Abstract
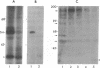
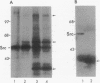
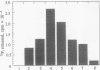
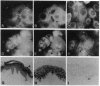
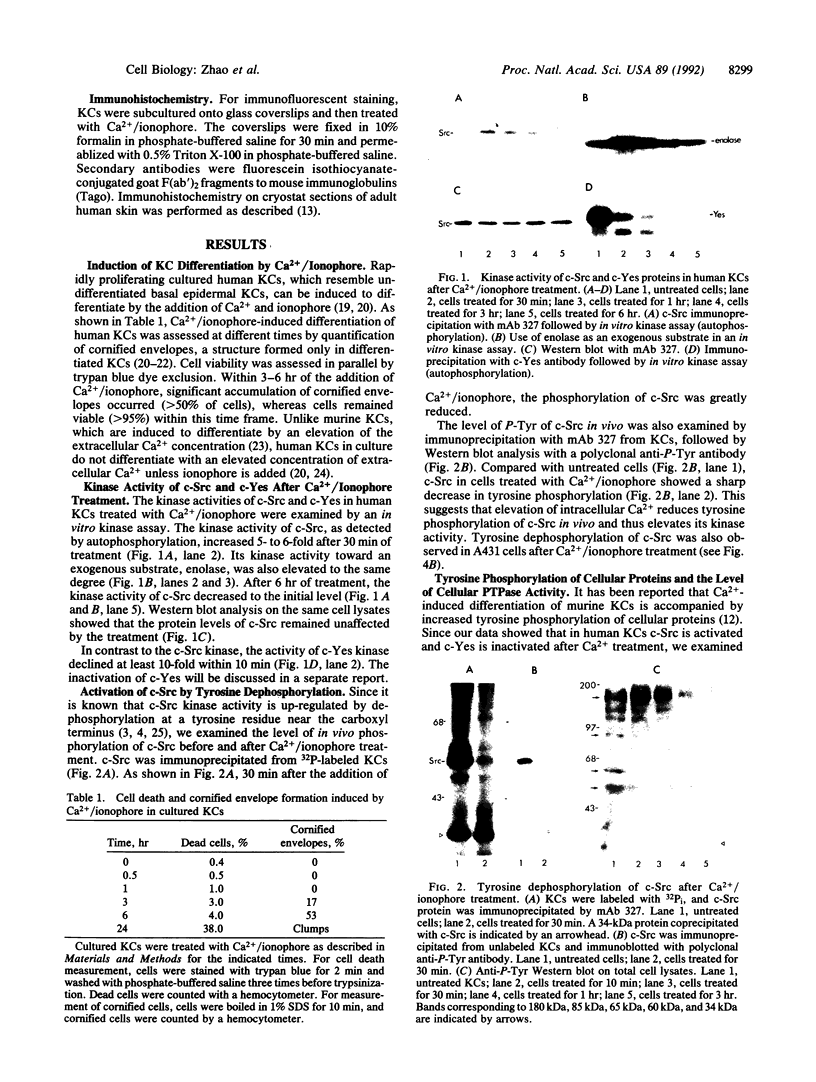
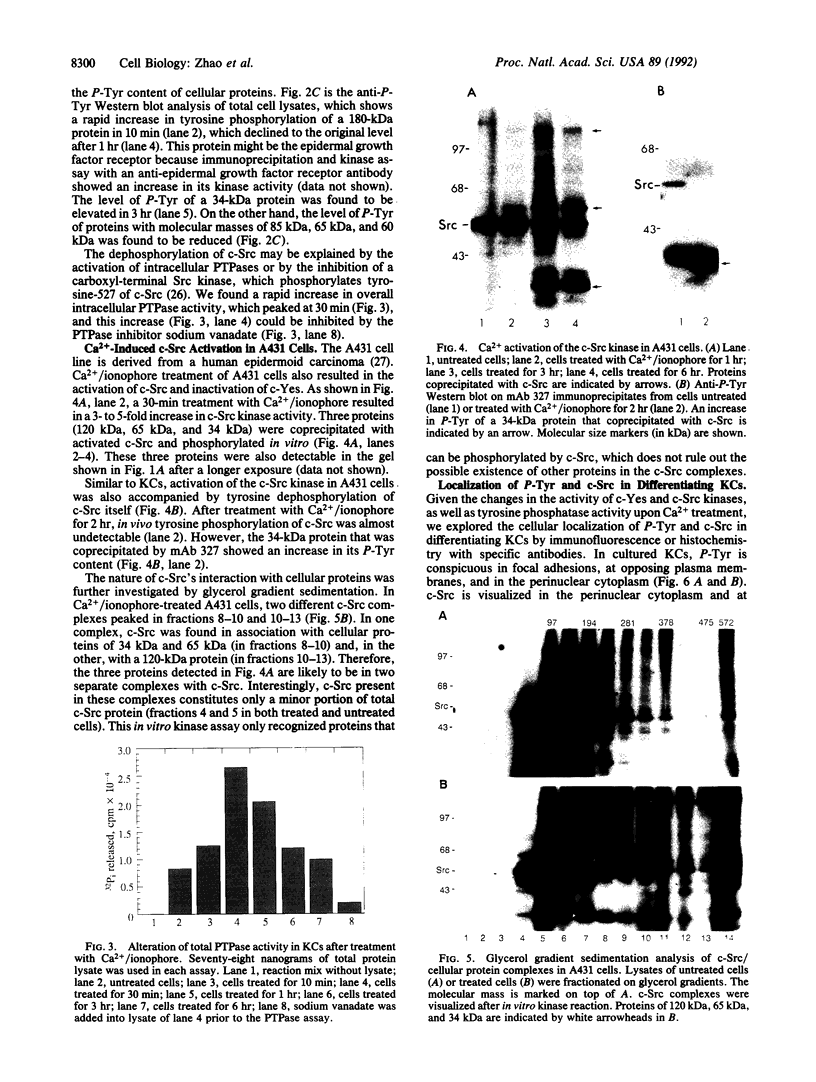
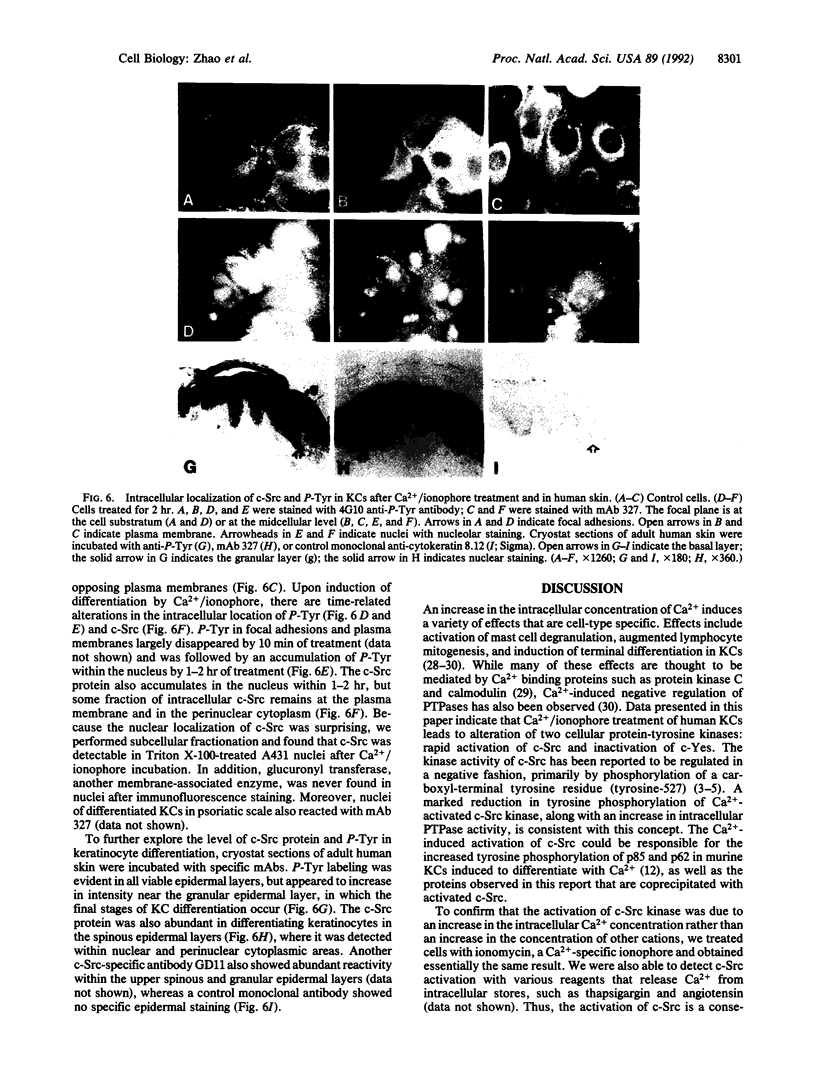
In cultured human epidermal keratinocytes, induction of differentiation by Ca2+ and ionophore treatment was found to result in rapid elevation of c-Src tyrosine kinase activity and inactivation of the c-Yes tyrosine kinase. Activation of c-Src kinase was accompanied by tyrosine dephosphorylation, which might be explained by a rapid increase in intracellular protein-tyrosine phosphatase activity. Ca(2+)-induced differentiation was also associated with altered tyrosine phosphorylation of several cellular proteins and correlated with a marked redistribution of intracellular phosphotyrosine from membrane and adhesion sites to the nucleus. Some of the c-Src protein was also found in the nucleus after Ca2+ treatment, and Ca(2+)-activated c-Src bound to three cellular proteins (120 kDa, 65 kDa, and 34 kDa). In agreement with these results, immunohistochemistry on human epidermis revealed an increase in c-Src expression and tyrosine phosphorylation in cells undergoing differentiation, which strongly suggests a possible role of non-receptor tyrosine kinases in epithelial cell maturation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B. Signal transduction by the SRC family of tyrosine protein kinases in hemopoietic cells. Cell Growth Differ. 1991 Aug;2(8):409–414. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Pfeuty T., Nouvian-Dooghe Y. Immunolocalization of the cellular src protein in interphase and mitotic NIH c-src overexpresser cells. J Cell Biol. 1990 Dec;111(6 Pt 2):3097–3116. doi: 10.1083/jcb.111.6.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLapp N. W., Dieckman D. K. Effect of basic fibroblast growth factor (bFGF) and insulin-like growth factors type I (IGF-I) and type II (IGF-II) on adult human keratinocyte growth and fibronectin secretion. J Invest Dermatol. 1990 Jun;94(6):777–780. doi: 10.1111/1523-1747.ep12874637. [DOI] [PubMed] [Google Scholar]
