Abstract
DREADDs, designer receptors exclusively activated by designer drugs, are engineered G protein-coupled receptors (GPCR) which can precisely control GPCR signaling pathways (for example, Gq, Gs and Gi). This chemogenetic technology for control of GPCR signaling has been successfully applied in a variety of in vivo studies, including in mice, to remotely control GPCR signaling, for example, in neurons, glia cells, pancreatic beta-cells, or cancer cells. In order to fully explore the in vivo applications of the DREADD technology we generated hM3Dq and hM4Di strains of mice which allow for Cre recombinase-mediated restricted expression of these pathway-selective DREADDs. With the many Cre driver lines now available, these DREADD lines will be applicable to studying a wide array of research and preclinical questions.
Keywords: chemogenetic, G protein–coupled receptors, hM3Dq, hM4Di, GsD
RESULTS AND DISCUSSION
Using directed evolution in a yeast mutagenesis system mutant G protein coupled muscarinic receptors were isolated which lost the ability to respond to the natural ligand acetylcholine, but gained the ability to respond to an inert compound (clozapine-N-oxide; CNO) with nanomolar potencies (Armbruster et al. 2007). We also demonstrated that CNO is both pharmacologically inert and metabolically stable in C57Bl6 mice (Alexander et al. 2009). Importantly, mutant receptors were selected to have low basal activity thereby providing a maximal dynamic range in agonist-induced responses (Armbruster et al. 2007). Currently, the existing DREADDs include the Gq, Gi and Gs (e.g. hM3Dq, hM4Di, and GsD respectively) signaling pathways, allowing for remote and non-invasive activation, inhibition and modulation of GPCR signaling pathways and neurotransmission in a variety of cells in vivo (Rogan and Roth 2011; Roth 2016).
Applications of this technology in mice in vivo have utilized either tet-dependent expression of hM3Dq or hM4Di in transgenic mice (Alexander et al. 2009; Zhu et al. 2014), or Cre-dependent (Krashes et al. 2011) or Cre-independent (Zhu et al. 2014) viral vectors (see Urban and Roth, 2014, for review). For the widest utilization of this system in vivo, including in developing organisms, we took advantage of the progress made in mouse genetic technology by generating strains of mice which express an activating (hM3Dq) and an inhibiting (hM4Di) DREADD, respectively, under control of a strong ubiquitous promoter, which is separated from the DREADD by a loxP site-flanked Stop signal (Sauer 1998). Mating of these strains with any Cre driver line will remove the Stop signal only in the cell type specified by the Cre driver used; this cell-specifically expressed DREADD can then be activated by parenteral or oral application of the ligand, CNO.
We generated targeting constructs placing hM3Dq, hM4Di, and GsD under control of the strong ubiquitous CAG promoter, separated from the DREADD coding sequence by a floxed stop cassette (Fig. 1). We based our constructs on the Allen Brain Institute’s Ai targeting vector which has been demonstrated to afford transgene expression at a robust level after Cre-mediated excision of a floxed STOP cassette, without expression in the presence of the STOP cassette (Madisen et al. 2010). Each DREADD is HA-tagged as well as bi-cistronic (Fig. 1). The HA-tag allows one to determine, immunohistochemically, the detailed spatial localization of the receptors in the cell after Cre-mediated removal of the STOP cassette. The DREADD sequence is followed by a P2A sequence (Kim et al. 2011) and a reporter (mCitrine; Griesbeck et al. 2001) allowing to visualize cells expressing DREADDs by fluorescence microscopy.
FIG. 1.
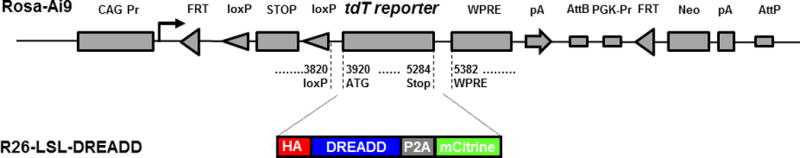
DREADD conditional targeting vectors. Schematic of Ai9 targeting vector (drawing and sequence annotations after Madison et al., 2010). Replacement of the tdTomato sequence by HA-DREADD-P2A-mCitrine generated R26-LSL-DREADD vectors.
