Abstract
Background
A number of studies have reported that depression is associated with lower relative left frontal activity in the alpha band (i.e., frontal asymmetry, or FA), as measured by electroencephalogram. FA has also been hypothesized to be a vulnerability marker for depression. If this is the case, FA should be evident in offspring of depressed mothers, a group at elevated risk for depression. However, the results of previous offspring studies have been inconsistent and none of these studies has considered whether the relationship between FA and risk changes over development in children.
Method
We assessed FA twice, at ages 3 and 6, in 253 never-depressed children from a community sample. Maternal history of depressive disorders was determined by a diagnostic interview completed by the mothers at the first assessment.
Results
There was a significant interaction between maternal depression and offspring age at assessment, indicating that FA exhibits different developmental trajectories depending on level of familial risk for depression. Offspring of depressed mothers exhibited a decreasing relative left FA over the course of early childhood, while offspring of non-depressed mothers exhibited relatively similar, symmetrical, levels of frontal alpha activity at both assessment points.
Conclusions
These results suggest that changes in FA from early to middle childhood distinguish those at risk for depression and that cross-sectional assessment of FA may have limited value in understanding risk. These results highlight the importance of considering development in understanding the role of FA in depression.
Keywords: Psychophysiology, maternal depression, developmental psychopathology, EEG, child development
Introduction
Davidson (1994, 1998) hypothesized that lateralization of alpha activity in the frontal region, as measured by electroencephalography (EEG), is linked to biobehavioral systems associated with approach and withdrawal behavior. Specifically, he proposed that left frontal brain activity is related to an appetitive/reward system associated with approach behavior such that greater relative left activity leads to positive affect/approach behavior, whereas right frontal activity is linked to a withdrawal/avoidance system, such that greater relative right activity leads to negative affect/avoidance. Davidson also hypothesized that depression is associated with approach deficits indicated by lower left than right frontal activity. Consistent with this model, Henriques and Davidson (1991) and others have reported that during rest, individuals with major depressive disorder (MDD) or elevated depressive symptoms exhibit relative left frontal hypoactivation (typically indexed by the difference between medial [F3-F4] and/or lateral [F7-F8] electrode sites). These findings were supported by a meta-analysis, which concluded that reduced relative left frontal asymmetry (FA) is associated with depression with a small-medium effect size (Thibodeau, Jorgensen, & Kim, 2006).
The link between FA and depression is not confined to current episodes and symptoms. Several studies indicate that individuals who have remitted from depression also exhibit left frontal hypoactivation relative to never depressed controls (e.g. Henriques & Davidson, 1990, Stewart, Bismark, Towers, Coan, & Allen, 2010). Moreover, FA has been reported to prospectively predict the first lifetime onset of depression (Nusslock et al., 2011). As FA has moderate test-retest stability in adults (e.g. Hagemann, Hewig, Seifert, Naumann, & Bartussek, 2005; Vuga et al., 2006) and is modestly heritable (e.g. Gao, Tuvblad, Raine, Lozano, & Baker, 2009), FA has been considered a putative vulnerability marker and endophenotype for depression (Allen & Reznik, 2015; Goldstein & Klein, 2014).
Most studies have been based on adult or adolescent samples; however if FA is a vulnerability marker, it is important to determine whether it is evident prior to the period of increased risk for depression, and especially in young children, where early detection and intervention may have particularly powerful effects (Luby, Lenze, & Tillman, 2012). Parental history of depression is arguably the best-established risk factor for depression (Gotlib & Colich, 2014). Thus, if FA is a vulnerability marker it should be evident in the offspring of depressed parents compared to offspring of parents with no history of depression.
There is a large literature that consistently finds lower relative left FA in infant offspring of depressed mothers compared to infants of never depressed mothers (e.g. Dawson et al., 1997; Field, Fox, Pickens, & Nawrocki, 1995). Indeed, a meta-analysis of 13 studies (including over 1000 infants) found lower relative left FA in infants of depressed mothers, and that the magnitude of this effect was comparable to the effect seen in depressed compared to non-depressed adults (Thibodeau et al., 2006). Fewer studies have used child and adolescent samples. Several studies found evidence for lower left FA in children and adolescents (Jones, Field, & Davalos; 2000; Lopez-Duran, Nusslock, George, & Kovacs, 2012; Tomarken, Dichter, Garber, & Simien; 2004); however, other studies failed to find associations (Bruder et al., 2005; Dawson, et al., 2003; Forbes, et. al., 2006).
