Abstract
Rationale
Tobacco smoking is a leading preventable cause of premature death in the United States. Menthol is a significant flavoring additive in tobacco products. Clinical evidence suggests that menthol may promote tobacco smoking and nicotine dependence. However, it is unclear whether menthol enhances the reinforcing actions of nicotine and thus facilitates nicotine consumption. This study employed a rat model of nicotine self-administration to examine the effects of menthol on nicotine-taking behavior.
Methods
Male Sprague-Dawley rats were trained in daily 1-h sessions to press a lever for intravenous nicotine self-administration under a fixed-ratio 5 schedule of reinforcement. In separate groups, rats self-administered nicotine at four different doses (0.0075, 0.015, 0.03, and 0.06 mg/kg/infusion). Five minutes prior to the two test sessions, menthol (5 mg/kg) or its vehicle was administered intraperitoneally in all rats in a counterbalanced design within each group. In separate rats that self-administered 0.015 mg/kg/infusion nicotine, menthol dose-response function was determined. Menthol was also tested on food self-administration.
Results
An inverted U-shaped nicotine dose-response curve was observed. Menthol pretreatment shifted the nicotine dose-response curve to the left. The facilitating effect of menthol on the self-administration of 0.015 mg/kg/infusion nicotine was dose-dependent, whereas it produced similar effects at doses above the threshold of 2.5 mg/kg. Menthol tended to suppress the self-administration of food pellets.
Conclusions
These data demonstrate that menthol enhances the reinforcing effects of nicotine, and the effect of menthol was specific to nicotine. The findings suggest that menthol directly facilitates nicotine consumption, thereby contributing to tobacco smoking.
Keywords: Dose-response, menthol, nicotine, rats, reinforcement, self-administration
Introduction
Tobacco-related diseases are a major problem worldwide, with substantial human health and economic consequences (WHO 2015). In the United States, tobacco smoking is a leading preventable cause of premature death, accounting for the loss of 450,000 lives annually and economic costs of $289 to $333 billion (Alberg et al. 2014; Holford et al. 2014; Warren et al. 2014). Currently, approximately 42.1 million American adults are smokers, representing 18.1% of the population (CDC 2014). Among these smokers, one-third use menthol cigarettes, with the highest prevalence (77–79%) in African Americans (Caraballo and Asman 2011; Curtin et al. 2014; Giovino 2010; Giovino et al. 2004; Pearson et al. 2012; SAMHSA 2009).
Since the enactment of the Family Smoking Prevention and Tobacco Control Act (FSPTCA) in 1999, there has been an increasing number of epidemiological and observational studies and clinical trials that have investigated the effects of menthol on smoking initiation, addiction, and cessation (Hoffman 2011; Hoffman and Miceli 2011; Hoffman and Simmons 2011). Evidence has shown a significant impact of menthol on perpetuation of the tobacco epidemic, with increases in experimentation and regular smoking and a decrease in smoking cessation success, particularly in youths and African Americans (Delnevo et al. 2011; Delnevo et al. 2015; Fagan et al. 2010; Fagan et al. 2015; Giovino et al. 2015; Tobacco 2011). Recent reports, including a report by the Food and Drug Administration Tobacco Products Scientific Advisory Committee, have concluded that menthol cigarettes promote the initiation of smoking behavior, facilitate the progression to regular smoking, and weaken the motivation to quit smoking in established smokers (Anderson 2011a; b; Benowitz and Samet 2011; TPSAC 2011). Compared with nonmenthol cigarette smokers, young menthol cigarette smokers are prone to nicotine dependence, demonstrated by several measurements of nicotine dependence, such as time to first cigarette, smoking craving, and quit rates (Collins and Moolchan 2006; D’Silva et al. 2012; Hersey et al. 2006; Hersey et al. 2010; Levy et al. 2011; Nonnemaker et al. 2013; Smith et al. 2014; Wackowski and Delnevo 2007). Similarly, adult smokers who use menthol cigarettes presented heightened nicotine addiction and were more likely to smoke their first cigarette sooner after waking and inhale more deeply (Ahijevych and Parsley 1999; Fagan et al. 2010; Hoffman and Simmons 2011; Hymowitz et al. 1995; Muscat et al. 2009; Okuyemi et al. 2003; Richter et al. 2008), leading to elevated levels of both serum nicotine and exhaled carbon monoxide (Williams et al. 2007). However, the results that have been reported by both clinical and basic laboratory studies have been equivocal (Gardiner and Clark 2010; Hoffman 2011), particularly reports that have originated from tobacco companies (e.g., Wang et al. 2010; Werley et al. 2007) that showed that menthol does not increase the number of puffs or volume or uptake of carbon monoxide and tar, suggesting that menthol does not affect smoking initiation, maintenance, progression, or cessation compared with non-menthol cigarettes. One of the most pressing tasks of the Food and Drug Administration Center for Tobacco Products, mandated by the FSPTCA, is to consider a ban on menthol in tobacco products. However, it is imperative to strengthen efforts to investigate the effects of menthol on nicotine intake and smoking addiction.
Menthol (cyclohexanol-5-methyl-2-[1-methylethyl] or [1R,2S,5R]-2-Isopropyl-5-methylcyclohexanol) is a monocyclic terpene alcohol that exists in plants of the Mentha genus (also known as mint). It occurs as four optical isomers, among which (−)-menthol is the most abundant in nature and produces the characteristic peppermint odor and cooling sensation when applied topically (Eccles 1994; 2000; Watson et al. 1978). Menthol has a minty and candy-like flavor and aroma, with topical cooling and anesthetic properties through the activation of transient receptor potential TRPM8 and TRPA1 ion channels (Gentry et al. 2010; Karashima et al. 2007; Liu et al. 2015; McKemy et al. 2002; Peier et al. 2002). These menthol-sensitive receptors are also called ―cold sensors,‖ which are expressed in peripheral tissues, especially sensory nerve terminals in epithelia and the skin (Fonfria et al. 2006; Liu et al. 2015; Reid and Flonta 2001). In many tobacco products, menthol is the most significant flavoring additive and the only one that is widely advertised by the tobacco industry among a total of approximately 600 additives (Heck 2010; Rabinoff et al. 2007). Menthol-labeled cigarettes account for approximately one-quarter of the total cigarette market. Menthol content in popular brands of menthol cigarettes in the United States is as high as 19.6 mg/cigarette (Ai et al. 2015; Best 1993; Celebucki et al. 2005; Kreslake et al. 2008). Almost all nonmenthol-labeled commercial tobacco products contain a certain amount of menthol (73.5 µg/cigarette; i.e., 100-to 1000-fold lower than menthol-labeled cigarettes; (Ai et al. 2015; Farco and Grundmann 2013; FTC 2009; Gordon et al. 2011; Hopp 1993; Wayne and Connolly 2004).
Emerging evidence in recent years has shown that menthol can act at the level of the central nervous system. For example, menthol modulates the function of several ionotropic neurotransmitter receptors, such as nicotinic acetylcholine receptors (nAChRs), γ-aminobutyric acid-A receptors, and strychnine-sensitive glycine receptors (Anand and Lin 2001; Hall et al. 2004; Pan et al. 2012; Tani et al. 2010; Zhang et al. 2008). In rodents, menthol-enhanced ambulation is sensitive to the pharmacological blockade of dopamine receptors, suggesting that menthol facilitates dopamine neurotransmission (Umezu and Morita 2003). Menthol directly influences the function of α4β2 nAChRs (Anand and Lin 2001; Ashoor et al. 2013; Hans et al. 2012). Neuroimaging studies have reported a 28% increase in α4β2 nAChR expression in the brain in menthol cigarette smokers compared with smokers who used non-menthol cigarettes, indicating significantly higher upregulation of these receptors after menthol cigarette smoking (Brody et al. 2013).