We homologously targeted both C57BL/6 ES cells (JM8A3.N1; Pettitt et al. 2009) and 129X1×129S1 ES cells (R1; Nagy et al. 1993) with our conditional DREADD constructs. We initially wanted to make sure that there is no expression in the absence, but correct expression in the presence of Cre recombinase. Accordingly, we plated ES cells carrying the respective Rosa-CAG-LSL-HA-DREADD-P2A-mCitrine allele on coverslips in 24-well plates, and transfected half of the wells with a Cre plasmid (CMV-Cre). After 5–7 days, we fixed the cells in situ, and used them directly for fluorescence microscopy, or for immunohistochemistry. Fig. 2 shows individual ES cell colonies under bright light and under fluorescence light; only colonies which were transfected with Cre are green. In addition to being bi-cistronic, the DREADD constructs are HA-tagged, allowing one to determine the detailed spatial localization of the receptors in the cell after Cre-mediated removal of the Stop cassette. Fig. 2E and F show confocal images of individual ES cells after Cre transfection; the cytoplasm is green under fluorescence light (HA-DREADD-P2A-mCitrine), while the HA-fused DREADD is located in the cell membrane (HA-DREADD-P2A-mCitrine; red after staining with antibodies to HA). No signal was detected in cells which did not express Cre.
FIG. 2.
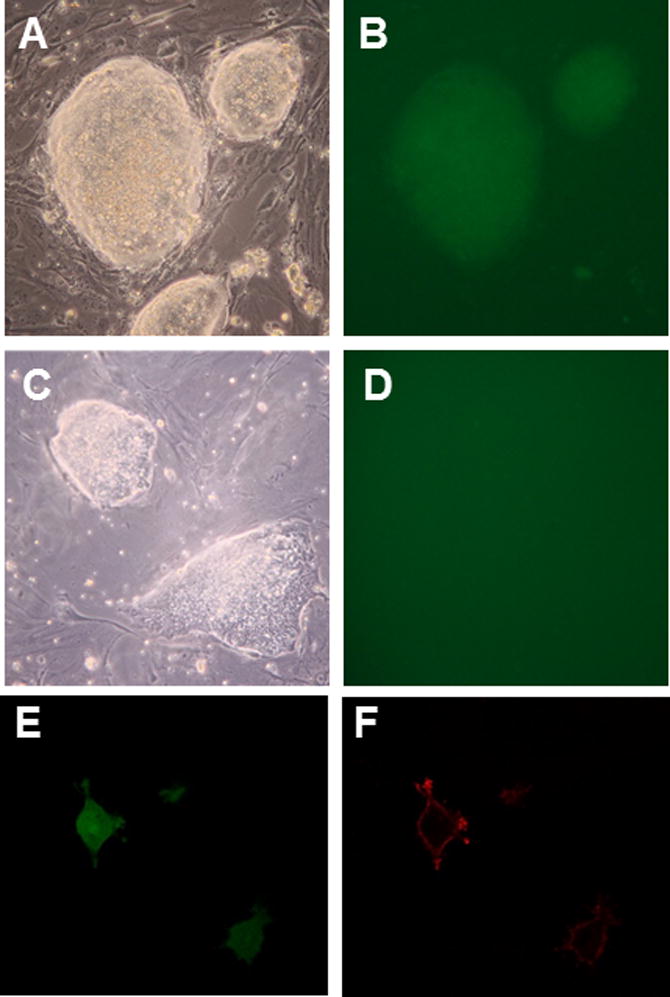
ES cells express DREAADs after Cre-mediated removal of STOP. Colonies from R26-LSL-hM4Di-DREADD-expressing ES cells were fluorescent only when transformed with CMV-Cre (A, B), while non-Cre transfected cells showed no fluorescence (C, D). To visualize membrane localization of DREADDs at the single cell level, Cre recombinase transformed ES cells were plated directly on gelatinized coverslips at low density; cells plated under these conditions show a flat, fibroblast-like morphology. Cells were fixed 2 days later, and treated with primary and secondary antibodies to HA. Confocal microscopy allowed visualizing, separately, cytoplasm and membrane of the same cells. Citrine (green) localizes to the cytoplasm (E), while HA (red) is fused to DREADDs and localizes to the cell membrane (F).