Changes in neurodevelopment from infancy through adolescence may pose challenges to interpreting the literature on FA and risk for depression (Galvan, 2010; Luciana, 2013; Raznahan et al., 2014). The stability of FA electrode pairs is quite modest in youth (correlations coefficients with small to medium effects; .21−.50), suggesting that there may be normative developmental changes in FA (Vuga, Fox, Cohn, Kovacs, & George, 2008; Müller, Kühn-Popp, Meinhardt, Sodian, & Paulus, 2015). Unfortunately, many of the studies of children and adolescents sampled a wide age range, and all published studies that we are aware of used cross-sectional designs. There is evidence that FA trajectories provide a more sensitive measure of individual differences than single assessments, particularly in high risk populations (McLaughlin, Fox, Zeanah, & Nelson, 2011). Hence, it may be important to compare the trajectories of FA in offspring of depressed and non-depressed parents across a uniform period of development. Moreover, if there are developmental changes in the association between FA and familial risk for depression, it may provide clues to the pathogenesis of depressive disorders and suggest periods when intervention may be most effective.
The present study examined the developmental trajectory of FA from age 3 to age 6 in a large community sample of offspring of mothers with and without a history of depression. To our knowledge, this is the first study to use a longitudinal design to address whether the associations between a child’s FA and maternal depression change across development.
Methods
Participants
A community sample of 559 families with a 3-year-old-child was recruited from Long Island, New York for a longitudinal study of risk for psychopathology (see Olino et al., 2010; Shankman et al., 2011). Only one child in each family participated. Mother’s provided written consent for participation. The study was approved by Stony Brook University Committee on Research Involving Human Subjects.
403 children completed a baseline EEG assessment when they were 3 years old, and their mothers participated in a diagnostic interview. 332 of these children also completed a second EEG assessment when they were 6 years old. For the current analyses, left-handed or ambidextrous children were excluded as is typical in the FA literature. Right handedness was defined by observation of the child, at ages 3 and 6, using their right hand or foot in at least 4 of 6 tasks: throwing a ball, drawing a circle, pretending to use scissor, opening a jar, kicking a ball, and looking through a paper towel roll. Of the children who completed both EEG assessments, 70 were left hand dominant at one of the two time points. One child was removed as an outlier, with some EEG values that were an order of magnitude larger than the next largest value observed in the other children. Another child’s data was lost due to technical problems. We also removed 7 children from analyses who had already met criteria for depressive disorders. Including o excluding these children did not change the pattern of results. Thus, the final sample is comprised of 253 non-depressed and stably right-handed children who completed both assessments and had data on maternal psychopathology.
Children in the final sample (N = 253) compared to the other families included in this project (N = 306) were similar on baseline age (M = 3.52 vs. M = 3.52, respectively; t (557) = .32, p >.05), proportion of non-Hispanic Caucasians (88.1% vs. 85.9%, respectively; χ² (1, N = 559) = 0.59, p > .05), and marital status of parents, (94.6% married vs. 94.2%, χ² (1, N = 517) = 0.02, p > .05). Importantly, the rate of maternal history of depressive disorders did not differ among children who were and were not included in the final sample (31.6% vs. 33.0%, respectively, χ² (1, N = 541) = 0.15, p > .05). However, children in the final sample were significantly more likely to be female (51.0% vs. 42.2%, χ² (1, N = 559) = 5.30, p < .05) and to have a parent with a college degree (76.2% vs. 66.8%, χ² (1, N = 518) = 4.73, p < .05) than the remaining children.
Demographic data for the final sample are shown in Table 1. Children with and without mothers with a history of depression did not differ significantly on any of the demographic variables.
Table 1.