Based on these recent experimental findings, one hypothesis is that menthol, in addition to its topical and sensory effects, strengthens the reinforcing actions of nicotine. This hypothesis was tested in the present study by examining whether menthol influences the self-administration of nicotine in a rat model of tobacco smoking. Specifically, rats were trained to establish stable nicotine self-administration at different unit doses, and the ability of menthol to alter nicotine self-administration behavior was examined. Five minutes prior to the test sessions, menthol was administered intraperitoneally to circumvent its topical and sensory effects.
Methods
Subjects
Male Sprague-Dawley rats were used (Charles River, Portage, MI, USA; 201–225 g upon arrival). The animals were individually housed in a humidity- and temperature-controlled (21–22°C) colony room on a reverse light/dark cycle (lights on at 8:00 PM, lights off at 8:00 AM). After 1 week of habituation, the rats were placed on a food-restriction regimen (20 g chow/day) throughout the experiments, which allowed the rats to maintain consistent but low weight gain at approximately 85% of their free-feeding weight. The rats had unlimited access to water. The training and experimental sessions were conducted during the dark phase at the same time each day (9:00 AM-3:00 PM). All of the experimental procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee.
Drugs
(−)-Nicotine hydrogen tartrate, (−)-menthol (cyclohexanol-5-methyl-2-[1-methylethyl), and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nicotine solutions for intravenous self-administration were prepared fresh each week by dissolving nicotine salt in physiological saline and adjusting the pH to 7.4 ± 0.4 with sodium hydroxide. The nicotine doses are reported as free-base concentrations. Menthol was dissolved in DMSO and diluted with deionized water to a final DMSO concentration being at 50% (V/V).
Self-administration apparatus
The experimental sessions were conducted in standard operant conditioning chambers that were located inside ventilated, sound-attenuating cubicles (Med Associates, St. Albans, VT, USA). The chambers were equipped with two retractable response levers on one side panel, a 28-V white light above each lever, and a red house light on top of the chambers. Between the two levers was a food pellet receptacle. Intravenous nicotine injections were delivered by a drug delivery system with a syringe pump (model PHM100-10 rpm, Med Associates). Experimental events and data collection were automatically controlled by an interfaced computer and software (Med-PC version IV, Med Associates).
Lever-press training
To facilitate operant responding for nicotine self-administration (see below), the rats underwent lever-press training. One day after beginning the food-restriction regimen, the rats were placed in the experimental chambers, and the training sessions began with introduction of the levers. Responding on the active lever was rewarded with the delivery of a food pellet (45 mg). The sessions lasted 1 h, with a maximum delivery of 45 food pellets on a fixed-ratio 1 (FR1) schedule of reinforcement. After the rats learned to respond, the reinforcement schedule was increased to FR5. Training ended after the rats earned 45 food pellets on the FR5 schedule. Successful lever-press training with food pellets was achieved within 2–5 sessions.
Surgery
Intravenous catheterization was performed after food training. The rats were anesthetized with isoflurane (1–3% in 95% O2 and 5% CO2). An indwelling catheter was inserted in the right external jugular vein. The catheters were constructed with a 15 cm piece of Silastic tubing (0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning Corporation, Midland, MI, USA) that was attached to a 22-gauge stainless-steel guide cannula. The guide cannula was bent and molded onto a tissue-compatible polypropylene mesh (Davol, Warwick, RI, USA) with dental cement to form the catheter base. Through an incision on the rat’s back, the catheter base was anchored beneath the skin at the level of the scapulae, and the catheter passed subcutaneously to the ventral lower neck region and was inserted into the right jugular vein (3.5 cm). The animals were allowed at least 7 days to recover from surgery. During the recovery period, the catheters were flushed daily with 0.1 ml of sterile saline that contained heparin (30 U/ml) and gentamicin (40 mg/ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with heparinized saline before and after the experimental sessions. On weekends when no experimental session was performed, the catheters were flushed once a day.
Nicotine self-administration training
After recovering from intravenous catheterization surgery, the rats underwent nicotine self-administration training sessions. In the daily 1-h sessions, the rats were placed in the operant conditioning chambers and connected to the intravenous drug infusion system. The sessions began with extension of the two levers and illumination of the red house light. Once the responses on the active lever reached the FR requirement, an intravenous infusion of nicotine (0.03 mg/kg, free base) was delivered in a volume of about 0.1 ml over approximately 1 s, depending on the rat’s body weight. Each nicotine infusion was accompanied by the presentation of auditory/visual stimuli that consisted of a 5-s tone and 20-s illumination of the light above the active lever. The stimuli signaled a 20-s timeout period following each nicotine infusion, during which time responses were recorded but not reinforced. An FR1 schedule was used for days 1–5. An FR2 schedule was used for days 6–8. An FR5 schedule was used for the remaining days of the experiments. Throughout the experiments, responses on the inactive lever were recorded but had no programmed consequences. All of the rats underwent 20 daily training sessions prior to the tests (described below).
Test 1: Nicotine dose-response function to maintain self-administration behavior
After establishing stable nicotine self-administration at 0.03 mg/kg/infusion, the rats were divided into four groups in a pseudorandom manner (n = 9–10 per group), based on similar lever responses that were emitted in the last three sessions of the self-administration training phase. In the subsequent 10 test sessions, rats in the different groups were provided nicotine at respectively different doses. One group of rats remained on 0.03 mg/kg/infusion of nicotine, whereas rats in the other three groups were allowed to self-administer nicotine at 0.0075, 0.015, or 0.06 mg/kg/infusion. The purpose of Test 1 was to establish nicotine dose-response functions that supported self-administration behavior in preparation for the subsequent menthol tests (described below in Test 2).
Test 2: Effect of menthol on nicotine self-administration dose-response curve
Following the 10 days of Test 1, the effects of menthol on nicotine self-administration were examined in two sessions. In these tests, rats in the four groups continued to self-administer nicotine at their respective doses. However, 5 min prior to the first test session, half of the rats in each group received an intraperitoneal injection of 5 mg/kg menthol, whereas the other half received vehicle (50% DMSO in deionized water) in a volume of 1 ml/kg. In the second test session, the order of menthol and vehicle administration was reversed. The two test sessions were separated by one nontreatment session. The menthol dose of 5 mg/kg was selected because it is approximately equivalent to the daily amount of menthol that is administered by moderate to heavy smokers (Ai et al. 2015; Best 1993; Celebucki et al. 2005; Kreslake et al. 2008).
Test 3: Dose-dependent effects of menthol on nicotine self-administration
Menthol produced the most marked changes in nicotine self-administration at 0.015 mg/kg/infusion (see Results section below). Therefore, the dose-response function for menthol was examined using a nicotine unit dose of 0.015 mg/kg/infusion. Eleven rats underwent nicotine self-administration training as described above, with the exception that the nicotine dose was switched from 0.03 to 0.015 mg/kg/infusion at the 11th session. After completing 10 sessions with nicotine at 0.015 mg/kg/infusion, the menthol test sessions began, in which the rats continued to self-administer nicotine at 0.015 mg/kg/infusion. Five minutes prior to the test session, menthol (0, 0.1, 1.0, 2.5, and 5 mg/kg) was administered intraperitoneally in a within-subjects Latin-square design. The test sessions were performed every other day, interspersed with one nontreatment session.
