Abstract
We present two experiments examining the universality and uniqueness of reduced context sensitivity in language processing in Autism Spectrum Disorders (ASD), as proposed by the Weak Central Coherence account (Happé & Frith, 2006, Journal of Autism and Developmental Disorders, 36(1), 25). That is, do all children with ASD exhibit decreased context sensitivity, and is this characteristic specific to ASD versus other neurodevelopmental conditions? Experiment 1, conducted in English, was a comparison of children with ASD with normal language and their typically-developing peers on a picture selection task where interpretation of sentential context was required to identify homonyms. Contrary to the predictions of Weak Central Coherence, the ASD-normal language group exhibited no difficulty on this task. Experiment 2, conducted in German, compared children with ASD with variable language abilities, typically-developing children, and a second control group of children with Language Impairment (LI) on a sentence completion task where a context sentence had to be considered to produce the continuation of an ambiguous sentence fragment. Both ASD-variable language and LI groups exhibited reduced context sensitivity and did not differ from each other. Finally, to directly test which factors contribute to reduced context sensitivity, we conducted a regression analysis for each experiment, entering nonverbal IQ, structural language ability, and autism diagnosis as predictors. For both experiments structural language ability emerged as the only significant predictor. These convergent findings demonstrate that reduced sensitivity to context in language processing is linked to low structural language rather than ASD diagnosis.
Keywords: Autism spectrum disorders, Language comprehension, Ambiguity resolution, Homonym, Weak central coherence, Context sensitivity, Typical development, Language impairment
1. Introduction
1.1. Autism, language and cognitive theories
Autism spectrum disorders (ASD) are neurodevelopmental disorders defined by impairments in social communication and interaction as well as restricted and repetitive behavior, interests or activities (APA, 2013). While structural language abilities, including vocabulary and grammar, are highly variable across the spectrum (Kjelgaard & Tager-Flusberg, 2001; Mawhood & Howlin, 2000), individuals with ASD are often reported to show difficulties in aspects of language comprehension (Hudry et al., 2010; Kjellmer et al., 2012; Kwok, Brown, Smyth, & Cardy, 2015), especially in understanding non-literal and ambiguous language (e.g., Happé, 1997).
Research on children with primary language impairment (LI), another neurodevelopmental disorder, shows that comprehension ability is a catalyst for further language growth and broader social development, as well as later academic achievement (e.g., Clegg, Hollis, Mawhood, & Rutter, 2005; Toppelberg & Shapiro, 2000). Thus, understanding the factors underlying language comprehension difficulties in neurodevelopmental disorders is an important objective.
There is a long tradition of searching for universal (shared by individuals across the spectrum), unique (to ASD and not to other disorders), and specific (domain-specific rather than general) cognitive markers of ASD, also called “core deficits” by Sigman and colleagues (e.g., Sigman, Dijamco, Gratier, & Rozga, 2004; see also Rajendran & Mitchell, 2007). The weak central coherence account of ASD (Frith, 1989; Happé & Frith, 2006) was proposed as such a marker and has become a popular explanation of communication challenges seen in this population. Frith (1989) first introduced the term “central coherence” referring to the drive to integrate pieces of information in order to achieve overall meaning in multiple domains (visual, non-speech auditory, and language processing). She applied the concepts of “local” to describe bottom-up processing of discrete information vs. “global” to describe top-down, meaning-based processing of information. Weak central coherence (WCC) therefore is an account that proposes a reduction of this drive in ASD.
Weak central coherence is an intuitively compelling explanation for some of the comprehension difficulties observed in ASD, since language comprehension involves the integration of literal language content with nonverbal communication and different types of contextual information. Happé and Frith (2006) proposed that WCC is a default cognitive style in ASD focused on detail or local processing, which can be overcome by providing explicit instructions to attend to context or global features (see also Happé & Booth, 2008). Models of language comprehension more generally also treat how different levels of linguistic and extra-linguistic information are integrated in the course of language processing (e.g., Bishop, 1997; Simpson, 1994).