- Deber C. M., Tom-Kun J., Mack E., Grinstein S. Bromo-A23187: a nonfluorescent calcium ionophore for use with fluorescent probes. Anal Biochem. 1985 May 1;146(2):349–352. doi: 10.1016/0003-2697(85)90550-0. [DOI] [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990 Oct;2(10):303–310. [PubMed] [Google Scholar]
- Ferris D. K., White G. A., Kelvin D. J., Copeland T. D., Li C. C., Longo D. L. p34cdc2 is physically associated with and phosphorylated by a cdc2-specific tyrosine kinase. Cell Growth Differ. 1991 Jul;2(7):343–349. [PubMed] [Google Scholar]
- Filvaroff E., Stern D. F., Dotto G. P. Tyrosine phosphorylation is an early and specific event involved in primary keratinocyte differentiation. Mol Cell Biol. 1990 Mar;10(3):1164–1173. doi: 10.1128/mcb.10.3.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990 Dec;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Hanafusa H. Requirement of phosphatidylinositol-3 kinase modification for its association with p60src. Mol Cell Biol. 1991 Apr;11(4):1972–1979. doi: 10.1128/mcb.11.4.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. Calcium and T lymphocyte activation. Cell. 1989 Oct 6;59(1):15–20. doi: 10.1016/0092-8674(89)90865-9. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Golsteyn E. J., Goren H. J., Lehoux J. G., Lefebvre Y. A. Phosphorylation and nuclear processing of the androgen receptor. Biochem Biophys Res Commun. 1990 Aug 31;171(1):336–341. doi: 10.1016/0006-291x(90)91398-c. [DOI] [PubMed] [Google Scholar]
- Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977 Jun;11(2):405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Hao Q. L., Ferris D. K., White G., Heisterkamp N., Groffen J. Nuclear and cytoplasmic location of the FER tyrosine kinase. Mol Cell Biol. 1991 Feb;11(2):1180–1183. doi: 10.1128/mcb.11.2.