Test 4: Effect of menthol on nicotine self-administration under a progressive-ratio schedule
We measured the effects of menthol pretreatment on rats’ motivation for nicotine self-administration at 0.015 mg/kg/infusion under a progressive-ratio (PR) schedule of reinforcement. The PR schedule was modified from the formula 5(02 × infusion number) − 5 (Depoortere et al. 1993). Thus, the response requirements for successive food pellet delivery were 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, and so on. This test were performed using the 10 rats that self-administered 0.015 mg/kg/infusion nicotine and completed the test with 5 mg/kg/infusion in Test 2 (see above). The reason why we reused these rats was to allow direct comparisons of the results that were obtained under the FR5 schedule vs. PR schedule. Specifically, two test sessions were performed every other day, interspersed with one nontreatment session. Menthol (0 or 5 mg/kg) was administered 5 min prior to the test sessions in a counterbalanced manner. During the session, the rats self-administered nicotine at 0.015 mg/kg/infusion.
Test 5: Effect of menthol on food self-administration
Twelve rats underwent 20 daily food self-administration sessions on an FR5 schedule. To obtain an adequate control, it was optimal to obtain lever-press responses that were similar to those emitted by nicotine self-administering rats. Thus, rats that self-administered food pellets were subjected to a 150-s timeout period after each food pellet delivery. Starting on self-administration day 21, the effects of menthol on food self-administration were examined. The menthol test sessions were conducted every other day using a within-subjects Latin-square design, interspersed with one nontreatment session. To eliminate redundant tests, menthol was tested only at doses (2.5 and 5 mg/kg) that exerted significant effects in the prior nicotine self-administration tests.
Statistical analyses
The data are expressed as the mean ± SEM number of lever responses and nicotine infusions. For Test 1, a one-way analysis of variance (ANOVA) was used to analyze the data that were averaged across the final three sessions, with nicotine dose as the factor. For Test 2, a two-way repeated-measures ANOVA was used to analyze the data, with nicotine dose as the between-subjects factor and menthol treatment as the within-subjects factor. Significant main effects and interactions in the ANOVA were followed by further analyses using a one-way ANOVA. For Tests 3 and 4, the data were analyzed using a one-way ANOVA, with menthol dose as the factor. Significant main effects in the ANOVAs were followed by Fisher’s PLSD post hoc test to verify differences among individual means.
Results
Nicotine self-administration
Within the 20 daily 1-h sessions, the rats developed steady lever-press behavior for intravenous nicotine self-administration at 0.03 mg/kg/infusion. Averaged across the final three sessions (sessions 18–20), the rats (n = 39, used subsequently for Test 1) emitted a mean ± SEM number of responses of 86 ± 18 on the active lever and 11 ± 5 responses on the inactive lever. The rats self-administered 15 ± 3 infusions of nicotine at a unit dose of 0.03 mg/kg/infusion.
Dose-response function of nicotine self-administration
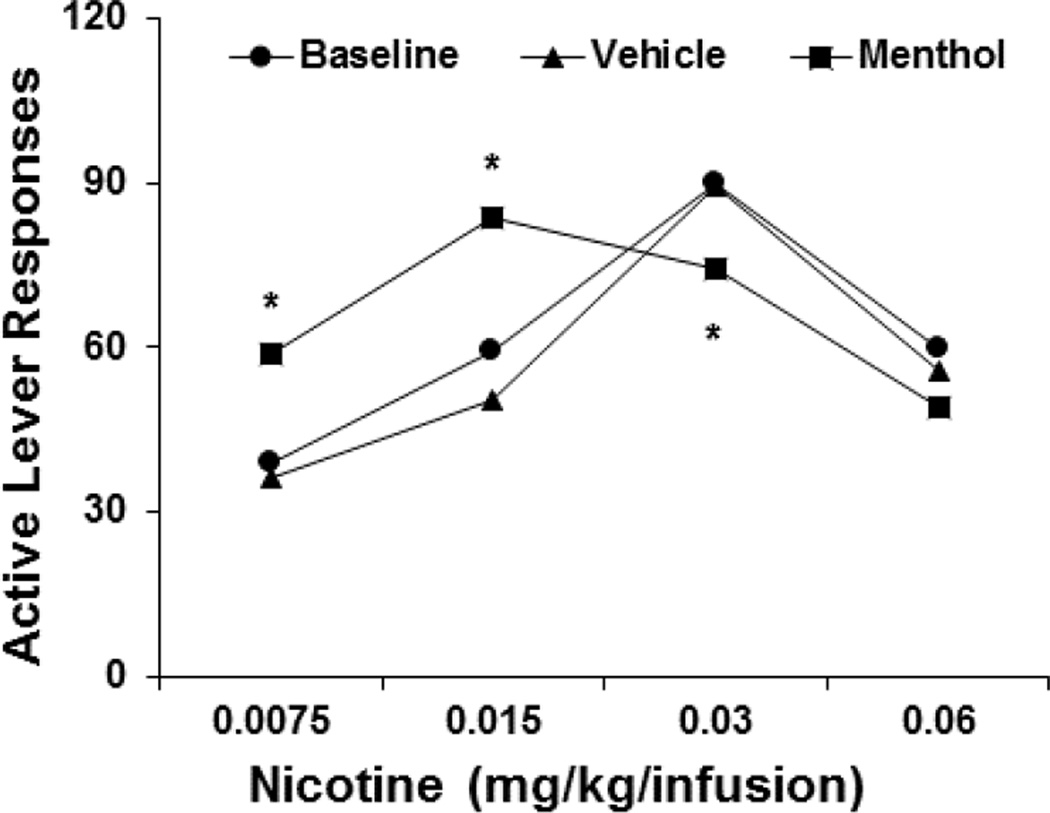
For the nicotine dose-response test, the rats were divided into four groups in a pseudorandom manner. The lever-press response profiles were similar among groups (data not shown). After the nicotine unit dose was switched to 0.0075, 0.015, or 0.06 mg/kg/infusion in three different groups of rats, with 0.03 mg/kg/infusion nicotine maintained in one group, the rats in each group continued to self-administer nicotine. However, their self-administration responses, averaged across the final three sessions, were maintained at different levels. The one-way ANOVA revealed a significant effect of nicotine dose on the number of responses on the active lever (F3,35 = 5.94, p < 0.01). Significant differences were found between the dose of 0.03 mg/kg/infusion and the other three doses (p < 0.001, vs. 0.0075 mg/kg/infusion; p < 0.01, vs. 0.015 mg/kg/infusion; p < 0.05, vs. 0.06 mg/kg/infusion). An inverted U-shaped nicotine dose-response curve was observed, with 0.03 mg/kg/infusion nicotine producing the most lever-press responses (Fig. 1, Baseline). A similar statistical outcome was obtained for the number of nicotine infusions that the rats self-administered during the 1-h sessions (F3,35 = 5.01, p < 0.05). Responses on the inactive lever remained low and indistinguishable among the four different groups (F3,35 = 1.35, p > 0.05).
Fig. 1.
Effect of menthol on the nicotine dose-response curve that supported operant self-administration behavior. Five minute prior to the test sessions, the rats (n = 9–10) in the different nicotine dose groups received an intraperitoneal injection of 5 mg/kg menthol. During the sessions, the rats responded on the active lever for nicotine self-administration on an FR5 schedule of reinforcement. For the sake of clarity, the figure does not show the SEM. *p < 0.05, significant difference from vehicle treatment.
