128.8±14.8 |
| Diastolic BP (mm of Hg) | 80.2±9.5 |
| AHI (events/hour) | Moderate, 22.9±4.9 (n = 10) |
| Severe, 58.3±23.7 (n = 9) | |
| MoCA | Normal, 28.0±1.5 (n = 13) |
| Impaired, 20.2±4.5 (n = 6) | |
| PSQI | Normal, 3.8±0.8 (n = 6) |
| Deprived, 9.5±2.8 (n = 13) | |
| ESS | Normal, 5.6±2.0 (n = 13) |
| Deprived, 13.2±3.1 (n = 6) | |
| BDI-II | Normal, 3.1±2.5 (n = 14) |
| Depressive, 12.2±1.8 (n =5) | |
| BAI | Normal, 3.1±2.7 (n = 15) |
| Anxious 14.8±2.8 (n = 4) | |
| Trial A (sec) | Normal, 23.1±3.7 (n = 13) |
| Impaired, 39.0±3.7 (n = 6) | |
| Trial B (sec) | Normal, 44.3±12.1 (n = 10) |
| Impaired, 94.5±31.1 (n = 9) |
BMI: Body Mass Index, AHI: Apnea-Hypopnea Index, MoCA: Montreal Cognitive Assessment, PSQI: Pittsburgh Sleep Quality Index, ESS: Epworth Sleepiness Scale, BDI-II: Beck Depression Inventory II, BAI: Beck Anxiety Inventory, BP: Blood Pressure.
Correlations between regional MTR values and AHI scores
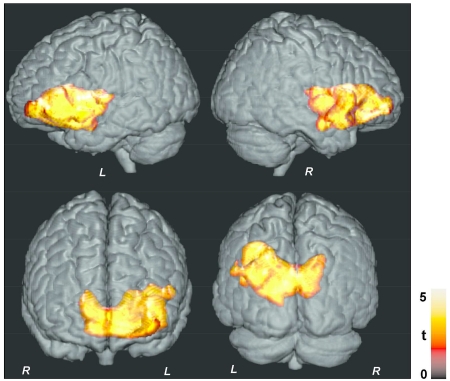
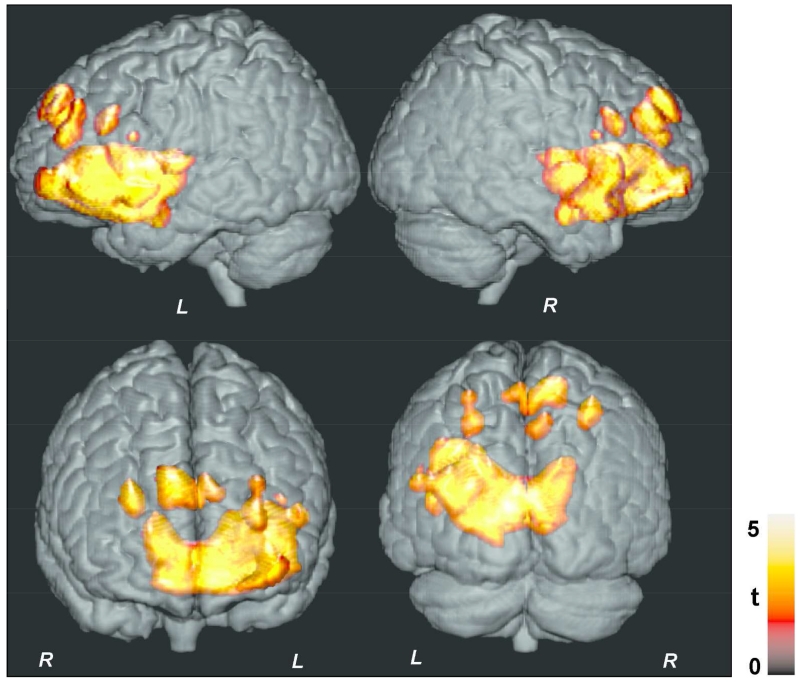
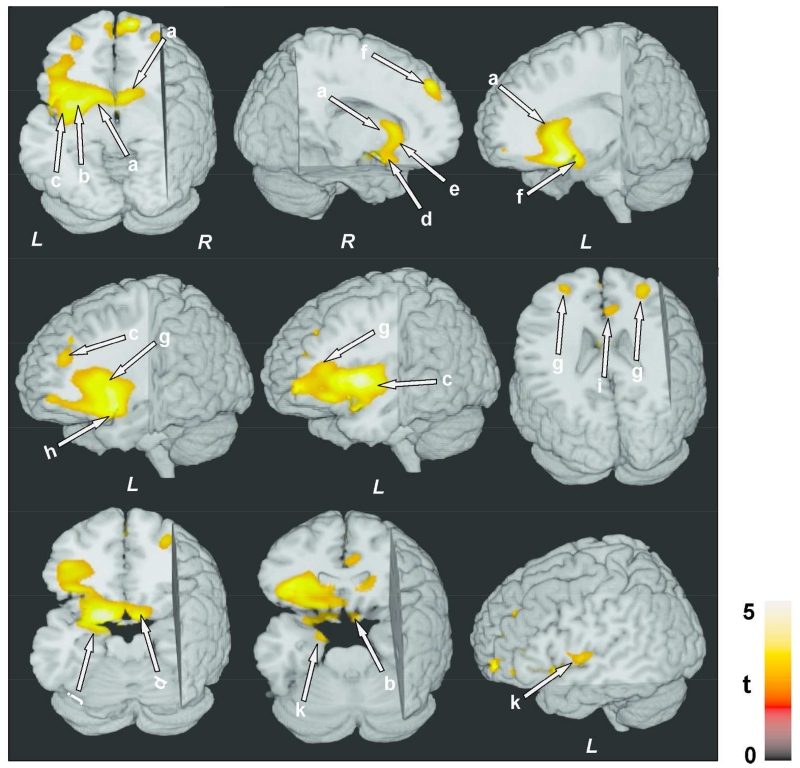
Multiple brain sites showed significant negative correlations between MTR values and AHI scores, indicating significant effects of AHI on tissue integrity, and these correlations were predominantly lateralized on the left side (Fig. 1). The affected sites included the bilateral caudate nuclei (Fig. 2a), putamen, extending to internal and external capsules (Fig. 2b), left anterior, mid, and posterior insular cortices (Fig. 2c), ventral medial prefrontal cortex (Fig. 2d), genu of corpus callosum (Fig. 2e), bilateral superior frontal cortices, extending to the surrounding white matter (Fig. 2f), anterior cingulate and cingulum bundle (Fig. 2i), prefrontal cortices (Fig. 