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Esquenazi-Behar S., Soter N. A., Lim H. W. Mast-cell heterogeneity: functional comparison of purified mouse cutaneous and peritoneal mast cells. J Invest Dermatol. 1990 Aug;95(2):178–185. doi: 10.1111/1523-1747.ep12477951. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Jove R., Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- Krane J. F., Gottlieb A. B., Carter D. M., Krueger J. G. The insulin-like growth factor I receptor is overexpressed in psoriatic epidermis, but is differentially regulated from the epidermal growth factor receptor. J Exp Med. 1992 Apr 1;175(4):1081–1090. doi: 10.1084/jem.175.4.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Garber E. A., Goldberg A. R. Differences in intracellular location of pp60src in rat and chicken cells transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4142–4146. doi: 10.1073/pnas.77.7.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J., Zhao Y. H., Murphy D., Sudol M. Differential expression of p62c-yes in normal, hyperplastic and neoplastic human epidermis. Oncogene. 1991 Jun;6(6):933–940. [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Aubry M., Shores C. G., Frame L., Pfenninger K. H. c-src gene product in developing rat brain is enriched in nerve growth cone membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5001–5005. doi: 10.1073/pnas.85.14.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., Magid M., King L. E., Jr Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986 Mar;86(3):260–265. doi: 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- Okada M., Nakagawa H. Identification of a novel protein tyrosine kinase that phosphorylates pp60c-src and regulates its activity in neonatal rat brain. Biochem Biophys Res Commun. 1988 Jul 29;154(2):796–802. doi: 10.1016/0006-291x(88)90210-0. [DOI] [PubMed] [Google Scholar]