Effect of 5 mg/kg menthol on nicotine dose-response curve
The pre-session administration of 5 mg/kg menthol altered nicotine self-administration behavior (Fig. 1, Menthol). The two-way repeated-measures ANOVA revealed significant effects of nicotine dose (F3,35 = 4.31, p < 0.01) and menthol treatment (F2,70 = 3.63, p < 0.05) and a significant nicotine dose × menthol treatment interaction (F6,70 = 6.62, p < 0.0001). Significant changes were found in menthol-induced active lever responses that were maintained by low but not high doses of nicotine. At both 0.0075 and 0.015 mg/kg/infusion nicotine, the rats emitted significantly (p < 0.05) more responses after menthol treatment compared with baseline and vehicle. In contrast, at 0.03 mg/kg/infusion, the number of active lever responses after menthol pretreatment decreased significantly (p < 0.05). At 0.06 mg/kg/infusion, the rats emitted fewer active lever responses, although this effect failed to reach statistical significance (p > 0.05). Thus, a leftward shift of the nicotine dose-response curve was observed after menthol pretreatment (Fig. 1). The same statistical analyses were applied to the number of nicotine infusions that the rats self-administered at the individual nicotine unit doses. The analysis revealed significant main effects of nicotine dose (F3,35 = 4.05, p < 0.01) and menthol treatment (F2,70 = 3.85, p < 0.05) and a significant nicotine dose × menthol treatment interaction (F6,70 = 5.47, p < 0.001). Responses on the inactive lever remained low and unchanged across the different pretreatment conditions.
Dose-dependent effects of menthol on nicotine self-administration
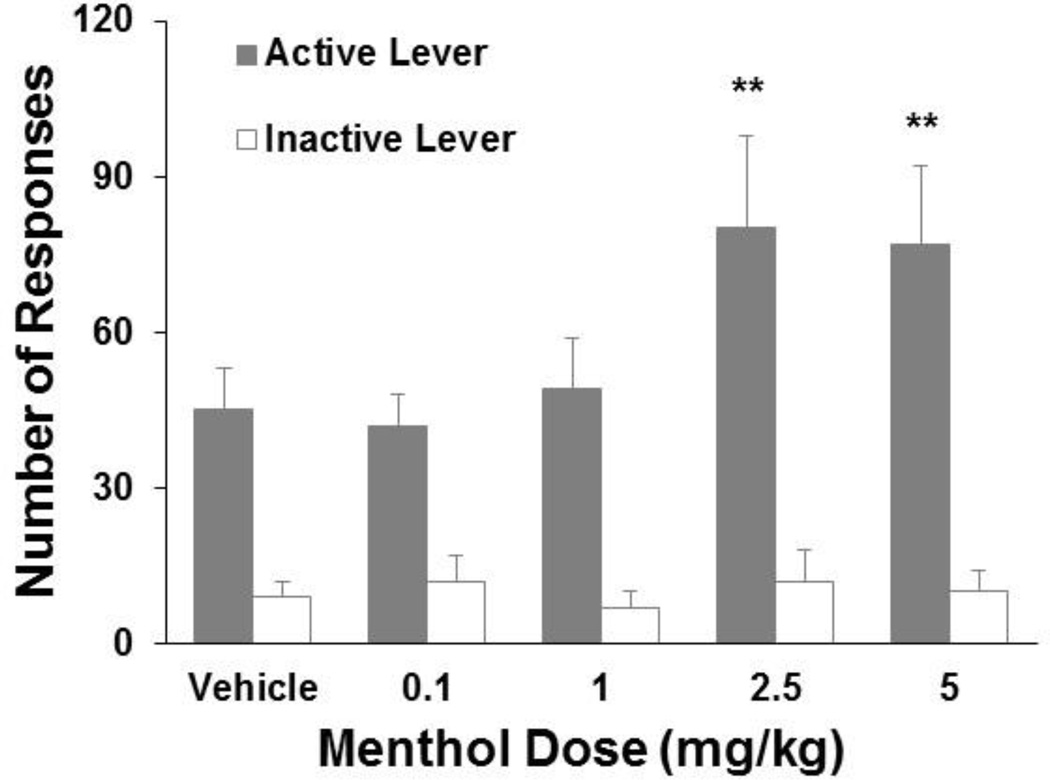
Nicotine at a unit dose of 0.015 mg/kg/infusion maintained steady self-administration behavior. Menthol treatment prior to the sessions dose-dependently enhanced responses on the active lever (Fig. 2). The one-way repeated-measures ANOVA of the number of active lever responses revealed a significant effect of menthol dose (F4,40 = 8.32, p < 0.001). The number of responses significantly increased after menthol pretreatment at 2.5 and 5 mg/kg compared with vehicle and compared with 0.1 and 1.0 mg/kg menthol (p < 0.01 to p < 0.001). Thus, menthol at the two high doses produced similar enhancing effects on nicotine self-administration at 0.015 mg/kg/infusion, whereas the two low doses of menthol did not alter nicotine self-administration behavior. Menthol exerted a similar effect on the number of nicotine infusions (F4,40 = 7.35, p < 0.001). Responses on the inactive lever did not change after menthol pretreatment at any of the doses tested.
Fig. 2.
Menthol dose-response function for enhancement of nicotine self-administration at 0.015 mg/kg/infusion. Five minutes prior to the test sessions, the rats (n = 11) received an intraperitoneal injection of menthol at different doses in a Latin-square design. During the sessions, the rats responded on the active lever for nicotine self-administration at 0.015 mg/kg/infusion on an FR5 schedule of reinforcement. The data are expressed as mean ± SEM. **p < 0.01, significant difference from vehicle treatment.
Effect of menthol on nicotine self-administration under a progressive-ratio schedule
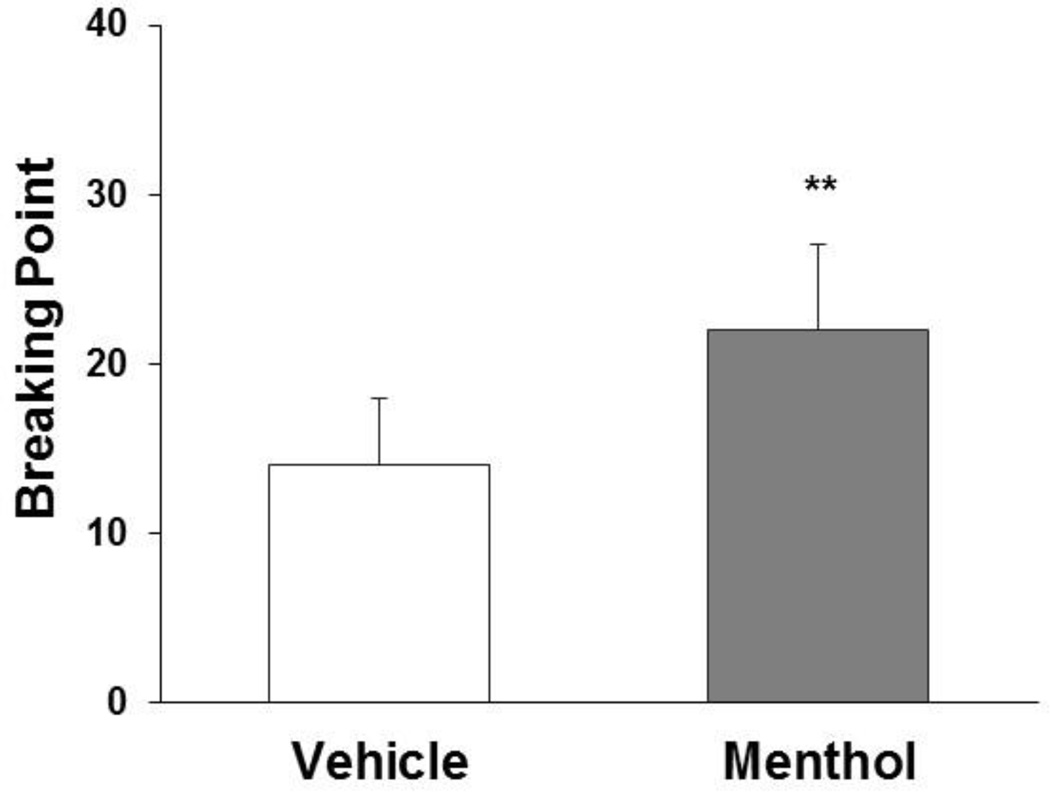
As shown in Fig. 3, pretreatment with 5 mg/kg menthol increased lever-press responses under a PR schedule of reinforcement. The maximum number (breakpoint) of responses on the active lever for nicotine self-administration at 0.015 mg/kg/infusion was 31 ± 6 after menthol pretreatment and 17 ± 4 in the vehicle condition. The increase in breakpoint was confirmed by a one-way repeated-measures ANOVA (F1,9 = 11.84, p < 0.01).