We proceeded with ES cell lines of two of the three DREADDs (hM3Dq and hM4Di) to generate chimeras by blastocyst injection of targeted R1 ES cells. Mice heterozygous for the Rosa-CAG-LSL-HA-DREADD-P2A-mCitrine conditional alleles are viable and fertile. Heterozygotes were mated to various Cre driver mice, and organs were collected from offspring, sectioned, and used directly for fluorescence microscopy or for staining with anti-GFP and anti-HA antibodies. Fig. 3 shows sections of several peripheral tissues under fluorescent light from offspring of LSL-hM4Di × CMV-Cre crosses, revealing the expected CMV-promoter driven ubiquitous expression. We observed similar expression patterns for offspring of LSL-hM3Dq × CMV-Cre crosses (data not shown). While DREADD expression in peripheral organ systems was easily detected by fluorescence microscopy, this was not the case for brain sections following paraformaldehyde fixation. Cre-mediated expression in the brain was revealed only after staining of sections with anti-GFP antibodies.
FIG. 3.
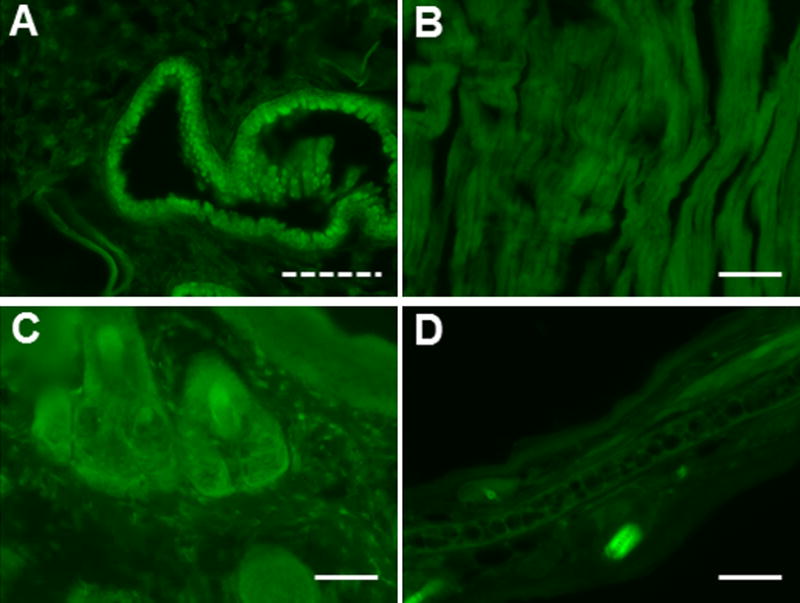
Conditional DREAAD mice express ubiquitously after CMV-Cre-mediated removal of STOP. R26-LSL-hM4Di mice were crossed with CMV-Cre driver mice. Various organs from offspring were collected, fixed, and sectioned. Shown are sections of lung (A), heart (B), tail (C), and ear (D) imaged under fluorescent light. Solid scale bar, 100 μm; dashed scale bar, 50 μm.
Fig. 4 shows anti-GFP staining of brain sections from both hM3Dq and hM4Di offspring of LSL-DREADD × CamkIIa-Cre crosses. The images demonstrate robust expression of DREADDs in cell types expressing CamKIIa-driven Cre. Overall, expression of both DREADDs was consistent with the known expression patterns of this Cre driver line. Specificity of Cre-induced expression was further confirmed by analyzing offspring from mating conditional hM3Dq and hM4Di mice to another Cre driver, Emx1-Cre (Fig. 5). This Cre line drives recombination in over 80% of the neurons of the neocortex and hippocampus, mimicking the pattern of expression of the endogenous gene. Brain sections were stained with antibodies against GFP and HA, validating the expected Cre-mediated expression of both DREADDs. Our analyses insured that each line has robust expression in the presence and no background expression of the respective DREADD in the absence of Cre, and that the Cre-induced expression of each DREADD is consistent with the pattern of expression of the Cre driver.
FIG. 4.