Demographic characteristics of offspring without and with depression history
| Without Maternal Depression History (N = 173) |
With Maternal Depression History (N = 80) |
|
|---|---|---|
| Child Gender (% male) | 50.3 % | 46.3 % |
| Marital status (% married) | 93.5 % | 93.2 % |
| At least one parent with a 4-year degree (%) |
79.8 % | 68.4 %+ |
| Race/ethnicity (% white-non Hispanic) |
87.9 % | 88.75 % |
| 1st assessment | ||
| Child age in years; M (SD) |
3.52 (.27) | 3.51 (.25) |
| 2nd assessment | ||
| Child age in years; M (SD) |
6.03 (.37) | 6.03 (.41) |
p < .10
Child psychopathology
At both time points child depressive disorder diagnoses using modified criteria for early childhood were evaluated using the Preschool Age Psychiatric Assessment (PAPA; Egger & Angold, 2004). The PAPA is a parent report diagnostic interview for DSM-IV disorders in preschoolers, and has been demonstrated to possess adequate test-retest reliability (Egger et al., 2006). At the age 3 assessment, interviews were completed over the phone by an advanced graduate. Reliability was excellent as Kappa values were 1.00 for depressive diagnoses based on 21 interviews coded by the interviewer and a second rater. At the age 6 assessment, the interviews were conducted face-to-face and Kappa values were 0.64 for depressive disorders based on 35 interviews (see Bufferd, Dougherty, Carlson, Rose, & Klein, 2012).1
Maternal psychopathology
As part of the age 3 assessment, the lifetime history of psychopathology of mothers was assessed by two Masters-level raters using the Structured Clinical Interview for DSM-IV, Non-Patient version (SCID-NP; First, Spitzer, Gibbon, & Williams, 1996) over the phone, which has been found to be comparable to face-to-face interviewing (Rohde, Lewinsohn, & Seeley, 1997). The kappa for inter-rater reliability, based upon 30 interview recordings, was .93 for depressive disorders. Of the 253 participants included in analyses, 80 mothers had a history of depressive disorders. Specifically, 54 mothers had a lifetime history of MDD, 16 had a lifetime history of dysthymic disorder, and 10 mothers had lifetime histories of MDD and dysthymic disorder. Of mothers with a history of either depressive disorder, 44 (55.0%) had a chronic or recurrent course. In addition, 36 mothers with depressive diagnoses (45.0%) had a history of at least one anxiety disorder, and 26 (32.5%) had a lifetime history of substance use.
EEG data acquisition and analysis
The EEG recording procedures have been described in detail elsewhere (see Shankman et al., 2011), and were identical at both the age 3 and age 6 assessment waves. However, the data reported here and questions addressed in the current study differ from our prior study, which utilized data from only the first assessment wave and explored associations between temperament and FA (Shankman et al., 2011).
EEG was recorded for 6 minutes with children alternating between 1 minute blocks with their eyes open or closed. During both conditions, children were instructed to sit as still and quietly as possible. Using the Active Two system (Biosemi, Amsterdam, The Netherlands), EEG was acquired with a 32 electrode channel Lycra cap following the international 10/20 labeling system (American Electroencephalographic Society, 1994). Two electrodes were placed behind the left and right ears resting on the mastoid bones. Additional electrodes were placed around the eyes to capture eye blinks: two located approximately one cm above and below the left eye, a third approximately one cm to the left of the left eye and a fourth approximately one cm to the right of the right eye. Another electrode was placed on the nose to be used as a reference. A 0.16–40 Hz bandpass filter was applied to all electrode channels at. Two reference montages were computed offline, a nose reference and a reference using the average signal.
Using the program PolyRex (Kayser, 2003), participants’ Biosemi data was converted to Neuroscan 4.1 (Charlotte, NC). The data was segmented into 1.024 second epochs at 0.512 second intervals, which yields epochs that overlap by 50% with one another. This was done to retain as much power as possible since power attenuated at the beginning of an epoch is represented in the overlapping windows. Epochs contaminated by blinks, eye movements, and movement-related artifacts were excluded with a semi-automated procedure and direct visual inspection of the data. A Fast Fourier transform was used to compute power spectra (only alpha is reported). Alpha power was defined by 6–10 Hz band, which was selected according to past research (Marshall, Bar-Haim, & Fox, 2002), and then alpha power was averaged at each recording site for accepted epochs.
Statistical analyses
The FA measures were computed the following way. The data collected at F3, F4, F7, and F8 were natural log (Ln) transformed to reduce positive skewness. Difference scores to reflect asymmetry were created for each of the two pairs of electrodes, Ln(Right) – Ln(Left), such that positive scores indicate greater relative left brain activity and negative scores indicate lower relative left brain activity (as alpha is a putative inverse of brain activity). To explore the effect of maternal history of depression and alpha asymmetry over time, we computed a three-level mixed linear model (MLM; Mplus version 7.31) using maximum-likelihood estimation. This approach accounts for fact that the data were clustered within individuals and within an individual’s assessment recording session. In the present analyses, we selected only the cases with complete EEG recordings from both time points. Maternal history of depression, eye condition (open or closed), reference scheme (nose or average), and electrode site (medial or lateral, which corresponds to either the F3-F4 or F7-F8 pairs, respectively) were included in our models as fixed effects. Instead of using assessment wave, we used the participants’ age at the time of each assessment to produce estimates that were more sensitive to developmental changes; these were mean-centered on the samples’ average age at the first time point (M=3.52). Age was treated as a random effect, such that the effect of age on FA could vary for an individual (Singer & Willet, 2003).