1.2. Reduced sensitivity to context in language comprehension
Using ambiguous words or sentences is a classic way to test context sensitivity. Single ambiguous words can be homophones, like bank or fan, or homographs, which are words with the same spelling, but different pronunciations depending on the intended meaning, e.g. tear. Further, phrases or sentences can be ambiguous due to single words (lexical ambiguity, like “He goes to the bank.”) or due to their syntactic structure, e.g. “The man hit the boy with a stick.” In such cases, both global context and word frequency highly influence how we understand or disambiguate the sentence (Beveridge & Marsh, 1991; MacDonald, Pearlmutter, & Seidenberg, 1994; Swaab, Brown, & Hagoort, 2003). A particularly strong test of reliance on sentence context is afforded by the processing of rare versions of biased homonyms that have one much more frequent meaning than the other. Since the rare meaning of a homonym is disfavored based on lexical frequency, coming to the adequate interpretation could only follow from integrating global information offered by sentence context.
Frith and Snowling (1983) were the first to investigate how children with autism interpreted ambiguous sentences in a homograph reading task (e.g., “There was a big tear in her dress”). Unlike controls, children with autism made more errors and used the common pronunciation more often regardless of the sentence context, which was explained as a weaker use of sentence context or diminished global processing. This seminal WCC finding is well replicated (Happé, 1997; Jolliffe & Baron-Cohen, 1999; López & Leekam, 2003). Although often cited as evidence for weak global processing in ASD specifically, Snowling and Frith (1986) already questioned whether this was an autism-specific pattern or if it was instead related to underlying cognitive abilities.
However, more recent studies on language processing in ASD have yielded inconsistent results, calling into question the explanatory adequacy of the WCC account. For instance, Hoy, Hatton, and Hare (2004) found that children with autism made more errors on the rare condition of a homophone task, but performance was correlated with verbal ability rather than with autism symptoms. Norbury (2005) used a paradigm to examine both contextual facilitation and suppression of irrelevant meanings and tested children with autism as well as children with specific language impairment (SLI). The SLI group and those children with ASD who also showed language difficulties were less context sensitive, whereas no difference was observed between children with autism and normal language abilities and the control group. A similar result was reported by Brock, Norbury, Einav, and Nation (2008) who contributed novel information regarding on-line language processing by measuring participants’ eye movements during a listening task. While most previous investigations focused on global or context sensitive responses, a recent study by Riches, Loucas, Baird, Charman, and Simonoff (2016) tested for local processing bias in a forced choice comprehension task with syntactically ambiguous sentences. Contrary to WCC predictions, participants with ASD did not display a greater tendency for local processing.
This pattern of results, and the extreme variability observed in the structural language abilities of individuals with ASD, lead to a possible explanation for inconsistent findings: context sensitivity in language processing may be linked to structural language ability rather than being a cognitive processing style specific to ASD. Individuals with ASD with very high structural language have been shown to be sensitive to context in their default processing (Brock et al., 2008; Nadig, Vivanti, & Ozonoff, 2009; Norbury, 2005), challenging the universality of this potential marker. In addition, the specificity of reduced context sensitivity to ASD has been questioned given that other groups, e.g., children with primary language impairment, also face language comprehension difficulties in similar ways (Norbury and Bishop, 2002).
Finally, there are some methodological features of work in this area that limit our understanding of context sensitivity in children with ASD. The classic homograph tasks (Frith & Snowling, 1983; Happé, 1997; Jolliffe & Baron-Cohen, 1999; López & Leekam, 2003), for instance, all contained strong lexical associates (e.g., fish-bank) of the ambiguous word in their stimuli to differentiate one meaning of ambiguous words from the other. However this leaves open the possibility that the task could be solved by lower level lexical priming, rather than by integrating contextual information per se. In addition, the majority of previous studies used reading instead of spoken language tasks. Thus, results were influenced by reading abilities and may not be generalizable to oral comprehension. More research is needed to clarify the WCC prediction of reduced context sensitivity in language comprehension in ASD.
1.3. Objectives and research questions
We present two experiments to further examine the universality and uniqueness of reduced context sensitivity in language processing in ASD. That is, do all children with ASD exhibit decreased sensitivity to context, and is this characteristic specific to ASD as opposed to other neurodevelopmental conditions, especially primary language disorder?