Fig. 3.
Effect of menthol on breakpoints of active lever responses. The breakpoint was defined as the maximum number of responses under a PR schedule of reinforcement that rats (n = 10) emitted within the 1-h sessions for the delivery of nicotine at 0.015 mg/kg/infusion. Menthol (5 mg/kg) was administered 5 min prior to the test sessions. The data are expressed as mean ± SEM. **p < 0.01, significant difference from vehicle treatment.
Effect of menthol on operant food self-administration
Pretreatment with menthol at the doses that reduced nicotine self-administration (≥2.5 mg/kg, see above) decreased the number of food pellet deliveries. The one-way repeated-measures ANOVA of the number of active lever responses revealed a trend toward an effect of menthol dose (F3,33 = 3.28, p = 0.057). Menthol pretreatment at 2.5 and 5.0 mg/kg prior to the test sessions decreased the number of responses on the active lever, but this effect did not reach statistical significance.
Discussion
The present study found an effect of menthol on intravenous nicotine self-administration in rats. Menthol significantly increased lever-press responses for nicotine self-administration at a unit dose of 0.015 mg/kg/infusion or lower, whereas an opposite effect of menthol was observed at higher nicotine doses (0.03 mg/kg/infusion and higher). Thus, menthol pretreatment resulted in a leftward shift of the inverted U-shaped nicotine dose-response curve. The facilitating effect of menthol on the self-administration of 0.015 mg/kg/infusion nicotine was dose-dependent, with a threshold menthol dose of 2.5 mg/kg. These data suggest that menthol enhances the reinforcing actions of nicotine, thereby facilitating nicotine self-administration and tobacco smoking.
Menthol has been the most widely used flavoring additive in tobacco products. Tobacco mentholation can mask the harshness of smoke inhalation, increase the ease of smoking, and provide an appealing oral sensation and social acceptance of its minty aroma (Anderson 2011b for a review of tobacco industry documents). The topical sensory actions of menthol were reported by a recent animal study that showed that menthol, via its cooling effect, facilitated nicotine self-administration (Wang et al. 2014). However, an important question remains unanswered, namely whether menthol directly enhances the reinforcing effects of nicotine and promotes nicotine self-administration and tobacco smoking. The intravenous self-administration paradigm that was used in the present study is the gold standard for measuring the reinforcing actions of nicotine. Rats exhibited reliable and dose-dependent lever-press responses for nicotine self-administration. An inverted U-shaped dose-response curve was observed, which is consistent with other rat studies (Clemens et al. 2015; Donny et al. 1995; Hopkins et al. 2012; Watkins et al. 1999). Importantly, the present study further demonstrated that intraperitoneal pretreatment with menthol shifted the nicotine dose-response curve to the left. Menthol increased the number of lever-press responses for the two lower doses of nicotine but reduced the number of lever-press for the higher doses of nicotine. The two lower nicotine doses are on the ascending limb of its dose-response curve, whereas one of the higher doses (0.03 mg/kg/infusion) is at the peak and the other higher dose (0.06 mg/kg/infusion) is on the descending limb of the curve. These findings indicate that menthol enhances the reinforcing actions of nicotine. Indeed, the menthol-induced enhancement of nicotine’s reinforcing effects was confirmed by the results of the PR schedule. Menthol pretreatment significantly elevated breakpoints (i.e., the maximum number of lever-press responses that the rats willingly emitted to obtain a nicotine infusion) on the active lever to obtain nicotine at 0.015 mg/kg/infusion. This indicates a higher reinforcing effect of nicotine and thus greater motivation to obtain nicotine. Furthermore, the dose-dependent manner by which menthol produced its effect on nicotine self-administration is quite similar to a previous study that found that menthol offset the pain response that was induced by nicotine in mice (Alsharari et al. 2015). In this previous study, menthol at doses of 100 mg/kg or higher produced the same analgesic effect, whereas it did not display an effect at doses of 50 mg/kg or lower.
Menthol produces its topical sensory cooling effects via the activation of TRPM8 ion channels (Journigan and Zaveri 2013; Keh et al. 2011; Liu et al. 2013; Liu et al. 2015; McKemy et al. 2002; Peier et al. 2002; Rath et al. 2015). Another chemosensory cation channel, TRPA1, mediates the perception of topical irritant stimuli, such as the unsaturated aldehydes that are contained in tobacco smoke (Andre et al. 2008; Bandell et al. 2004; Bautista et al. 2006; Jordt et al. 2004; Karashima et al. 2007). Nicotine itself also activates TRPA1 (Talavera et al. 2009). Menthol acts as a modulator of TRPA1 (Karashima et al. 2007; Xiao et al. 2008), thereby reducing the nociceptive response to irritants and nicotine in tobacco smoke (Karashima et al. 2007). In the present study, menthol was administered intraperitoneally. Therefore, the observed effect of menthol on nicotine self-administration behavior was unlikely to involve the topical sensory effects of menthol in the oral, laryngeal, and throat areas. No studies to date have reported any functional expression of these TRP channels in the peritoneum. Therefore, it may be presumed that menthol did not produce any local or topical sensory effects after intraperitoneal administration. The present study found that menthol enhanced the reinforcing actions of nicotine independently of its topical sensory effects.
One possibility is that menthol interacts with nicotine at the pharmacokinetic level. In rodents, peak blood menthol levels are reached within 2 h after intraperitoneal administration (Daniel and Rycroft 1976). Because of its lipophilicity, menthol readily crosses the blood-brain barrier and immediately appears in brain tissues (Pan et al. 2012). In smokers, menthol is mostly (80–90%) eliminated from the body within 4 h after menthol cigarette smoking (Lugton et al. 1978). However, there are no consistent reports on the possible interactions between menthol and nicotine with regard to the metabolism of these substances. Menthol was found to either slow or not change the metabolism of nicotine in humans and rodents (Abobo et al. 2012; Alsharari et al. 2015; Benowitz et al. 2004; Benowitz et al. 1999; Heck 2009). Menthol may function as a very weak inhibitor of the CYP2A6 liver enzyme, which is responsible for converting nicotine to cotinine (Kramlinger et al. 2012; MacDougall et al. 2003). A recent rodent study (Abobo et al. 2012) excluded the possibility that menthol significantly changes the metabolism of nicotine within the same time window of the self-administration tests that were performed in the present study. The aforementioned rodent studies injected animals with menthol at doses of 100 mg/kg or higher. These menthol doses were substantially higher than the doses (0.1–5 mg/kg) that were used in the present study. Thus, we may conclude that the changes in nicotine self-administration behavior after menthol treatment occurred through a mechanism apart from pharmacokinetic interactions.