A full factorial model was specified so that all possible interactions could be tested, producing a fully saturated model with 31 predictors. Only main effects and significant interaction effects are discussed below (parameter estimates for all effects are available in the online supplementary Table S1). To follow up interactions from multilevel regression-based analyses, we used Preacher’s approach (Preacher, Curran, & Bauer, 2006), which is similar to Aiken and West’s (1991) simple slopes method. Values were selected for one of the independent variables (IV; referred to as W) that makes up the interaction and this allows for an interpretation of the relationship of the second IV (referred to as X) to the dependent variable at various levels of W. The significance and the direction of the estimated parameter for the outcome variable is interpretable as the effect of X at each level of W in that particular follow-up test (Preacher et al., 2006).
Results
Table 2 provides descriptive data for average alpha power at each electrode site by maternal depression history. Table 3 reports intraclass correlations among EEG FAs over time for each conditions and reference scheme. Stability from age 3 to 6 was modest, but generally statistically significant, ranging from .07 to .26 across conditions (eyes open vs. closed and when using a nose or average reference).
Table 2.
Frontal alpha power by maternal history status
| No maternal Depression History (N= 173) |
Maternal Depression History (N=80) |
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| F4 age 3 (right) | 1.86 | .39 | 1.88 | .36 |
| F8 age 3 (right) | 1.59 | .34 | 1.63 | .32 |
| F3 age 3 (left) | 1.85 | .36 | 1.87 | .37 |
| F7 age 3 (left) | 1.62 | .33 | 1.64 | .34 |
| F4 age 6 (right) | 1.68 | .39 | 1.63 | .33 |
| F8 age 6 (right) | 1.38 | .36 | 1.34 | .32 |
| F3 age 6 (left) | 1.66 | .39 | 1.70 | .44 |
| F7 age 6 (left) | 1.38 | .37 | 1.35 | .35 |
Table 2 Shows the mean and standard deviations for each electrode site as a function of maternal depression status for each time point (averaged across, eye recording condition and reference scheme). Odd numbers correspond to the left hemisphere and even numbers correspond to the right hemisphere.
Table 3.
Frontal Asymmetry stability from age 3 to age 6
| Test-retest Correlations |
||
|---|---|---|
| Nose | Average | |
| F3-F4 (closed) | .07 | .14* |
| F3-F4 (opened) | .08 | .15* |
| F7-F8 (closed) | .26** | .23** |
| F7-F8 (opened) | .20** | .19** |
Table 3 reports the stability (single measures interclass-correlation; ICC) of asymmetry from age 3 to 6 by electrode location, for each recording eyes open/closed condition, and for each reference (N=253)
p < .01;
p < .001.
For the MLM analysis of FA we have included the online supplemental Table S1 to provide details of the effects of each predictor, but focus here on the main effects and significant interactions. The MLM analysis for FA revealed that the main effect of eye condition was significant at a trend level (b = 0.01, standard error [SE] = .01, p < .10) and eye condition did not significantly interact with any other variables. There were significant main effects for reference scheme (b = 0.04, SE = .01, p <.001), and electrode site (b = −0.04, SE = .01, p < .001). However, they were qualified by reference by electrode site (b = 0.03, SE = .02, p < .05) and age by electrode site (b = 0.02, SE = .004, p < .001) interactions. As these interactions were not hypothesized or of substantive interest, we did not conduct follow-up analyses.