The first experiment, conducted in English, compared the performance of children with ASD with normal language and their typically-developing peers on a task where interpretation of sentential context was required to identify a homonym. Homonym stimuli were of different types: common or rare meanings of biased homonyms and either meaning of balanced homonyms. This allowed for an examination of whether reduced context sensitivity would be observed in children with ASD with normal structural language abilities, and how context sensitivity interacts with lexical frequency. A regression analysis was conducted to further investigate which factors (nonverbal IQ, structural language, ASD diagnosis) predict sensitivity to context in language comprehension.
The second experiment was conducted in German and built on Experiment 1 by including a group of children with primary language impairment, a group of children with ASD who had variable language abilities, and typically-developing participants, addressing the question of the uniqueness of decreased context sensitivity to ASD. Once again we conducted a regression analysis to identify the factor(s) contributing to reduced sensitivity.
A central methodological goal was to disentangle effects of sentential context from lexical priming, as individuals with autism have been shown to have intact lexical priming for homographs specifically (Hala, Pexman, & Glenwright, 2007), as well as for unambiguous words (López & Leekam, 2003). Consequently, the context sentences in our stimuli controlled for lexical associates of the homonyms. That is, for the homonym “bank” the sentence did not contain words like “money.” In addition, since daily communication is rooted in oral language both experiments tested spoken rather than written language processing. Experiment 1 was entirely a comprehension task, while Experiment 2 required comprehension of context sentences as well as production of a sentence completion.
2. Experiment 1
The first experiment was part of a larger study on contextual language processing in ASD that took place in California, USA. Study participation involved three approximately 2-h lab visits, scheduled within two weeks of each other. Experiment 1 was administered at either the second or third visit, counterbalanced across participants.
2.1. Method
2.1.1. Participants
All participants were native speakers of North American English. Participants were recruited using a university research institute’s database. The first group of participants were 19 children with ASD and normal language abilities (ASD-NL) between the ages of 9 and 14 years. In the literature a sample of this nature is often described as children with high functioning autism. The second group were 19 typically-developing children (TYP) between the ages of 8 and 14 years. Structural language ability was evaluated with the Clinical Evaluation of Language Fundamentals Fourth Edition (CELF-4, Semel, Wiig, & Secord, 2003), which provides comprehensive assessment of both expressive and receptive language ability. Performance IQ was assessed using the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999). Groups did not differ statistically with respect to age, gender, structural language or performance IQ. Details on participant characteristics are provided in Table 1.
Table 1.
Participant characteristics for Experiment 1.
| Group | TYP (n = 19) | ASD-NL (n = 19) | p | D |
|---|---|---|---|---|
| Mean (SD) range | Mean (SD) range | |||
| Age (years) | 10;10 (1;11) | 11;04 (1;09) | .404 | .27 |
| Gender | 15 male/4 female | 17 male/2 female | .374 | |
| CELF-4 Core language score | 114.32 (12.44) 88–129 | 108.68 (13.08) 92–134 | .182 | .44 |
| WASI Performance IQ | 113.68 (13.47) 88–135 | 108.74 (17.45) 67–133 | .085 | .57 |
| SCQ Social comm. Questionnaire | 2.21 (3) 0–7 | 26.32 (5.46) 16–34 | <.001 | 5.66 |
| ADOS Algorithm score | N/A | 13.95 (3.73) 7–20 |
Note: The p-values were calculated using t-tests except for the categorical variable of gender where a Chi-square test was used. Effect size is given using Cohen’s d for continuous variables.
Diagnoses of ASD were confirmed within the study by administration of the Autism Diagnostic Observation Schedule, ADOS-Module 3 (Lord, Rutter, DiLavore, & Risi, 1999) and the Social Communication Questionnaire, SCQ-Lifetime (Rutter, Bailey, Berument, Lord, & Pickles, 2003). All participants in the ASD-NL group met full criteria for DSM-IV Autistic Disorder, as established by a combination of current performance on the ADOS, developmental history from the SCQ, and clinical judgment.
Exclusion criteria for all participants were the presence of major medical conditions or physical disability. Further, for the TYP participants a history of developmental problems or delay and autistic symptoms, as assessed with the SCQ, were ruled out.