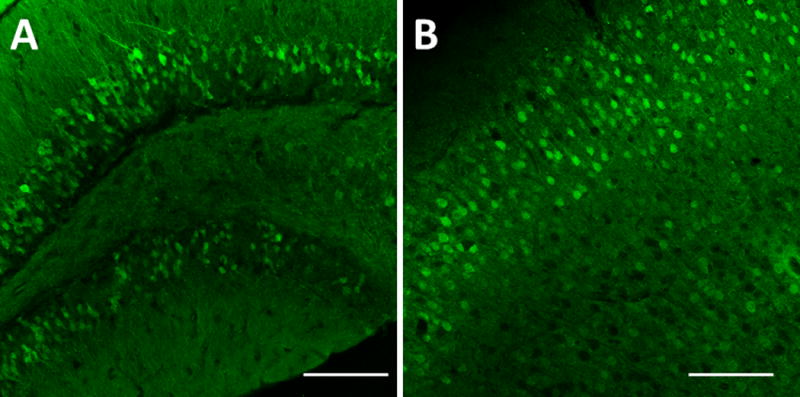
Conditional DREAAD mice express in targeted brain regions after CamKIIa-Cre-mediated removal of STOP. R26-LSL-hM4Di- and –hM3Dq-DREADD mice were crossed with CamKIIa-Cre driver mice. Coronal sections of offspring (A – hM4Di, B – hM3Dq) were stained with anti-GFP antibody and imaged (20×). (A) Dentate gyrus of the hippocampus shows granular cells fluorescent. (B) Cerebral cortex shows pyramidal cells fluorescent. Scale bar: 100 μm.
FIG. 5.

Conditional DREAAD expression in targeted brain regions after Emx1-Cre-mediated removal of STOP. R26-LSL-hM4Di- and –hM3Dq-DREADD mice were crossed with Emx1-Cre driver mice. (A, B) Coronal sections of offspring were stained for mCitrine (GFP antibody) or HA. Tiled images of coronal sections of Emx1-Cre+/hM3Dq+ and Emx1-Cre-/hM3Dq+ (A) and of Emx1-Cre+/hM4Di+ and Emx1-Cre-/hM4Di+ (B) showing no transgene expression in the absence of Cre-recombinase. (C) Confocal images of neurons expressing HA-tagged hM3Dq at the membrane of mCitrine positive neurons in the presence of Cre-recombinase. (D) 10× tiled-image of an Emx1-Cre+/hM4Di+ mouse (white arrow indicating the mossy-fiber projections to CA3 from the dentate gyrus) and confocal images of HA-tagged hM4Di at the membrane of mCitrine positive CA3 targeting axons in the presence of Cre-recombinase.
To test for functionality of the conditional DREADD, LSL-hM3Dq × Emx1-Cre offspring carrying Emx1-Cre and either ROSA26-hM3Dq or the wildtype ROSA26 allele were tested in an open field setting. As Emx1 is expressed in excitatory cortical glutamatergic projection neurons (Gorski et al. 2002), activation of these pathways can be expected to increase motor activity. Both sets of animals received CNO or vehicle. Only mice which expressed hM3Dq in Emx1 neurons and received CNO (0.2 mg/kg) showed a significant increase in movement (Fig. 6 A, B). We also assessed effects of CNO-induced silencing of neurons on contextual conditioned fear as prior studies using CaMKIIα-hM4Di expressing mice showed a robust effect in this paradigm (Zhu et al. 2014) and expression of CaMKIIα and Emx1 overlap in glutamatergic pyramidal neurons. As expected (Fig 6 G) CNO suppressed contextual fear conditioning only in animals expressing hM4Di.
FIG. 6.
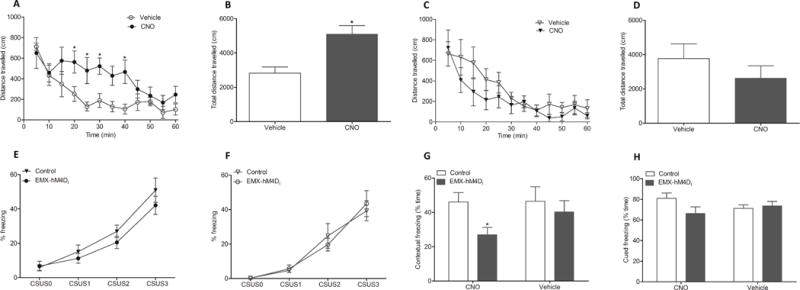
Conditional DREADD mice display behavioral changes after clozapine-n-oxide (CNO) injection. R26-LSL-hM3Dq or R26-LSL-hM4Di mice were crossed with Emx1-Cre driver mice to obtain offspring with DREADD expression (EMX-hM3Dq or EMX-hM4Di) and respective control littermates without DREADD expression. In the open field test (A–D), the EMX-hM3Dq mice (circles) show significantly increased ambulation starting from 20 and lasting to 40 minutes after CNO injection (0.2 mg/kg, closed circles, n = 7) compared to vehicle injection (open circles, n = 7) (A, B). The control mice without hM3Dq display no difference in ambulation with CNO (closed triangles, n = 7) compared to vehicle (open triangles, n = 7) (C, D). In a fear conditioning test (E–H), the EMX-hM4Di mice showed significantly disrupted contextual memory compared to the control littermates. Mice were conditioned on day 1 and vehicle or CNO (2 mg/kg) administed in three subsequent injections at 0, 2 and 4 hours of conditioning. Freezing response to conditioning was similar in hM4Di mice (circles) and control mice (triangles) in both CNO (n = 10, each; filled symbols) (E) or vehcle (n = 8, each; open symbols) (F) treatment groups (CS, conditioned stimulus; US, unconditioned stimulus). Evaluation of contexual memory after 24 hours revealed that CNO treatment significantly disrupted contexual learning (p < 0.05) in the EMX-hM4Di mice (grey bars) than in the control mice (white bars) (G). The cued memory tested after 48 hours show no difference in cued learning between the EMX-hM4Di or the control mice (H). Data were expressed as Mean ± S.E.