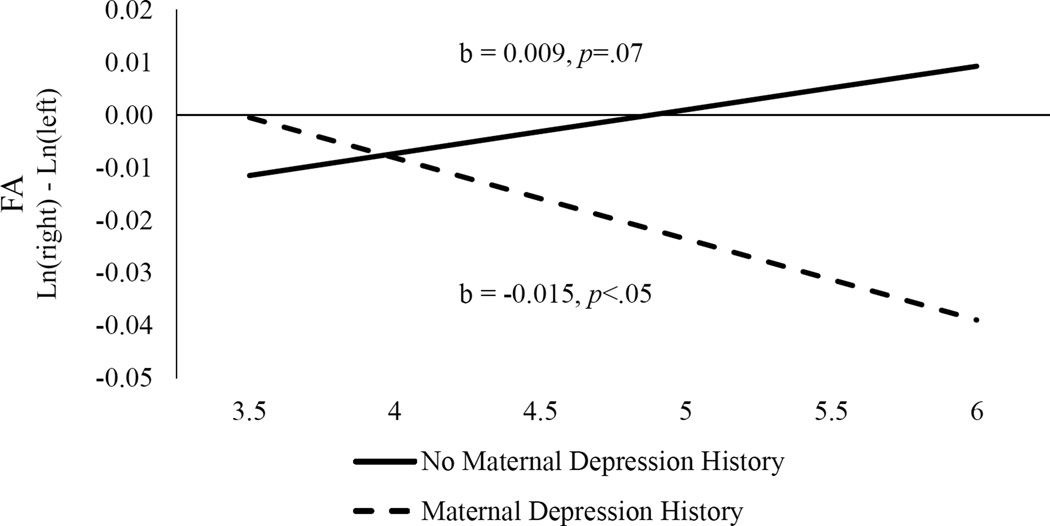
The main effects of child age (b = <0.001, SE = .004, p > .05) and maternal depression history (b = 0.01, SE = .02, p > .05) were not significant. However, as indicated in Figure 1, there was a significant interaction between child age and maternal depression (b = −.02, SE = .01, p < .01). This interaction was followed up by computing simple slopes effects for each level of maternal depression status. The simple slope effect of child age was significant at a trend level for offspring of non-depressed mothers (b = 0.009, SE = 0.005, p = .07), suggesting that there was a tendency for FA to become slightly more positive (greater left than right activity) with age. As the intercept for this slope was −0.01, however, it appears that both hemispheres exhibited relatively equal levels of activity. In contrast, the simple slope for offspring of mothers with depression history was significant (b = −0.015, SE = 0.007, p < .05), indicating that FA became more negative (lower left than right activity) with age. At the age 3 assessment, offspring of mothers with depression history did not significantly differ from offspring of non-depressed mothers (b = 0.01, SE = 0.016, p >.05). However, at the age 6 assessment offspring of mothers with a history of depression had a significantly lower relative left FA than offspring of mothers without depression history (b = −0.049, SE = 0.02, p <.05). There was no age by maternal depression history by reference interaction (p = 0.14) suggesting that this effect did not vary by reference.2
Figure 1.
Frontal asymmetry interaction of age and maternal depression
Positive scores indicate greater left than right activity and negative scores indicate greater right than left activity. The b values are given for the slopes of offspring of mothers with and without maternal depression history over time.
Discussion
We examined if maternal history of depression is related to children’s FA and whether this relationship changes between ages 3 and 6. We found a significant interaction between maternal depression and child age indicating that offspring of mothers with a history of depression developed significantly lower left than right FA over time. In contrast, offspring of mothers without depression showed relatively similar, symmetrical, pattern of frontal activity at both time points. As a result, group differences were not evident at age 3, but emerged at age 6. These findings indicate that the developmental trajectories of FA in early childhood differ between offspring of mothers with and without a history of MDD, and that the two groups diverge from the preschool to the early school-age period. Further follow-up is necessary to determine whether the groups separate even further in later childhood and adolescence, and whether relative left FA predicts the onset of depression.
Previous studies conflict as to whether decreased relative left FA is present in offspring of depressed mothers. There is evidence that lower relative left FA is present in infants of depressed mothers (e.g. Dawson et al., 1997; Field et al., 1995), but the effect may weaken in later infancy (Thibodeau, Jorgensen, & Kim, 2006). Furthermore, studies using children and adolescents report inconsistent findings (Bruder et al., 2005; Dawson et al., 2003; Forbes et al., 2003; Jones et al., 2000; Lopez-Duran et al., 2012; Tomarken et al., 2004). The inconsistencies may be attributed to study differences in age and ethnicity of offspring, parental education/social class, assessment of maternal depression (diagnoses based on semi-structured interviews versus elevated scores on self-report inventories), definition of alpha band frequency, and reference montage. In addition, some studies expose children to stimuli during the resting EEG assessment (e.g. blowing bubbles near the child, Dawson et al., 2003; emotional film clips, Lopez-Duran et al., 2012). Finally, depression is a heterogeneous syndrome and samples may differ in the nature and characteristics of parental depression and the presence of comorbid conditions, such as anxiety disorders. The present study cannot fully resolve these inconsistencies; although we did not find effects with maternal anxiety in the model. Nonetheless, our findings suggest that the results of comparisons of FA between offspring of depressed and non-depressed mothers may be age dependent, and that cross-sectional comparisons may obscure differences between the two groups’ developmental trajectories. Indeed, when our findings are taken together with the literature on infants of depressed mothers, it raises the possibility that there are non-linear relationships between FA and familial risk for depression over the course of development.