- Ostergaard H. L., Trowbridge I. S. Negative regulation of CD45 protein tyrosine phosphatase activity by ionomycin in T cells. Science. 1991 Sep 20;253(5026):1423–1425. doi: 10.1126/science.1654595. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., Creutz C. E. p60c-src activity detected in the chromaffin granule membrane. Biochem Biophys Res Commun. 1986 Jan 29;134(2):736–742. doi: 10.1016/s0006-291x(86)80482-x. [DOI] [PubMed] [Google Scholar]
- Pawson T., Bernstein A. Receptor tyrosine kinases: genetic evidence for their role in Drosophila and mouse development. Trends Genet. 1990 Nov;6(11):350–356. doi: 10.1016/0168-9525(90)90276-c. [DOI] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Highly specific antibody to Rous sarcoma virus src gene product recognizes a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985 Feb;100(2):409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Thai T., Tang M., Saito H. Distinct functional roles of the two intracellular phosphatase like domains of the receptor-linked protein tyrosine phosphatases LCA and LAR. EMBO J. 1990 Aug;9(8):2399–2407. doi: 10.1002/j.1460-2075.1990.tb07415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M., Alvarez-Buylla A., Hanafusa H. Differential developmental expression of cellular yes and cellular src proteins in cerebellum. Oncogene Res. 1988 May;2(4):345–355. [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. L., Handel L. M., Nelson W. J. Elevated expression of pp60c-src alters a selective morphogenetic property of epithelial cells in vitro without a mitogenic effect. Mol Cell Biol. 1988 Feb;8(2):632–646. doi: 10.1128/mcb.8.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zhao Y. H., Baker H., Walaas S. I., Sudol M. Localization of p62c-yes protein in mammalian neural tissues. Oncogene. 1991 Oct;6(10):1725–1733. [PubMed] [Google Scholar]
- Zhao Y. H., Krueger J. G., Sudol M. Expression of cellular-yes protein in mammalian tissues. Oncogene. 1990 Nov;5(11):1629–1635. [PubMed] [Google Scholar]
- Zhong X. H., Howard B. D. Phosphotyrosine-containing lactate dehydrogenase is restricted to the nuclei of PC12 pheochromocytoma cells. Mol Cell Biol. 1990 Feb;10(2):770–776. doi: 10.1128/mcb.10.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]