2.1.2. Stimuli
Homonym stimuli were noun/noun homonyms with two picture-able meanings (see Appendix A, Table A1). Three types of homonyms were included. Biased homonyms were those that had one meaning that was much more frequent than the other. This designation was established using English relative meaning frequency norms (Gawlick-Grendell & Woltz, 1994; Nelson, McEvoy, & Schreiber, 1998; Twilley, Dixon, Taylor, & Clark, 1994). In these norming studies, participants are asked to say or write the first word that came to mind when presented with a homograph. The relative frequency of each meaning, or proportion of people who provided that meaning, was established. For biased homonyms (e.g., fan) this difference in relative frequency between the two most common meanings was large—always greater than .53. Biased homonyms were further divided into the common (for the current stimuli relative frequencies were .75 or higher) and rare (.20 or lower) meanings. Balanced homonyms, where the difference in the relative frequency of the two meanings was less than .45 (e.g., cell), were also included. In order to avoid priming effects (see Section 1.3) the context sentences did not contain any lexical associates of the homonyms (norms from Nelson et al., 1998). Two lists of stimuli were prepared with one version of each homonym per list, and list was counterbalanced across participants. Participants therefore encountered only one version of each homonym.
Color pictures of each version of homonym stimuli and filler objects were found using ClipArt (openclipart.org). Stimuli were normed with 10 6- to 8-year-olds (selected to be younger than the participants using the rationale that if they were familiar with the stimuli older children would be as well). Context sentences were printed on a sheet of paper, followed by the homonym, and then pictures of both versions of the homonym. Children were asked to pick the picture that goes best with each sentence and the word that comes after it. Pictures and context sentences were retained if at least 80% of respondents chose the correct picture for the context sentence.
2.1.3. Procedure
Participants were seated at a computer. The experiment was presented via Eprime software and responses were collected via mouse click. While viewing a blank screen participants heard a context sentence presented auditorally via recordings by a female speaker who was not involved in testing, e.g., “The noise of the game and cheering kept her up all night”. Then four pictures appeared, one in each quadrant of the screen, with a red square in the middle (see Fig. 1). Participants were instructed to click on the red square with the computer mouse; this action activated an auditory recording of the homonym e.g., “fan”. Finally, participants were instructed to “Click on the picture that went best with everything you heard.” Response times were collected when the participant clicked on the square as well as when they clicked on a picture; and the accuracy of the picture choice was collected. Eye-tracking data was collected with an ASL head-mounted eyetracker during the task but is not presented here as our focus is on behavioral responses. Participants completed 40 trials of this task in a fixed random order, hearing sentences for 12 balanced homonyms, 13 biased homonyms and 15 filler stimuli, where context sentences described a pictured word that was shown with three unrelated pictures (e.g., for the target word “key”, “Jeff had to wait for his roommate to get into his house.”).
Fig. 1.
Example display for biased homonym “fan,” displayed after hearing either a or b. (a) Common: The whirring noise and summer heat kept her up all night. (b) Rare: The noise of the game and cheering kept her up all night.
2.1.4. Predictions
It was predicted that the TYP group should generally select the picture that was congruent with the context sentence, and that the ASD-NL group would largely follow this pattern as well, given the findings of Norbury (2005). However, following the WCC account we hypothesized that the ASD-NL group would take longer to select the context-congruent picture for rare homonyms if global processing was not their default mode. Finally, we predicted that both groups would be sensitive to lexical frequency and therefore would have the best performance on common versions of biased homonyms, followed by balanced homonyms, and finally rare versions of biased homonyms.
2.2. Results
Analyses were conducted with mixed ANOVAs with homonym type (common, balanced, rare) as a within-subjects factor and group (ASD-NL, TYP) as a between-subjects factor.
2.2.1. Accuracy of picture selection
There was a main effect of homonym type, F (2, 35) = 31.88, p < .001, ηp2 = .65. There was no main effect of group, F (1, 36) = 1.05, p = .313, ηp2 = .03. Finally, there was no interaction between group and homonym type, F (2, 35) = .04, p = .957, ηp2 < .01. As seen in Fig. 2, accuracy followed a pattern of lexical frequency in both groups as predicted, with most accurate answers on common versions of biased homonyms, followed by balanced homonyms and finally rare versions of biased homonyms.