The likely reason for lack of endogenous fluorescence of the mCitrine in brain sections is due to the well known ability of paraformaldehyde and other fixatives to denature and therefore inhibit GFP-like fluorescence. Despite the lack of fluorescence visualization in fixed sections, it may be visible in non-fixed sections as DREADD expression in the brain of these conditional mice results in observable effects upon administration of CNO in vivo (Fig. 6). For ex vivo slice experiments, mice could be crossed also with an independent strong fluorescence reporter strain in order to allow identification of individual DREADD-expressing cells acutely during recording. Because we utilized the Ai-targeting technique, these should be compatible with many common reporters.
In summary, we generated a widely applicable transgenic platform by creating hM3Dq and hM4Di strains of mice which allow for the Cre recombinase-mediated restricted expression of these pathway-selective DREADDs. The mice have been deposited in the Jackson Laboratory’s repository and are available to all interested investigators. With the ever growing number of Cre driver lines becoming available these DREADD lines will be applicable to studying a wide array of research questions.
METHODS
Targeting vectors
The ROSA26 targeting vector is based on the Allen Brain Institute’s Ai vector series, specifically Ai9 (CAG-floxed tdTomato; Addgene plasmid 22799; contributed by Hongkui Zeng; Madisen et al. 2010). Accordingly, the Rosa-CAG-LSL-HA-DREADD-P2A-mCitrine targeting vector was designed with (from 5′ to 3′) a CMV-IE enhancer/chicken beta-actin/rabbit beta-globin hybrid promoter (CAG), a FRT site, a loxP-flanked STOP cassette (LSL; with stop codons in all 3 reading frames and a triple polyA signal), a hemagglutinin epitope tag (HA), the hM3Dq, hM4Di, and GsD sequence, respectively (DREADD), a P2A sequence (GenBank GQ293238.1), the mCitrine variant of enhanced yellow fluorescent protein, a woodchuck hepatitis virus post-transcriptional regulatory element (WPRE; to enhance the mRNA transcript stability; (Zufferey et al. 1999), a BGH polyA signal, and a PGK-FRT-Neo-polyA cassette. The PGK-neo marker is flanked by a pair of PhiC31 recognition sites (attB/attP) and can be deleted from the reporter lines by crossing with PhiC31 deleter mice (Stock #007743, Jackson Laboratory; Raymond and Soriano 2007).
Targeting ES cells and generation of chimeric mice
The entire Rosa-CAG-LSL-HA-DREADD-P2A-mCitrine-WPRE targeting vector was homologously inserted between exons 1 and 2 of the Gt(ROSA)26Sor locus (Zambrowicz et al. 1997) via electroporation of (129X1/SvJ × 129S1/Sv)F1-Kitl+-derived R1 embryonic stem (ES) cells (Nagy et al. 1993) and of C57BL/6N JM8A3.N1 ES cells (Pettitt et al. 2009). Homologous integration was identified by PCR (reaction included 5% DMSO; 95 °C-30 seconds, 62 °C-30 seconds, 72 °C-90 seconds) using primers from the ROSA genomic sequence 5′ of the short arm (5′-CCTCGGCTAGGTAGGGGATCG) and from the CAG promoter sequence (5′-TGGGCTATGAACTAATGACCCCGTA), and confirmed by Southern blot analysis. Correctly targeted R1 ES cells for hM3Dq and hM4Di were injected into recipient blastocysts from superovulated C57BL/6 mice (Harlan Laboratories). The resulting chimeric animals were crossed to C57BL/6 mice; heterozygotes were further crossed to C57BL/6.