2g), hypothalamus (Fig. 2f), basal forebrain, amygdala and hippocampus (Fig. 2j), left ventral temporal lobe (Fig. 2k), and inferior frontal cortex and surrounding white matter (Fig. 2h). Except only few superior frontal regions, above listed sites showed significant correlations even after correction for multiple comparisons (Fig. 3).
Figure 1.
Brain sites with significant negative correlations between MTR values and AHI scores in OSA subjects with voxel-level uncorrected threshold (p < 0.005). Clusters are overlaid onto a 3D whole-brain cortical surface for structural identification, and are shown in coronal and sagittal views (L = Left; R = Right). Color bar indicates t-statistic values.
Figure 2.
Negative correlations between MTR values and AHI scores in OSA subjects. Brain sites included the caudate nuclei (a), putamen, extending to internal and external capsules (b), left insular cortex (c), ventral medial prefrontal cortex and surrounding white matter (d), genu of corpus callosum (e), bilateral superior frontal cortices, extending to the surrounding white matter (f), anterior cingulate and cingulum bundle (i), prefrontal cortices (g), hypothalamus (f), amygdala and hippocampus (j), left ventral temporal lobe (k), inferior frontal cortex, and surrounding white matter (h). All images are in neurological convention (L = Left; R = Right). Color bar indicates t-statistic values.
Figure 3.
Brain sites with significant negative correlations between MTR values and AHI scores with cluster-level family wise error correction for multiple comparisons (p < 0.05). Clusters are overlaid onto a 3D whole-brain cortical surface for structural identification, and are shown in coronal and sagittal views similar to Figure 1.