FA is hypothesized to index tendencies in approach and/or withdrawal behavior (e.g. Davidson, 1994, 1998). It may also be related to reward processing, a facet of approach, which is implicated in depression (Pizzagalli, 2013; Treadway & Zald, 2011). Indeed, when FA was assessed during a reward task, left hemisphere activity increased in healthy controls, but not in individuals with early onset depression (Shankman, Klein, Tenke, & Bruder, 2007; Shankman et al., 2013). The present findings are also consistent with evidence from event-related potential and functional magnetic resonance imaging (fMRI) studies revealing reward processing deficits in older children and adolescents of depressed mothers (Gotlib et al., 2010; Kujawa, Proudfit, & Klein, 2014; Olino et al., 2014; Sharp et al., 2014), and findings that diminished reactivity to reward predicts increases in depressive symptoms (Morgan, Olino, McMakin, Ryan, & Forbes, 2013) and first-onsets of MDD (Bress et al., 2013) in adolescents and children. Human and non-human studies typically find that the same regions (such as the ventral striatum) are associated with reward processing through development, but that neural activity in response to reward and dopamine function increases during adolescence, which is often thought of as a period of reward hypersensitivity (Galvan, 2010). However, recent modeling of reward areas using structural MRI also suggest substantial non-linear changes during the childhood period included in this study (Raznahan et al., 2014).
Interestingly, there is some evidence that rates of depression, although low, increase between the preschool and school-age periods (Bufferd et al., 2012; Luby, Gaffrey, Tillman, April, & Belden, 2014), paralleling the present findings regarding FA. However, this does not account for our results, as children with depressive disorders were excluded from the analyses. However, it is consistent with the possibility of an increase in underlying risk processes, perhaps associated with atypical development of the approach system.
This study has several strengths; in particular, it is the largest sample to examine FA in children of depressed parents and is the only study that examines the relationship between familial risk for depression and the developmental trajectory of FA. However, it also has several limitations. Many participants from the overall sample did not complete the EEG procedure at one or both time points. Also, we had only two data points and captured a limited part of development. As a result, we cannot identify non-linear trajectories or draw conclusions about other developmental periods. Indeed, as FA is more consistently found in younger infants samples than children, it is possible that the relationship between FA and risk status does not follow a linear trajectory. To address, this future studies should measure EEG FA repeatedly and frequently from infancy through adolescence. Finally, consistent with the literature, we focused on maternal depression; further work examining the influence of paternal depression on offspring FA is also warranted.
In conclusion, our results suggest that the association between FA and familial risk for depression may change as a function of development, and that this link becomes stronger over the course of early childhood. However, additional studies are needed to determine the clinical utility and impact of our findings as well as why and when FA changes in at-risk offspring. The present findings suggest that conclusions about the role of FA in risk for depression at any one point in development must be very circumscribed, and that FA may be a dynamic marker of risk that changes with development.
Supplementary Material
Key points.
Previous studies examining lower left than right EEG frontal asymmetry (FA) as a vulnerability marker in young offspring of depressed mothers have been inconsistent, possibly as a result of developmental changes.
The current study of a large community sample used a longitudinal design that included two FA assessments, when children were approximately 3 years-old and again when they were approximately 6.
An age by maternal history of depressive disorder interaction was found, suggesting that at risk offspring developed significantly lower left than right FA over time whereas offspring of never depressed mothers showed symmetrical FA at both time points.
This study highlights the importance longitudinal designs when determining whether FA is a vulnerability marker for depression.
Acknowledgments
This work was supported by a National Institute of Mental Health grant (R01 MH069942) awarded to D.N.K.
Footnotes
Dimensional symptom scores for depression were created by summing raters’ responses, which were created to test whether children’s symptoms were associated with FA at the time of recording. None of the correlations were statistically significant suggesting that any observed effects are due to maternal depression history alone.
We also conducted two secondary MLMs to test the consistency of our finding. First, we excluded mothers with a history of dysthymic disorder without MDD from the maternal depression history group. The pattern of findings was similar to that reported above. Second, we substituted maternal anxiety history for maternal depression history in the MLM, and found that the main effect for maternal anxiety and the age by maternal anxiety interaction were not significant, indicating that our effects are specific to maternal depression.
Some investigators have also observed a pattern of lower relative right posterior activity (or posterior asymmetry) in depressed samples (Henriques & Davidson, 1990), which has been hypothesized to be associated with reduced emotional arousal (Heller, 1993). While some investigators (Bruder et al., 2005) have reported a similar pattern of posterior asymmetry (PA) in offspring of depressed mothers, most studies have not found differences between children of depressed and non-depressed mothers on PA (e.g. Dawson et al., 2007; Field et al., 1995; Tomarken et al., 2004). Therefore, we focused on FA in this paper. Nevertheless, we analyzed PA at ages 3 and 6 as a function of maternal history of depression using a multilevel model and have summarized the results in the online supplemental Table S2. These analyses revealed a significant electrode site by maternal depression interaction indicating that the relative difference between offspring of depressed and non-depressed mothers was greater at P3/P4 than at P7/P8. Importantly, however, the groups did not differ significantly at either electrode site. In addition, there was a trend for a site X age X maternal depression interaction. However, the two groups of offspring did not differ significantly at either electrode site at either age.