Mice
Mice were maintained as approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Production of mice and all handling were approved by the Institutional Animal Care and Use Committee (IACUC) at Duke University (NC) or UNC Chapel Hill (NC). Camk2a-Cre (Stock #005359) (Tsien et al. 1996), CMV-Cre (Stock #006054) (Schwenk, Baron, and Rajewsky 1995), and Emx1-Cre (Stock #005628) (Gorski et al. 2002) mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Genotyping
DNA from tail tips was prepared following the protocol by Laird et al. 1991. PCR reactions for detecting the presence of the conditional alleles were done using forward primer 5′ ATGTCTGGATCCCCATCAAG for both hM3Dq and hM4Di, and reverse primers 5′-gatgttgccgatgatggtcac (hM3Dq) and 5′-gaaggcgcctatgatgagatc (hM4Di). Excision of the STOP sequence was confirmed by PCR using a forward primer upstream of the STOP sequence (5′-CTCTGCTAACCATGTTCATGC) with the DREADD specific reverse primers (see above).
ES cell histology
ES cells carrying the respective Rosa-CAG-LSL-HA-DREADD-P2A-mCitrine allele were plated on coverslips in 24-well plates, and half of the wells were transfected with a Cre plasmid (CMV-Cre), using Lipofectamine 2000 (Invitrogen). After 5–7 days, cells were fixed in situ with 4% paraformaldehyde, and directly used for fluorescence microscopy, or for immunohistochemistry, followed by confocal microscopy.
Slide Preparation and Immunohistochemistry
Animals were deeply anesthetized with an overdose of ketamine (400 mg/kg) and xylazine (40 mg/kg) and transcardially perfused first with phosphate-buffered saline (PBS) and subsequently 4% PFA, both at 4°C and pH 7.0. Brains were dissected and post-fixed overnight at 4°C in PFA. Brains were subsequently incubated in 30% sucrose/0.1M phosphate-buffer for 48 hours, and sectioned coronally at 40 μm on a Leica SM2010 R Sliding microtome. Floating tissue sections were washed three times in PBS, followed by permeabilization in 0.5% Triton X-100 (PBST) for 1 hour. Tissues were blocked in PBST containing 5% donkey serum, and then incubated with primary antibodies (Rabbit anti-HA, Cell-signaling #3724, 1:500; Goat anti-GFP, Rockland Immunochemicals #600-101-215, 1:500) overnight at room temperature. Tissues were then washed three times for 10 minutes in PBST before incubating with secondary antibodies at 1:250 (Alexa 488 donkey anti-goat; Alexa 568 donkey anti-rabbit) for two hours. Finally, tissues were washed twice in PBST for 10 minutes, once with PBS containing 500 nM DAPI for 5 minutes, and a final two washes in PBS for 10 minutes. Tissues were slide mounted and coverslipped.
Fluorescence and confocal microscopy
Tissue sections from various organs or coverslips with fixed cells were viewed using an Olympus BX60 upright microscope, equipped with 1200 W xenon illumination and appropriate filter sets. Confocal images of ES cells were obtained in the Duke Light Microscopy Core Facility, using a Zeiss Axio Observer inverted confocal microscope with oil immersion objective. Brain sections were imaged at the UNC neuroscience imaging center using either a Nikon Eclipse 80i, or on an Olympus laser-scanning confocal microscope (FV-1000). Confocal images were acquired with a 10× air-objective (N.A. 0.4) and a 40× oil-immersion objective (1.3 N.A.).