ROI Analyses
Regional correlation coefficients between MTR values and AHI scores are listed in Table 2. Only correlation coefficients for those regions that showed any overlap between findings based on cluster corrected voxel-level results and the Neuromorphometrics atlas are reported. These findings also demonstrated significant negative correlations, consistent with results derived from voxel-by-voxel analyses.
Table 2.
Regional correlation values between MTR and AHI scores in OSA subjects, corrected for age and gender.
| Brain Region | CC | Extent (N) |
MNI Coordinates (x, y, z) |
P-value |
|---|---|---|---|---|
| L Caudate | −0.69 | 369 | (−18, 19, −2) | < 0.002 |
| R Caudate | −0.67 | 537 | (9, 16, −3) | < 0.003 |
| L Putamen | −0.71 | 1138 | (−29, 10, −2) | < 0.001 |
| L Anterior insula | −0.75 | 1603 | (−33, 11, 2) | < 0.001 |
| L Posterior insula | −0.69 | 224 | (−39, −5, 0) | < 0.002 |
| L Hippocampus | −0.65 | 18 | (−14, −5, −23) | <0.005 |
| L Amygdala | −0.68 | 95 | (−15, −4, −21) | < 0.001 |
| L Hypothalamus | −0.65 | 31 | (−8, 5, −14) | <0.005 |
| L Basal forebrain | −0.73 | 146 | (−14, 5, −18) | < 0.001 |
| R Basal forebrain | −0.67 | 66 | (9, 5, −17) | < 0.003 |
| L Ventral-medial prefrontal cortex |
−0.65 | 72 | (−5, 19, −20) | < 0.005 |
| L Genu of Corpus Callosum |
−0.65 | 136 | (−14, 17, −12) | <0.005 |
| R Genu of Corpus Callosum |
−0.64 | 112 | (8, 16, −8) | <0.005 |
| L Genu of Cingulate | −0.72 | 378 | (−14, 11, −20) | <0.001 |
| R Genu of Cingulate | −0.68 | 306 | (9, 11, −20) | <0.003 |
| L Frontal white matter | −0.70 | 3224 | (−18, 13, −18) | <0.002 |
| L Globus Pallidus | −0.62 | 27 | (−24, −4, 0) | < 0.008 |
| L Superior temporal cortex |
−0.64 | 54 | (−47, 14, −12) | < 0.005 |
OSA: obstructive sleep apnea, SD: Standard Deviation, CC: Correlation Coefficient, N: number of voxels, L: Left, R: Right.
DISCUSSION
Overview
We assessed the relationships between white matter integrity and disease severity (AHI) in newly-diagnosed, treatment-naïve OSA subjects using MT-based procedures. Several brain sites showed negative correlations between MTR values and AHI scores, including the insula, basal-ganglia, basal-forebrain, frontal, internal and external capsules, hypothalamus, amygdala, hippocampus, and temporal sites, suggesting reduced white matter integrity in those areas. The correlations were preferentially widespread on the left over the right hemisphere. The pathological mechanisms for altered white matter integrity with disease severity may include hypoxic and perfusion changes in the condition (1999; Fanfulla et al. 2008; Yadav et al. 2013).
OSA and Brain Injury
Since MT imaging provides an estimate of macromolecule content, white matter deficits show lower MTR values, and indicate loss of white matter integrity. Earlier studies, based on various imaging and spectroscopy procedures, showed tissue injury, including myelin changes in frontal, parietal, temporal, insular and cingulum bundle, thalamus, hippocampus, pontine, and cerebellar areas (Chen et al. 2015; Joo et al. 2010; Kamba et al. 1997; Kumar et al. 2012; Kumar et al. 2014; Macey et al. 2008; Morrell et al. 2010; Tummala et al. 2015). In this study, several white matter and sub-cortical sites demonstrated predominantly negative correlations with AHI, including the external and internal capsules, corpus callosum, frontal and temporal white matter, and cingulum bundle, indicating increased axonal injury with disease severity. These findings indicate that MT imaging is especially effective for evaluating white matter integrity with disease severity in OSA subjects.