The authors have declared that they have no conflicts of interest in relation to this article.
Supporting information
Additional supporting information may be found in the online version of this article:
Table S1. Parameter estimates of full MLM of FA
Table S2. Parameter estimates of full MLM of PA
References
- Allen JJB, Reznik SJ. Frontal EEG asymmetry as a promising marker of depression vulnerability: summary and methodological considerations. Current Opinion in Psychology. 2015;4:93–97. doi: 10.1016/j.copsyc.2014.12.017. http://dx.doi.org/10.1016/j.copsyc.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Electroencephalographic Society. American electroencephalographic society guidelines in electroencephalography, evoked potentials, and polysomnography. Journal of Clinical Neurophysiology. 1994;11(1):1–142. [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. http://dx.doi.org/10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, Weissman MM. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biological Psychiatry. 2005;57(4):328–335. doi: 10.1016/j.biopsych.2004.11.015. http://dx.doi.org/10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: Continuity from ages 3 to 6. American Journal of Psychiatry. 2012;169(11):1157–1164. doi: 10.1176/appi.ajp.2012.12020268. http://doi.org/10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1994;6(4):741–758. http://dx.doi.org/10.1017/S0954579400004764. [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12(3):307–330. http://dx.doi.org/10.1080/026999398379628. [Google Scholar]
- Dawson G, Ashman SB, Panagiotides H, Hessl D, Self J, Yamada E, Embry L. Preschool outcomes of children of depressed mothers: Role of maternal behavior, contextual risk, and children’s brain activity. Child Development. 2003;74(4):1158–1175. doi: 10.1111/1467-8624.00599. http://dx.doi.org/10.1111/1467-8624.00599. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, HessI D. Infants of depressed mothers exhibit atypical frontal brain activity a replication and extension of previous findings. Journal of Child Psychology and Psychiatry. 1997;38(2):179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x. http://dx.doi.org/10.1111/j.1469-7610.1997.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, Klein DN. Psychiatric disorders in preschoolers: Continuity from ages 3 to 6. American Journal of Psychiatry. 2012;169(11):1157–1164. doi: 10.1176/appi.ajp.2012.12020268. http://doi.org/10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A. The Preschool Age Psychiatric Assessment (PAPA): A structured parent interview for diagnosing psychiatric disorders in preschool children. Handbook of Infant, Toddler, and Preschool Mental Health Assessment. 2004:223–243. [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the preschool age psychiatric assessment (PAPA) Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(5):538–549. doi: 10.1097/01.chi.0000205705.71194.b8. http://doi.org/10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Field T, Fox NA, Pickens J, Nawrocki T. Relative right frontal EEG activation in 3-to 6-month-old infants of” depressed” mothers. Developmental Psychology. 1995;31(3):358. http://dx.doi.org/10.1037/0012-1649.31.3.358. [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology and Psychiatry. 2006;47(1):79–87. doi: 10.1111/j.1469-7610.2005.01442.x. http://dx.doi.org/10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:116–124. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. The teenage brain: sensitivity to rewards. Current Directions in Psychological Science. 2013;22(2):88–93. doi: 10.1177/0963721413476512. http://dx.doi.org/10.1177/0963721413480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Tuvblad C, Raine A, Lozano DI, Baker LA. Genetic and environmental influences on frontal EEG asymmetry and alpha power in 9-10-year-old twins. Psychophysiology. 2009;46(4):787–796. doi: 10.1111/j.1469-8986.2009.00815.x. http://dx.doi.org/10.1111/j.1469-8986.2009.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Third. New York: Guilford Press; 2014. pp. 240–258. [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. http://dx.doi.org/10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W. Neuropsychological mechanisms of individual differences in emotion, personality, and arousal. Neuropsychology. 1993;7(4):476–489. http://doi.org/10.1037/0894-4105.7.4.476. [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99(1):22–31. doi: 10.1037//0021-843x.99.1.22. http://dx.doi.org/10.1037/0021-843X.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100(4):535–545. doi: 10.1037//0021-843x.100.4.535. http://dx.doi.org/10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Davalos M. Right frontal EEG asymmetry and lack of empathy in preschool children of depressed mothers. Child Psychiatry and Human Development. 2000;30(3):189–204. doi: 10.1023/a:1021399605526. http://dx.doi.org/10.1023/A:1021399605526. [DOI] [PubMed] [Google Scholar]
- Kayser J. Polygraphic Recording Data Exchange—PolyRex. New York State Psychiatric Institute: Department of Biopsychology; 2003. [Google Scholar]
- Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology. 2014;123(2):287–297. doi: 10.1037/a0036285. http://dx.doi.org/10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Nusslock R, George C, Kovacs M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology. 2012;49(4):510–521. doi: 10.1111/j.1469-8986.2011.01332.x. http://dx.doi.org/10.1111/j.1469-8986.2011.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of preschool disorders to full DSM depression at school age and early adolescence: Continuity of preschool depression. American Journal of Psychiatry. 2014;171(7):768–776. doi: 10.1176/appi.ajp.2014.13091198. http://doi.org/10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Lenze S, Tillman R. A novel early intervention for preschool depression: Findings from a pilot randomized controlled trial. Journal of Child Psychology and Psychiatry. 2012;53(3):313–322. doi: 10.1111/j.1469-7610.2011.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development in normality and psychopathology. Development and Psychopathology. 2013;25(4):1325–1345. doi: 10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113(8):1198–1208. doi: 10.1016/s1388-2457(02)00163-3. http://dx.doi.org/10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Adverse rearing environments and neural development in children: The development of frontal electroencephalogram asymmetry. Biological Psychiatry. 2011;70(11):1008–1015. doi: 10.1016/j.biopsych.2011.08.006. http://dx.doi.org/10.1016/j.biopsych.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. http://dx.doi.org/10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller BCN, Kühn-Popp N, Meinhardt J, Sodian B, Paulus M. Long-term stability in children’s frontal EEG alpha asymmetry between 14-months and 83-months. International Journal of Developmental Neuroscience. 2015;41:110–114. doi: 10.1016/j.ijdevneu.2015.01.002. http://dx.doi.org/10.1016/j.ijdevneu.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: Common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120(2):497–503. doi: 10.1037/a0022940. http://dx.doi.org/10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468–478. doi: 10.1037/a0020112. http://dx.doi.org/10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, Forbes EE. Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. http://dx.doi.org/10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology. 2013;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. http://dx.doi.org/10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. http://dx.doi.org/10.3102/10769986031004437. [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Giedd JN. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences. 2014;111(4):1592–1597. doi: 10.1073/pnas.1316911111. http://dx.doi.org/10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews in assessing axis I and II disorders. American Journal of Psychiatry. 1997;154(11):1593–1598. doi: 10.1176/ajp.154.11.1593. http://dx.doi.org/10.1176/ajp.154.11.1593. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116(1):95–104. doi: 10.1037/0021-843X.116.1.95. http://dx.doi.org/10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Torpey DC, Olino TM, Dyson MW, Kim J, Tenke CE. Do positive and negative temperament traits interact in predicting risk for depression? A resting EEG study of 329 preschoolers. Development and Psychopathology. 2011;23(2):551–562. doi: 10.1017/S0954579411000022. http://dx.doi.org/10.1017/S0954579411000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322–338. doi: 10.1037/a0030747. http://dx.doi.org/10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Kim S, Herman L, Pane H, Reuter T, Strathearn L. Major depression in mothers predicts reduced ventral striatum activation in adolescent female offspring with and without depression. Journal of Abnormal Psychology. 2014;123(2):298–309. doi: 10.1037/a0036191. http://dx.doi.org/10.1037/a0036191. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119(3):502–512. doi: 10.1037/a0019196. http://dx.doi.org/10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115(4):715–729. doi: 10.1037/0021-843X.115.4.715. http://dx.doi.org/10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Garber J, Simien C. Resting frontal brain activity: Linkages to maternal depression and socio-economic status among adolescents. Biological Psychology. 2004;67(1–2):77–102. doi: 10.1016/j.biopsycho.2004.03.011. http://dx.doi.org/10.1016/j.biopsycho.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011;35(3):537–555. doi: 10.1016/j.neubiorev.2010.06.006. http://dx.doi.org/10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NAF, Cohn J, George CJ, Levenstein RM, Kovacs M. Long-term stability of frontal electroencephalographic asymmetry in adults with a history of depression and controls. International Journal of Psychophysiology. 2006;59(2):107–115. doi: 10.1016/j.ijpsycho.2005.02.008. http://dx.doi.org/10.1016/j.ijpsycho.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology. 2008;67(1):70–77. doi: 10.1016/j.ijpsycho.2007.10.007. http://dx.doi.org/10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.