Behavioral studies
Mice used for behavior studies were housed in a humidity controlled room with 12 hour dark-light cycle. Age and gender matched littermates (8–12 weeks) were used for open field and fear conditioning tests. Open field tests were carried out in locomotor activity assessment chambers using fusion operating software (Omnitech Electronics Inc., Columbus, OH, USA). Mice were injected with 0.2 mg/kg of CNO dissolved in 0.9% saline or 0.9% saline as a vehicle, and placed in open field for an hour. Average distance travelled during individual five minute-bins and cumulative distance travelled over an hour were measured. Fear conditioning tests were carried out in a contextual NIR video fear conditioning system for mouse (Med Associates Inc., St. Albans, VT, USA) using a three day paradigm as described (Zhu et al. 2014). On day one, mice were conditioned with tone and shock followed by administration of CNO (2 mg/kg) or vehicle at 0, 2 and 4 hours. On day two contextual learning and on day three cued learning were evaluated. Consolidation of fear memory was evaluated for Emx1-hM4Di mice and their littermate controls in two groups either by administering CNO or vehicle.
Availability
Conditional DREADD lines for hM3Dq and hM4Di can be obtained directly from Jackson Laboratory (R26-LSL-Gi-DREADD, Stock# 26219; R26-LSL-Gq-DREADD, Stock# 26220). Targeting vectors and ES cell lines carrying conditional alleles of hM3Dq, hM4Di, and GsD, in C57BL/6 and in 129Sv background, can be obtained directly from UH.
Acknowledgments
Supported by NIH grant R21MH093914 (UH), UO1MH104974 (BLR) and NIH NINDS National Research Service Award (F31NS093917-01) to RHJO.
LITERATURE CITED
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote Control of Neuronal Activity in Transgenic Mice Expressing Evolved G Protein-Coupled Receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the Lock to Fit the Key to Create a Family of G Protein-Coupled Receptors Potently Activated by an Inert Ligand. Proc Natl Acad Sci USA. 2007;104:5163–68. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JLR, Jones KR. Cortical Excitatory Neurons and Glia, but Not GABAergic Neurons, Are Produced in the Emx1-Expressing Lineage. J Neurosci. 2002;22:6309–14. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the Environmental Sensitivity of Yellow Fluorescent Protein. Mechanism and Applications. J Biol Chem. 2001;276:29188–94. doi: 10.1074/jbc.M102815200. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SR, Li LH, Park HJ, Park JH, Lee KY, Kim MK, Shin BA, Choi SY. High Cleavage Efficiency of a 2A Peptide Derived from Porcine Teschovirus-1 in Human Cell Lines, Zebrafish and Mice. PloS One. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye CP, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, Reversible Activation of AgRP Neurons Drives Feeding Behavior in Mice. J Clin Invest. 2011;121:1424–28. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified Mammalian DNA Isolation Procedure. Nucl Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A Robust and High-Throughput Cre Reporting and Characterization System for the Whole Mouse Brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of Completely Cell Culture-Derived Mice from Early-Passage Embryonic Stem Cells. Proc Natl Acad Sci USA. 1993;90:8424–28. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt SJ, Liang Q, Rairdan XY, Moran JL, Prosser HM, Beier DR, Lloyd KC, Bradley A, Skarnes WC. Agouti C57BL/6N Embryonic Stem Cells for Mouse Genetic Resources. Nat Methods. 2009;6:493–95. doi: 10.1038/nmeth.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Soriano P. High-Efficiency FLP and PhiC31 Site-Specific Recombination in Mammalian Cells. PloS One. 2007;2:e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote Control of Neuronal Signaling. Pharmacological Reviews. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Inducible Gene Targeting in Mice Using the Cre/lox System. Methods. 1998;14:381–92. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A Cre-Transgenic Mouse Strain for the Ubiquitous Deletion of loxP-Flanked Gene Segments Including Deletion in Germ Cells. Nucl Acids Res. 1995;23:5080–81. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and Cell Type-Restricted Gene Knockout in Mouse Brain. Cell. 1996;87:1317–26. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Ann Rev Pharmacol Toxicol. 2015;55:399–417. doi: 10.1146/annurev-pharmtox-010814-124803. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of Overlapping Transcripts in the ROSA Beta Geo 26 Gene Trap Strain Leads to Widespread Expression of Beta-Galactosidase in Mouse Embryos and Hematopoietic Cells. Proc Natl Acad Sci USA. 1997;94:3789–94. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, Roth BL. Chemogenetic Inactivation of Ventral Hippocampal Glutamatergic Neurons Disrupts Consolidation of Contextual Fear Memory. Neuropsychopharmacology. 2014;39:1880–92. doi: 10.1038/npp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Donello JE, Trono D, Hope TJ. Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element Enhances Expression of Transgenes Delivered by Retroviral Vectors. J Virol. 1999;73(4):2886–92. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