Magnetization Transfer vs. Other Imaging Procedures
Although other imaging procedures can be used to examine brain tissue integrity, including high-resolution T1-weighted imaging and diffusion tensor imaging (DTI), we used MT imaging procedures here due to better white matter sensitivity and in-plane resolution over other methods. Voxel-based-morphometry procedures, based on T1-weighted imaging, require partitioning into gray and white matter probability maps, and normalizing gray matter probability maps to a standard template with a higher voxel size. These data processing steps yield final gray matter maps with poorer resolution. Diffusion tensor imaging procedures also are inherently lower resolution, and data are collected with an assumption of Gaussian diffusion across the brain. However, white matter tract regions follow non-Gaussian diffusion due to complex microstructures and crossing fibers, and thus, may provide less accurate information of regional white matter integrity. Also, MT imaging offers better in-plane resolution than DTI. These aspects of better in-plane resolution and superior sensitivity to characterize white matter suggest that MT imaging procedures are better suited for use here.
Negative Correlations between MTR with AHI values
Significant negative correlations appeared between MTR values and AHI scores in OSA subjects in regions responsible for regulation of autonomic functions (e.g., blood pressure, heart rate), including insular, hypothalamic, basal-forebrain, and ventral medial prefrontal regions (Berntson et al. 1998; Harper et al. 1998; Oppenheimer et al. 1992; Soltis and DiMicco 1991). The left insula is primarily responsible for parasympathetic, and right insula, for sympathetic regulation (Oppenheimer et al. 1992), and both left and right areas interact with the hypothalamus, an area that also contributes to body temperature and sleep cycle control. The basal-forebrain, a site that influences autonomic action and quiet sleep regulation, and the ventral medical prefrontal cortex and surrounding white matter, a region heavily responsible for blood pressure control, showed enhanced injury with increased OSA severity.
Several motor activities, including periodic limb and upper airway movements, diminished in OSA subjects, can result from injury in the insular, putamen, caudate, and globus pallidus regions (Ackermann and Riecker 2004; Crutcher and DeLong 1984). Specifically, the anterior insula may perturb motor activities related to speech articulation and swallowing, as reported earlier (Ackermann and Riecker 2004). The putamen, and its integration with the caudate nucleus via surrounding white matter tracts, is responsible for motor integration and learning, and motor-cognitive activities, showed wide-spread damage with disease severity here (Packard and Knowlton 2002). The caudate nuclei also serve cognitive, affective, and motor control functions (Levitt et al. 2002; Villablanca 2010), and this structure also showed associations between tissue injury and disease severity. The globus pallidus, which contributes to the fiber bundles to the sub-thalamic nuclei and vice versa (Canteras et al. 1990), modifies voluntary motor activities, and also showed increased injury with disease severity.
Cognitive and mood dysfunctions commonly reported in OSA subjects may result from the hippocampus, amygdala, basal-forebrain, and frontal and ventral medial prefrontal areas (Maren 1999; Matsumura et al. 1999; Pribram et al. 1952). The inferior frontal cortex, hippocampus, basal-forebrain, and ventral medial prefrontal areas, sites responsible for higher-order cognition and mood regulation, demonstrated increased injury with OSA severity. Other areas, including the amygdala and ventral medial prefrontal cortex that regulate anxiety symptoms, commonly-reported issue in the condition, showed injury as negative correlations with disease severity. Also, the genu of the corpus callosum, containing white matter tracts responsible for integration of cognitive (Banich and Brown 2000), and somatosensory activities showed more tissue damage with disease severity.
Lateralization of Injury
White matter changes, based on negative correlations between MTR and AHI scores, were principally lateralized on the left side, including the insula, internal and external capsules, caudate, putamen, and cingulate and cingulum bundle. The left insula regulates parasympathetic nervous system activity, reduces blood pressure and heart rate, and showed wide-spread injury. Failure of parasympathetic control may lead to increased risk of cardiac arrhythmias and hypertension, as found in OSA subjects. The white matter tracts, including internal and external capsules, and the cingulum bundle, responsible for regulation of autonomic and motor activities, showed lateralized injury.