Menthol at doses that effectively altered nicotine self-administration appeared to also suppress lever-press responses for food. Although the suppressing effects of menthol on food self-administration did not reach statistical significance (p = 0.057), such an effect still deserves discussion. One possibility is that the rats were unable to emit operant responses for food. Menthol may have produced nonspecific impairments in locomotor activity, arousal state, or the motivation for rewards. However, these possibilities are unlikely because menthol enhanced lever-press responses for nicotine self-administration at lower doses and shifted the nicotine dose-response curve to the left. Furthermore, menthol is considered to not produce adverse effects at similarly low doses as those used in the present study. Menthol administration by intragastric gavage at doses up to 200 mg/kg for 28 days did not produce noticeable changes in the brain in rats (Thorup et al. 1983). In the present study, operant responses for food served as a control condition, so it was optimal to match the number of lever responses across nicotine and food reinforcers. A longer timeout period after each food pellet delivery was set compared with the timeout period for nicotine self-administration. Such a modification in the timeout period made lever-press responses to some extent comparable between food and nicotine self-administration. Thus, the differences in menthol’s effect on nicotine and food self-administration cannot be explained by potential response rate-dependent actions of menthol. The suppressant effect of menthol on food reinforcement remains to be further investigated.
To our knowledge, only one published animal study has examined the influence of menthol on nicotine self-administration in rats (Wang et al. 2014). In that study, the response-contingent delivery of menthol strengthened rats’ licking responses for nicotine self-administration at 0.03 mg/kg/infusion. Both Wang et al. and the present study experimentally support the hypothesis that menthol facilitates nicotine reinforcement and increases nicotine consumption. However, several differences between the two studies deserve discussion. Wang et al. investigated the effects of an oral menthol cue on nicotine self-administration, whereas the present study examined the direct effects of menthol via intraperitoneal administration, thus circumventing possible oral sensory actions of menthol. Wang et al. exploited rats’ innate licking behavior rather than acquired lever-press responses for nicotine self-administration. Rats naturally emit enormous numbers of lick responses, which may be less sensitive to such manipulations as changes in the dose of nicotine and menthol. In Wang et al., the rats drank menthol solutions from a sipper tube by licking a spout. Thus, it was difficult to measure the exact amount of menthol that the rats drank compared with the present study, in which specific doses of menthol were injected intraperitoneally. The overall sensory impact of menthol cigarettes was previously reported to depend on the dose of menthol, in which low doses exerted facilitative effects and high doses were aversive (Henningfield et al. 2003). Wang et al. also used adolescent female rats, whereas the present study used adult male rats. Despite these procedural differences, both Wang et al. and the present study provide empirical evidence that the mentholation of tobacco products promotes smoking behavior.
Several studies have shown that menthol interacts with nAChRs. The ability of menthol to regulate the response of these receptors to nicotine may underlie the menthol-induced enhancement of nicotine reinforcement that was observed in the present study. Hans et al. (2012) performed an electrophysiology study and found a direct inhibitory effect of menthol on α4β2 nAChRs on sensory neurons using whole-cell patch-clamp and single-channel recording. Menthol appeared to preferentially interact with these receptors in their closed state. Considering the widespread distribution of nicotinic interneurons in the ventral striatal area, including the nucleus accumbens, and their rich innervation of inhibitory γ-aminobutyric acid neurons, menthol may lead to the disinhibition of dopamine neurotransmission and thereby facilitate the reinforcing actions of nicotine. Ashoor et al. (2013) found that menthol inhibited the response of α7 nAChRs that were expressed in Xenopus oocytes to nicotine application. Further computational structure modeling predicted that the binding site of menthol was outside the nicotine-binding pocket, suggesting that menthol may act as an allosteric modulator of these receptors. Brunzell and McIntosh (2012) reported that the highly α7 nAChR-selective antagonist α-conotoxin ArlB [VIIL,VI6D] that was microinjected in the rat nucleus accumbens significantly increased nicotine self-administration behavior under a PR schedule of reinforcement, suggesting tonic α7 nAChR inhibition of nicotine reinforcement.
Menthol may weaken the inhibitory role of α7 nAChRs. As a result of the aforementioned disinhibition via actions on α4β2 and α7 nAChRs, menthol may increase dopamine activity in the mesolimbic system, thereby enhancing the reinforcing actions of nicotine. Moreover, when dopamine neurons are in a burst-firing state, the suppression or blockade of nAChRs that are located on dopamine terminals paradoxically enhances dopamine release (Rice and Cragg 2004; Zhang and Sulzer 2004). That may be another mechanism by which menthol inhibits nAChRs, resulting in an increase in dopamine neurotransmission and enhancement of nicotine reinforcement. Supporting the direct interaction between menthol and nAChRs, a recent study found that menthol-responsive thermosensory neurons in the dorsal root ganglia and trigeminal ganglia in adult rats expressed nAChRs (Teichert et al. 2014). Menthol was also found to alter dopamine neurotransmission in rodents by directly interacting with dopamine uptake (Umezu and Morita 2003). Altogether, menthol appears to directly interact with α4β2 and α7 nAChRs and enhance the reinforcing effects of nicotine. This argument keeps in line with a human study showing that nAChR upregulation in the brain was significantly higher in menthol cigarette smokers compared with nonmenthol cigarette smokers (Brody et al. 2013).
In summary, this study showed an enhancing effect of menthol on nicotine reinforcement in rat models of nicotine administration. In particular, menthol was administered via an intraperitoneal route so that it produced its effect independently of its topical and sensory actions. It is proposed that a direct interaction of menthol with brain nAChRs may present a neurobiological mechanism for the observed behavioral changes. Together with another recent rodent study reporting that menthol produced a facilitative effect on nicotine self-administration via acting as an oral sensory stimulus (Wang et al. 2014), these data demonstrated that menthol may strengthen the reinforcement by nicotine and thus promote nicotine consumption and tobacco smoking.