Fig. 2.
Boxplot of proportion of context sensitive responses by group and homonym type.
2.2.2. Response time for picture selection
There was again a main effect of homonym type, F (2, 35) = 12.20, p < .001, ηp2 = .25. There was no main effect of group, F (1, 36) = .09, p = .768, ηp2 < .01. Finally, there was no interaction between group and homonym type, F (2, 35) = 1.08, p = .344, ηp2 = .03. Fig. 3 shows that, mirroring results for accuracy, the median response time for both groups of participants was fastest in response to common versions of biased homonyms, followed by the balanced ones and slowest for rare versions of biased homonyms.
Fig. 3.
Boxplot of picture selection response time in milliseconds by group and homonym type.
2.2.3. Regression analysis
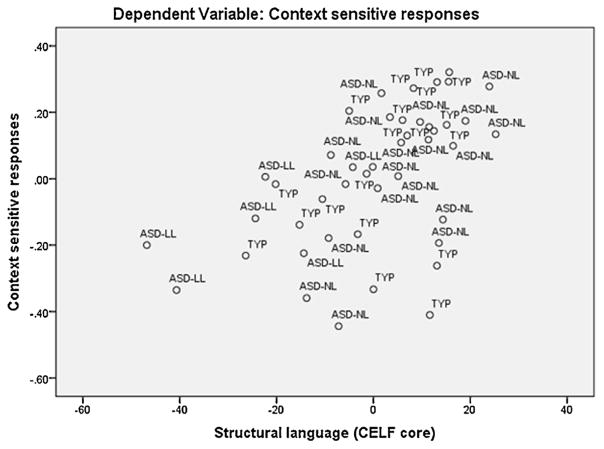
To directly investigate which factors predict sensitivity to context in language comprehension (specifically context sensitive responses for rare versions of homonyms), we ran a model including both groups of participants described above. In addition, we had data available from 6 participants with ASD who we were not able to include in the matched group due to their lower language abilities (ASD-LL; structural language scores ranging from 58 to 87; 2 were more than 2 SD below the mean, 2 were between 1 and 2 SD below the mean; and the remaining 2 were in the low average range, thus the group is not described as language impaired). Thus they had significantly lower structural language scores than both the TYP and ASD-NL groups (ps < .001). Their PIQ scores (range 74–114) were significantly lower than the TYP group (p = .01) but not the ASD-NL group (p = .223). Since we were interested in examining the effects of lower structural language abilities on contextual language processing we included these participants in the model as well, resulting in a total sample size of 44. A multiple regression with nonverbal IQ (measured by WASI PIQ), structural language ability (CELF core score), and ASD diagnosis (yes or no) as predictors was conducted using the enter method. The full model explained a significant amount of the variance in context sensitive responses (F (3, 40) = 5.653, p = .003, R2 = .298, R2Adjusted = .245). The analysis demonstrated that nonverbal IQ (Beta =−.100, t (40) =−.666, p = .509) and ASD diagnosis (Beta = .101, t (40) = .584, p = .562) were not significant predictors. However, structural language ability did significantly predict context sensitive responses (Beta = .635, t (40) = 3.579, p < .001)1. This result is depicted in Fig. 4.
Fig. 4.
Partial regression plot of the residuals of the outcome variable (context sensitive responses) and the predictor variable structural language, showing that structural language was a significant positive predictor of context sensitive responses. Note that some of the participants with the highest proportion of context sensitive responses were those from the ASD normal language group, whereas those with the lowest scores were from the ASD lower language group, as reflected in the finding that ASD diagnosis itself was not a significant predictor of performance.
2.3. Discussion
The results of Experiment 1 demonstrate clearly that, contrary to the predictions of WCC, children with ASD who had normal language abilities do not show decreased sensitivity to sentence context when interpreting ambiguous homonyms. Critically, this was shown in a task where lexical priming effects could not account for the results, and this pattern held even for accuracy on rare versions of biased homonyms. Our prediction that the ASD-NL group may show some subtle WCC effects in their response times to rare homonyms is not supported by the data which showed no significant differences in response time.