Limitations
There are several limitations in this study, including the small number of OSA subjects, procedural limitations, and contributions of BMI and hypertension on brain changes. These findings should be replicated on a larger population. Also, MT procedures offer poor resolution (3-4 mm slice thickness), and thus, the method is not useful to examine small brain sites. In addition, the small number of OSA subjects did not allow partitioning of BMI- and hypertension-induced brain changes.
Conclusions
Predominantly negative correlations emerged between regional brain MTR values and AHI scores in newly-diagnosed, treatment-naïve OSA subjects, indicating disease-dependent altered white matter integrity in the condition. Negative associations appeared in autonomic, cognitive, motor, and neuropsychologic control areas, including the basal-ganglia, insula, internal and external capsules, ventral medial prefrontal cortex and surrounding white matter, hypothalamus, hippocampus, and amygdala. These findings indicate the usefulness of MT imaging as a potential tool for examining and monitoring of OSA disease progression.
Significance.
The magnetization transfer (MT) imaging procedure is sensitive to brain white matter integrity, and has the potential to assess effects of disease severity (apnea-hypopnea index) on myelin integrity in obstructive sleep apnea (OSA) subjects. The procedure can also be used to monitor how white matter integrity changes with disease progression, and to evaluate effectiveness of interventions in OSA subjects, as well as in other neurological conditions.
Acknowledgements
We thank Ms. Karen Harada and Mr. Jose Palomares for their assistance in data collection.
Grant support: This research work was supported by National Institutes of Health R01 HL-113251 and R01 NR-015038.
Footnotes
Conflicts of Interest Statement
All authors have indicated no financial conflicts of interest to declare.
REFERENCES
- The Report of an American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89(2):320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Anik Y, Iseri P, Demirci A, Komsuoglu S, Inan N. Magnetization transfer ratio in early period of Parkinson disease. Acad Radiol. 2007;14(2):189–192. doi: 10.1016/j.acra.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Banich MT, Brown WS. A life-span perspective on interaction between the cerebral hemispheres. Dev Neuropsychol. 2000;18(1):1–10. doi: 10.1207/S15326942DN1801_1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res. 1998;94(2):225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Shammah-Lagnado SJ, Silva BA, Ricardo JA. Afferent connections of the subthalamic nucleus: a combined retrograde and anterograde horseradish peroxidase study in the rat. Brain Res. 1990;513(1):43–59. doi: 10.1016/0006-8993(90)91087-w. [DOI] [PubMed] [Google Scholar]
- Chen HL, Lu CH, Lin HC, Chen PC, Chou KH, Lin WM, Tsai NW, Su YJ, Friedman M, Lin CP, Lin WC. White matter damage and systemic inflammation in obstructive sleep apnea. Sleep. 2015;38(3):361–370. doi: 10.5665/sleep.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res. 1984;53(2):244–258. doi: 10.1007/BF00238154. [DOI] [PubMed] [Google Scholar]
- Fanfulla F, Grassi M, Taurino AE, D’Artavilla Lupo N, Trentin R. The relationship of daytime hypoxemia and nocturnal hypoxia in obstructive sleep apnea syndrome. Sleep. 2008;31(2):249–255. doi: 10.1093/sleep/31.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman RI, Gomori JM, Ramer KN, Lexa FJ, Schnall MD. Magnetization transfer: theory and clinical applications in neuroradiology. Radiographics. 1994;14(2):279–290. doi: 10.1148/radiographics.14.2.8190954. [DOI] [PubMed] [Google Scholar]