Acknowledgments
This work was supported by the National Institute on Drug Abuse and Food and Drug Administration Center for Tobacco Products (R01DA037277 to X. Liu). The funding source had no other role other than financial support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
References
- Abobo CV, Ma J, Liang D. Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res. 2012;14:801–808. doi: 10.1093/ntr/ntr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24:115–120. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR. Menthol Content in US Marketed Cigarettes. Nicotine Tob Res. 2016;18:1575–1580. doi: 10.1093/ntr/ntv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179:403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PloS one. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R, Lin L. Menthol modulates the functional properties of nicotinic receptors. Soc Neurosci Abstr. 2001;137:14. [Google Scholar]
- Anderson SJ. Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents. Tob Control. 2011a;20(Suppl 2):i20–i28. doi: 10.1136/tc.2010.041939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SJ. Menthol cigarettes and smoking cessation behaviour: a review of tobacco industry documents. Tob Control. 2011b;20(Suppl 2):i49–i56. doi: 10.1136/tc.2010.041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashoor A, Nordman JC, Veltri D, Yang KH, Al Kury L, Shuba Y, Mahgoub M, Howarth FC, Sadek B, Shehu A, Kabbani N, Oz M. Menthol binding and inhibition of alpha7-nicotinic acetylcholine receptors. PloS one. 2013;8:e67674. doi: 10.1371/journal.pone.0067674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;291:1196–1203. [PubMed] [Google Scholar]
- Benowitz NL, Samet JM. The threat of menthol cigarettes to U.S. public health. N Engl J Med. 2011;364:2179–2181. doi: 10.1056/NEJMp1103610. [DOI] [PubMed] [Google Scholar]
- Best FW. Effects of some cigarette construction parameters on menthol migration and transfer. Rec Adv Tob Sci. 1993;19:155–201. [Google Scholar]
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern MA. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol. 2013;16:957–966. doi: 10.1017/S1461145712001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo RS, Asman K. Epidemiology of menthol cigarette use in the United States. Tob Induc Dis. 2011;9(Suppl 1):S1. doi: 10.1186/1617-9625-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Current cigarettes moking among adults-UnitedStates. Morb. Mortal. Wkly Rep. 2014;63:29–34. [Google Scholar]
- Celebucki CC, Wayne GF, Connolly GN, Pankow JF, Chang EI. Characterization of measured menthol in 48 U.S. cigarette sub-brands. Nicotine Tob Res. 2005;7:523–531. doi: 10.1080/14622200500186270. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Lay BP, Holmes NM. Extended nicotine self-administration increases sensitivity to nicotine, motivation to seek nicotine and the reinforcing properties of nicotine-paired cues. Addict Biol. 2015 doi: 10.1111/adb.12336. (in press) [DOI] [PubMed] [Google Scholar]
- Collins CC, Moolchan ET. Shorter time to first cigarette of the day in menthol adolescent cigarette smokers. Addict Behav. 2006;31:1460–1464. doi: 10.1016/j.addbeh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Curtin GM, Sulsky SI, Van Landingham C, Marano KM, Graves MJ, Ogden MW, Swauger JE. Patterns of menthol cigarette use among current smokers, overall and within demographic strata, based on data from four U.S. government surveys. Regul Toxicol Pharmacol. 2014;70:189–196. doi: 10.1016/j.yrtph.2014.06.018. [DOI] [PubMed] [Google Scholar]
- D’Silva J, Boyle RG, Lien R, Rode P, Okuyemi KS. Cessation outcomes among treatment-seeking menthol and nonmenthol smokers. Am J Prev Med. 2012;43:S242–S248. doi: 10.1016/j.amepre.2012.07.033. [DOI] [PubMed] [Google Scholar]
- Daniel JW, Rycroft D. l-menthol: absorption, excretion and biotransformation in rats, mice, Syrian hamsters and guinea pigs. Brown and Williamson. 1976 570312869/2918. [Google Scholar]
- Delnevo CD, Gundersen DA, Hrywna M, Echeverria SE, Steinberg MB. Smoking-cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med. 2011;41:357–365. doi: 10.1016/j.amepre.2011.06.039. [DOI] [PubMed] [Google Scholar]
- Delnevo CD, Villanti AC, Wackowski OA, Gundersen DA, Giovenco DP. The influence of menthol, e-cigarettes and other tobacco products on young adults’ self-reported changes in past year smoking. Tob Control. 2015 doi: 10.1136/tobaccocontrol-2015-052325. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Eccles R. Role of cold receptors and menthol in thirst, the drive to breathe and arousal. Appetite. 2000;34:29–35. doi: 10.1006/appe.1999.0291. [DOI] [PubMed] [Google Scholar]
- Fagan P, Moolchan ET, Hart A, Jr, Rose A, Lawrence D, Shavers VL, Gibson JT. Nicotine dependence and quitting behaviors among menthol and non-menthol smokers with similar consumptive patterns. Addiction. 2010;105(Suppl 1):55–74. doi: 10.1111/j.1360-0443.2010.03190.x. [DOI] [PubMed] [Google Scholar]
- Fagan P, Pohkrel P, Herzog T, Pagano I, Vallone D, Trinidad DR, Sakuma KL, Sterling K, Fryer CS, Moolchan E. Comparisons of three nicotine dependence scales in a multiethnic sample of young adult menthol and non-menthol smokers. Drug Alcohol Depend. 2015;149:203–211. doi: 10.1016/j.drugalcdep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farco JA, Grundmann O. Menthol--pharmacology of an important naturally medicinal “cool”. Mini Rev Med Chem. 2013;13:124–131. [PubMed] [Google Scholar]
- Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- Federal Trade Commission. Cigarette report for 2006. Washington DC: Federal Trade Commision; 2009. [Google Scholar]
- Gardiner P, Clark PI. Menthol cigarettes: moving toward a broader definition of harm. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2010;12(Suppl 2):S85–S93. doi: 10.1093/ntr/ntq176. [DOI] [PubMed] [Google Scholar]
- Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain. 2010;6:4. doi: 10.1186/1744-8069-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA. Patterns of and recent trends in the use of mentholated cigarettes in the United States. Silver Pring, MD: US FDA Tobacco Product Scientific Advisory Board; 2010. [Google Scholar]
- Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, Cummings KM. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6(Suppl 1):S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Villanti AC, Mowery PD, Sevilimedu V, Niaura RS, Vallone DM, Abrams DB. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control. 2015;24:28–37. doi: 10.1136/tobaccocontrol-2013-051159. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Brinkman MC, Meng RQ, Anderson GM, Chuang JC, Kroeger RR, Reyes IL, Clark PI. Effect of cigarette menthol content on mainstream smoke emissions. Chem Res Toxicol. 2011;24:1744–1753. doi: 10.1021/tx200285s. [DOI] [PubMed] [Google Scholar]
- Hall AC, Turcotte CM, Betts BA, Yeung WY, Agyeman AS, Burk LA. Modulation of human GABAA and glycine receptor currents by menthol and related monoterpenoids. Eur J Pharmacol. 2004;506:9–16. doi: 10.1016/j.ejphar.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses. 2012;37:463–469. doi: 10.1093/chemse/bjr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JD. Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiol Biomarkers Prevent. 2009;18:622–629. doi: 10.1158/1055-9965.EPI-08-0550. [DOI] [PubMed] [Google Scholar]
- Heck JD. A review and assessment of menthol employed as a cigarette flavoring ingredient. Food Chem Toxicol. 2010;48(Suppl 2):S1–S38. doi: 10.1016/j.fct.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Ahijevych K, Garrett BE, Connolly GN, Wayne GF. Does menthol enhance the addictiveness of cigarettes? An agenda for research. Nicotine Tob Res. 2003;5:9–11. doi: 10.1080/1462220031000070543. [DOI] [PubMed] [Google Scholar]
- Hersey JC, Ng SW, Nonnemaker JM, Mowery P, Thomas KY, Vilsaint MC, Allen JA, Haviland ML. Are menthol cigarettes a starter product for youth? Nicotine Tob Res. 2006;8:403–413. doi: 10.1080/14622200600670389. [DOI] [PubMed] [Google Scholar]
- Hersey JC, Nonnemaker JM, Homsi G. Menthol cigarettes contribute to the appeal and addiction potential of smoking for youth. Nicotine Tob Res. 2010;12(Suppl 2):S136–S146. doi: 10.1093/ntr/ntq173. [DOI] [PubMed] [Google Scholar]
- Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob Induc Dis. 2011;9(Suppl 1):S7. doi: 10.1186/1617-9625-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Miceli D. Menthol cigarettes and smoking cessation behavior. Tob Induc Dis. 2011;9(Suppl 1):S6. doi: 10.1186/1617-9625-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tob Induc Dis. 2011;9(Suppl 1):S5. doi: 10.1186/1617-9625-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford TR, Meza R, Warner KE, Meernik C, Jeon J, Moolgavkar SH, Levy DT. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA. 2014;311:164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins TJ, Rupprecht LE, Hayes MR, Blendy JA, Schmidt HD. Galantamine, an Acetylcholinesterase Inhibitor and Positive Allosteric Modulator of Nicotinic Acetylcholine Receptors, Attenuates Nicotine Taking and Seeking in Rats. Neuropsychopharmacology. 2012;37:2310–2321. doi: 10.1038/npp.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp R. Menthol: its origin, chemistry, physiology and toxicological properties. Rec Adv Tob Sci. 1993;19:3–46. [Google Scholar]
- Hymowitz N, Mouton C, Edkholdt H. Menthol cigarette smoking in African Americans and Whites. Tob Control. 1995;4:194–195. [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Journigan VB, Zaveri NT. TRPM8 ion channel ligands for new therapeutic applications and as probes to study menthol pharmacology. Life Sci. 2013;92:425–437. doi: 10.1016/j.lfs.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keh SM, Facer P, Yehia A, Sandhu G, Saleh HA, Anand P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology. 2011;49:453–457. doi: 10.4193/Rhino11.089. [DOI] [PubMed] [Google Scholar]
- Kramlinger VM, von Weymarn LB, Murphy SE. Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, beta-nicotyrine and menthol. Chem Biol Interact. 2012;197:87–92. doi: 10.1016/j.cbi.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslake JM, Wayne GF, Alpert HR, Koh HK, Connolly GN. Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. Am J Public Health. 2008;98:1685–1692. doi: 10.2105/AJPH.2007.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DT, Blackman K, Tauras J, Chaloupka FJ, Villanti AC, Niaura RS, Vallone DM, Abrams DB. Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J Public Health. 2011;101:1241–1247. doi: 10.2105/AJPH.2011.300178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Fan L, Balakrishna S, Sui A, Morris JB, Jordt SE. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain. 2013;154:2169–2177. doi: 10.1016/j.pain.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, Lin YJ, Lee HF, Ho CY, Ruan T, Kou YR. Menthol suppresses laryngeal C-fiber hypersensitivity to cigarette smoke in a rat model of gastroesophageal reflux disease: the role of TRPM8. J Appl Physiol. 2015;118:635–645. doi: 10.1152/japplphysiol.00717.2014. [DOI] [PubMed] [Google Scholar]
- Lugton W, Dyas B, Binns R. Human volunteer smoking studies on mentholated cigarettes. Brown and Williamson. 1978 750312337/2364. [Google Scholar]
- MacDougall JM, Fandrick K, Zhang X, Serafin SV, Cashman JR. Inhibition of human liver microsomal (S)-nicotine oxidation by (−)-menthol and analogues. Chem Res Toxicol. 2003;16:988–993. doi: 10.1021/tx0340551. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP., Jr Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol Biomarkers Prevent. 2009;18:35–41. doi: 10.1158/1055-9965.EPI-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnemaker J, Hersey J, Homsi G, Busey A, Allen J, Vallone D. Initiation with menthol cigarettes and youth smoking uptake. Addiction. 2013;108:171–178. doi: 10.1111/j.1360-0443.2012.04045.x. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98:1387–1393. doi: 10.1046/j.1360-0443.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- Pan R, Tian Y, Gao R, Li H, Zhao X, Barrett JE, Hu H. Central mechanisms of menthol-induced analgesia. J Pharmacol Exp Ther. 2012;343:661–672. doi: 10.1124/jpet.112.196717. [DOI] [PubMed] [Google Scholar]
- Pearson JL, Abrams DB, Niaura RS, Richardson A, Vallone DM. A ban on menthol cigarettes: impact on public opinion and smokers’ intention to quit. Am J Public Health. 2012;102:e107–e114. doi: 10.2105/AJPH.2012.300804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Rabinoff M, Caskey N, Rissling A, Park C. Pharmacological and chemical effects of cigarette additives. Am J Public Health. 2007;97:1981–1991. doi: 10.2105/AJPH.2005.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath P, Hilton JK, Sisco NJ, Van Horn WD. Implications of Human Transient Receptor Potential Melastatin 8 (TRPM8) Channel Gating from Menthol Binding Studies of the Sensing Domain. Biochemistry. 2015;55:0114–0124. doi: 10.1021/acs.biochem.5b00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Flonta ML. Physiology. Cold current in thermoreceptive neurons. Nature. 2001;413:480. doi: 10.1038/35097164. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Richter P, Beistle D, Pederson L, O’Hegarty M. Small-group discussions on menthol cigarettes: listening to adult African American smokers in Atlanta, Georgia. Ethn Health. 2008;13:171–182. doi: 10.1080/13557850701784694. [DOI] [PubMed] [Google Scholar]
- SAMHSA. The NSDUH Report: Use of Menthol Cigarettes. Substance Abuse and Mental Health Services Administration. Rockville, MD: Office of Appied Studies; 2009. [Google Scholar]
- Smith SS, Fiore MC, Baker TB. Smoking cessation in smokers who smoke menthol and non-menthol cigarettes. Addiction. 2014;109:2107–2117. doi: 10.1111/add.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, Everaerts W, Benoit M, Janssens A, Vennekens R, Viana F, Nemery B, Nilius B, Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Tani M, Onimaru H, Ikeda K, Kawakami K, Homma I. Menthol inhibits the respiratory rhythm in brainstem preparations of the newborn rats. Neuroreport. 2010;21:1095–1099. doi: 10.1097/WNR.0b013e3283405bad. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Memon T, Aman JW, Olivera BM. Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass. Proc Natl Acad Sci USA. 2014;111:2319–2324. doi: 10.1073/pnas.1324019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup I, Wurtzen G, Carstensen J, Olsen P. Short term toxicity study in rats dosed with pulegone and menthol. Toxicol Lett. 1983;19:207–210. doi: 10.1016/0378-4274(83)90120-0. [DOI] [PubMed] [Google Scholar]
- Tobacco. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. Washington DC: US Food and Drug Administration; 2011. Tobacco Products Scientific Advisory Committee. [Google Scholar]
- TPSAC. Menthol cigarettes and public health: review of the scientific evidence and recommendations. Rockville, MD: Food and Drug Administration; 2011. [Google Scholar]
- Umezu T, Morita M. Evidence for the involvement of dopamine in ambulation promoted by menthol in mice. J Pharmacol Sci. 2003;91:125–135. doi: 10.1254/jphs.91.125. [DOI] [PubMed] [Google Scholar]
- Wackowski O, Delnevo CD. Menthol cigarettes and indicators of tobacco dependence among adolescents. Addict Behav. 2007;32:1964–1969. doi: 10.1016/j.addbeh.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Wang J, Roethig HJ, Appleton S, Werley M, Muhammad-Kah R, Mendes P. The effect of menthol containing cigarettes on adult smokers’ exposure to nicotine and carbon monoxide. Regul Toxicol Pharmacol. 2010;57:24–30. doi: 10.1016/j.yrtph.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking--50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120:1914–1916. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Watson HR, Hems R, Rowsell DG, Spring DJ. New compounds with the menthol cooling effect. J Cosmetic Chem. 1978;29:185–200. [Google Scholar]
- Wayne G, Connolly GN. Application, function, and effects of menthol in cigarettes: a servey of tobacco inductry documents. Nicotine Tob Res. 2004;6(suppl 1):S43–S54. doi: 10.1080/14622203310001649513. [DOI] [PubMed] [Google Scholar]
- Werley MS, Coggins CR, Lee PN. Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regul Toxicol Pharmacol. 2007;47:189–203. doi: 10.1016/j.yrtph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- WHO. 2015 http://apps.who.int/iris/bitstream/10665/178574/1/9789240694606_eng.pdf?ua=1.
- Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL. Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res. 2007;9:873–881. doi: 10.1080/14622200701484995. [DOI] [PubMed] [Google Scholar]
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang XB, Jiang P, Gong N, Hu XL, Fei D, Xiong ZQ, Xu L, Xu TL. A-type GABA receptor as a central target of TRPM8 agonist menthol. PloS one. 2008;3:e3386. doi: 10.1371/journal.pone.0003386. [DOI] [PMC free article] [PubMed] [Google Scholar]