This interpretation is bolstered by the results of the regression model including the ASD-NL and TYP groups as well as six additional children with ASD and lower language abilities (ASD-LL). The regression demonstrated that, in this combined group of children, structural language ability as measured by the CELF-4 significantly predicted context sensitive responses for rare versions of homonyms. Importantly, performance IQ was not a significant predictor, though children varied in this ability. Crucially, ASD diagnosis was also not a significant predictor, providing evidence against Weak Central Coherence in language processing as a universal marker of ASD.
3. Experiment 2
The second experiment was part of a larger study on contextual language processing (Eberhardt, 2014) with three different experimental tasks and took place close to Cologne, Germany. Study participation involved two sessions conducted at the child’s school or therapy center. Sessions lasted approximately 60–90 min each, scheduled at least 2 weeks apart from each other.
3.1. Method
3.1.1. Participants
Participants were recruited from local autism or language therapy centers, mainstream primary and secondary schools as well as special schools for students with speech and language impairment. All participants spoke German as their first language.
The ASD group consisted of 30 children, of whom 25 had a formal diagnosis of Asperger syndrome (AS) and 5 were diagnosed as having Autistic Disorder according to DSM-IV criteria. The typically-developing control group (TYP) consisted of 30 children. A second control group was included in Experiment 2, composed of 24 children with primary language impairment (LI). To confirm the presence of language impairment the Test for Reception of Grammar (TROG; German version TROG-D Fox, 2009) and a sentence repetition task (subtest of the Heidelberger language development test battery; Grimm & Schöler, 1991) were conducted. Children were included in the LI group only if they had a score below the percentile rank of 16 on one of the two tests and a score below the percentile rank of 25 in the second test. Conversely, children in the TYP control group had to score above a percentile rank of 25 on both language assessments. It is important to note that, unlike the ASD-NL group in Experiment 1, here the language abilities of the ASD group varied: 7 children with ASD also met the above mentioned criterion for LI. Exclusion criteria for all participants were the presence of sensory issues or physical disability which might impact task performance.
In Germany no comprehensive language test comparable to the CELF exists. Thus, in addition to the above mentioned assessments (TROG-D and sentence repetition task), the Peabody Picture Vocabulary Test (PPVT; German version Bulheller & Häcker, 2003) was administered. Since the three different language tests do not cover the full range of participants’ ages, raw scores were used for further analysis. Nonverbal ability was measured by Raven’s Standard Progressive Matrices (SPM; German standardization Heller, Kratzmeier, & Lengfelder, 1998). Details on participant characteristics and matching are provided in Table 2. As seen here the three groups did not differ significantly in age or gender proportion, but the LI group showed significantly lower scores on the Raven SPM than the TYP and ASD group. This pattern was also reported in previous studies (e.g. Norbury, 2005) and will be taken into account for all analysis. As expected per inclusion criterion, the LI group differed significantly from the TYP and ASD group in all language measures. The ASD scored significantly lower than the TYP group only on the TROG-D.
Table 2.
Participant characteristics for Experiment 2.
| Group | a. TYP (n = 30) | b. ASD (n = 30) | c. LI (n = 24) | a vs. b | a vs. c | b vs. c |
|---|---|---|---|---|---|---|
| p | p | p | ||||
| Mean (SD) Range | Mean (SD) Range | Mean (SD) Range | d | d | d | |
| Age (years) | 11;84 (1;58) 9;03–15 | 11;77 (1;67) 9–15;03 | 11;61 (1;72) 9;02–15;09 | .880 | .615 | .726 |
| −.043 | −.139 | −.094 | ||||
| Gender | 28 male/2 female | 28 male/2 female | 22 male/2 female | 1.00 | .816 | .816 |
| Raven SPM | 46.43 (8.39) 35–64 | 47.23 (9.15) 33–68 | 38.92 (4.96) 32–55 | .725 | <.001 | <.001 |
| .091 | −1.09 | −1.129 | ||||
| TROG-D | 19.13 (.90) 17–20 | 17.97 (2.04) 14–21 | 13.71 (1.85) 9–16 | .007 | <.001 | <.001 |
| −.736 | −3.726 | −2.188 | ||||
| Sentence repetition | 23.52 (.68) 22–24 | 23.03 (1.52) 18–24 | 14.96 (4.95) 0–21 | .108 | <.001 | <.001 |
| −.416 | −2.423 | −2.204 | ||||
| PPVT | 39.07 (13.23) 15–65 | 36.90 (15.51) 9–62 | 19.54 (9.88) 2–41 | .563 | <.001 | <.001 |
| −.151 | −1.673 | −1.335 |
Note: The p-values were calculated using t-tests except for the categorical variable of gender where a Chi-square test was used. Effect size is given using Cohen’s d for continuous variables.