- Harper RM, Gozal D, Bandler R, Spriggs D, Lee J, Alger J. Regional brain activation in humans during respiratory and blood pressure challenges. Clin Exp Pharmacol Physiol. 1998;25(6):483–486. doi: 10.1111/j.1440-1681.1998.tb02240.x. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Woo MA, Macey PM, Macey KE, Frysinger RC, Alger JR, Yan-Go F, Harper RM. Neural responses during Valsalva maneuvers in obstructive sleep apnea syndrome. J Appl Physiol. 2003;94(3):1063–1074. doi: 10.1152/japplphysiol.00702.2002. [DOI] [PubMed] [Google Scholar]
- Joo EY, Tae WS, Lee MJ, Kang JW, Park HS, Lee JY, Suh M, Hong SB. Reduced brain gray matter concentration in patients with obstructive sleep apnea syndrome. Sleep. 2010;33(2):235–241. doi: 10.1093/sleep/33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamba M, Suto Y, Ohta Y, Inoue Y, Matsuda E. Cerebral metabolism in sleep apnea. Evaluation by magnetic resonance spectroscopy. Am J Respir Crit Care Med. 1997;156(1):296–298. doi: 10.1164/ajrccm.156.1.9611063. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Rathouz PJ, Yan LL, Liu K, Lauderdale DS. Stability of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Questionnaires over 1 year in early middle-aged adults: the CARDIA study. Sleep. 2006;29(11):1503–1506. doi: 10.1093/sleep/29.11.1503. [DOI] [PubMed] [Google Scholar]
- Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res. 2012;90(10):2043–2052. doi: 10.1002/jnr.23083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, Harper RM. Neuroanatomic deficits in congenital central hypoventilation syndrome. J Comp Neurol. 2005;487(4):361–371. doi: 10.1002/cne.20565. [DOI] [PubMed] [Google Scholar]
- Kumar R, Pham TT, Macey PM, Woo MA, Yan-Go FL, Harper RM. Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep. 2014;37(4):723–732. doi: 10.5665/sleep.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, McCarley RW, Dickey CC, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Ciszewski AA, Kikinis R, Jolesz FA, Shenton ME. MRI study of caudate nucleus volume and its cognitive correlates in neuroleptic-naive patients with schizotypal personality disorder. Am J Psychiatry. 2002;159(7):1190–1197. doi: 10.1176/appi.ajp.159.7.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31(7):967–977. [PMC free article] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22(12):561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Mascalchi M, Ginestroni A, Bessi V, Toschi N, Padiglioni S, Ciulli S, Tessa C, Giannelli M, Bracco L, Diciotti S. Regional analysis of the magnetization transfer ratio of the brain in mild Alzheimer disease and amnestic mild cognitive impairment. Am J Neuroradiol. 2013;34(11):2098–2104. doi: 10.3174/ajnr.A3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura N, Nishijo H, Tamura R, Eifuku S, Endo S, Ono T. Spatial- and task-dependent neuronal responses during real and virtual translocation in the monkey hippocampal formation. J Neurosci. 1999;19(6):2381–2393. doi: 10.1523/JNEUROSCI.19-06-02381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ, Jackson ML, Twigg GL, Ghiassi R, McRobbie DW, Quest RA, Pardoe H, Pell GS, Abbott DF, Rochford PD, Jackson GD, Pierce RJ, O’Donoghue FJ, Corfield DR. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65(10):908–914. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Ann Rev Neurosci. 2002:25-563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Park BPJ, Woo MA, Kang DW, Macey PM, Yan-Go FL, Harper RM, Kumar R. Aberrant insular functional network integrity in patients with obstructive sleep apnea. Sleep. 2016a doi: 10.5665/sleep.5738. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BPJ, Woo MA, Kang DW, Macey PM, Yan-Go FL, Harper RM, Kumar R. Distruted functional brain network organization in patients with obstructive sleep apnea. Brain and Behav. 2016b doi: 10.1002/brb3.441. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike GB, De Stefano N, Narayanan S, Worsley KJ, Pelletier D, Francis GS, Antel JP, Arnold DL. Multiple sclerosis: magnetization transfer MR imaging of white matter before lesion appearance on T2-weighted images. Radiology. 2000;215(3):824–830. doi: 10.1148/radiology.215.3.r00jn02824. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Mishkin M, Rosvold HE, Kaplan SJ. Effects on delayed-response performance of lesions of dorsolateral and ventromedial frontal cortex of baboons. J Comp Physiol Psychol. 1952;45(6):565–575. doi: 10.1037/h0061240. [DOI] [PubMed] [Google Scholar]
- Ridha BH, Tozer DJ, Symms MR, Stockton KC, Lewis EB, Siddique MM, MacManus DG, Rossor MN, Fox NC, Tofts PS. Quantitative magnetization transfer imaging in Alzheimer disease. Radiology. 2007;244(3):832–837. doi: 10.1148/radiol.2443061128. [DOI] [PubMed] [Google Scholar]
- Ropele S, Schmidt R, Enzinger C, Windisch M, Martinez NP, Fazekas F. Longitudinal magnetization transfer imaging in mild to severe Alzheimer disease. Am J Neuroradiol. 2012;33(3):570–575. doi: 10.3174/ajnr.A2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rovira A, Grive E, Pedraza S, Rovira A, Alonso J. Magnetization transfer ratio values and proton MR spectroscopy of normal-appearing cerebral white matter in patients with liver cirrhosis. Am J Neuroradiol. 2001;22(6):1137–1142. [PMC free article] [PubMed] [Google Scholar]
- Scholz TD, Hoyt RF, DeLeonardis JR, Ceckler TL, Balaban RS. Water-macromolecular proton magnetization transfer in infarcted myocardium: a method to enhance magnetic resonance image contrast. Magn Reson Med. 1995;33(2):178–184. doi: 10.1002/mrm.1910330206. [DOI] [PubMed] [Google Scholar]
- Sibon I, Tourdias T, Felix S, Asselineau J, Bracoud L, Vivot A, Rouanet F, Renou P, Orgogozo JM, Dousset V. Magnetisation transfer parameters and stroke outcome. J Clin Neurosci. 2015;22(6):1012–1017. doi: 10.1016/j.jocn.2014.11.035. [DOI] [PubMed] [Google Scholar]
- Soltis RP, DiMicco JA. GABAA and excitatory amino acid receptors in dorsomedial hypothalamus and heart rate in rats. Am J Physiol. 1991;260(1 Pt 2):R13–20. doi: 10.1152/ajpregu.1991.260.1.R13. [DOI] [PubMed] [Google Scholar]
- Strollo PJ, Jr., Rogers RM. Obstructive sleep apnea. New Eng J Med. 1996;334(2):99–104. doi: 10.1056/NEJM199601113340207. [DOI] [PubMed] [Google Scholar]
- Tambasco N, Belcastro V, Sarchielli P, Floridi P, Pierguidi L, Menichetti C, Castrioto A, Chiarini P, Parnetti L, Eusebi P, Calabresi P, Rossi A. A magnetization transfer study of mild and advanced Parkinson’s disease. Eur J Neurol. 2011;18(3):471–477. doi: 10.1111/j.1468-1331.2010.03184.x. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tozer DJ, Rees JH, Benton CE, Waldman AD, Jager HR, Tofts PS. Quantitative magnetisation transfer imaging in glioma: preliminary results. NMR Biomed. 2011;24(5):492–498. doi: 10.1002/nbm.1614. [DOI] [PubMed] [Google Scholar]
- Tummala S, Palomares J, Kang DW, Park B, Woo MA, Harper RM, Kumar R. Global and regional brain non-Gaussian diffusion changes in newly diagnosed patients with obstructive sleep apnea. Sleep. 2015;39(1):51–57. doi: 10.5665/sleep.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard SJ, Dumas EM, Hart EP, Milles J, Reilmann R, Stout JC, Craufurd D, Gibbard CR, Tabrizi SJ, van Buchem MA, van der Grond J, Roos RA. Magnetization transfer imaging in premanifest and manifest huntington disease: a 2-year follow-up. Am J Neuroradiol. 2013;34(2):317–322. doi: 10.3174/ajnr.A3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca JR. Why do we have a caudate nucleus? Acta Neurobiol Exp. 2010;70(1):95–105. doi: 10.55782/ane-2010-1778. [DOI] [PubMed] [Google Scholar]
- Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- Yadav SK, Kumar R, Macey PM, Richardson HL, Wang DJ, Woo MA, Harper RM. Regional cerebral blood flow alterations in obstructive sleep apnea. Neurosci Lett. 2013:555-159–164. doi: 10.1016/j.neulet.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang D, Qin W, Li Q, Chen B, Zhang Y, Yu C. Altered resting-state brain activity in obstructive sleep apnea. Sleep. 2013;36(5):651–659B. doi: 10.5665/sleep.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]