3.1.2. Stimuli
As in Experiment 1 noun/noun homonyms with two picture-able meanings were used. These were chosen from a school dictionary to make sure that children would be familiar with the words. It was further checked that all words have a frequency class (defined as log2 x frequency of the most frequent word in German/frequency of a target word) of at least 9 (online corpus; University Leipzig, 2009). Not only the sentence context, but also the frequency of the single meanings may have an influence on understanding. Because no adequate frequency corpus exists in Germany, a pilot study on common vs. rare meaning of homonyms was conducted. 32 children aged 10.9 to 13.4 years who did not take part in the main study were asked to name the first thing they associate with a given word. Only biased homonyms for which one meaning was stated at least three times more often than the other meaning were included (criterion based on Jolliffe & Baron-Cohen, 1999). The remaining 12 homonyms are listed in Appendix A, Table A2. To minimize the influence of social cognitive abilities, no mental state verbs were used, and in order to avoid priming effects the context sentences did not include verbs that were co-occurrences to the homonym according to the University Leipzig corpus (2009).
The 24 items and six unambiguous filler items were pseudo-randomized for each participant with the condition that at least two distinct items appeared between the two versions of the same homonym. The items were audio recorded by a female native speaker who was not involved in testing.
3.1.3. Procedure and scoring
Each participant was tested alone in a quiet and non-distracting room by the first author or a specifically trained student. The participants were seated in front of a microphone. Via speakers they heard a context sentence followed by an ambiguous sentence stem, e.g.2
Timo browses the Internet. He grabs the mouse and. . .
Timo has bought a new cage. He grabs the mouse and. . .
They were instructed to “complete the second sentence without thinking about it too long”.
Children’s answers were recorded via the open source software Audacity. The task was repeated in a second test session in order to investigate effects of a different instruction, but this question is not our focus here and the data is presented elsewhere (Eberhardt & Nussbeck, 2015).
Similar to the procedure of Booth and Happé (2010) the completions were scored as context sensitive (global), context insensitive (local), unclear, or no completion. A context sensitive answer is defined as a completion that takes the context into account and therefore is semantically correct (e.g., Timo browses the Internet. He grabs the mouse and. . .clicks on a button). A context insensitive completion makes sense in the isolated second sentence but not within the context (e.g., [. . .] and strokes it.). Piloting showed that children sometimes also give unclear completions that don’t allow a judgement of global or local (e.g. [. . .] and he laughs.).
The completions were coded by the first author. A second independent person, who was blind to group membership and aims of the study scored 67% of the protocols. Overall agreement was high (96%, κ = .82).
3.1.4. Predictions
Following the WCC account it was predicted that the ASD group would make fewer context sensitive completions than the control group. For the LI group it was assumed a priori that they would perform less accurately than the TYP. There was no specific hypothesis about the extent to which they would differ from the ASD group. As in the first experiment, we predicted that all three groups would be sensitive to lexical frequency and therefore would make fewer context sensitive completions for rare as opposed to common versions of biased homonyms.
3.2. Results
The two items containing the homonym “card” could not be scored reliably, because the combination of the context sentence and the rare and the common version of the homonym allowed for more than two meanings. Therefore both the rare and the common stimulus item had to be removed and a total of 22 items remained.
An ANOVA showed a significant effect of group for the number of no completion responses F (2, 81) = 3.50, p < .05. Therefore the percentage of context sensitive responses over the completed sentences was calculated for further analysis.
3.2.1. Context sensitive responses
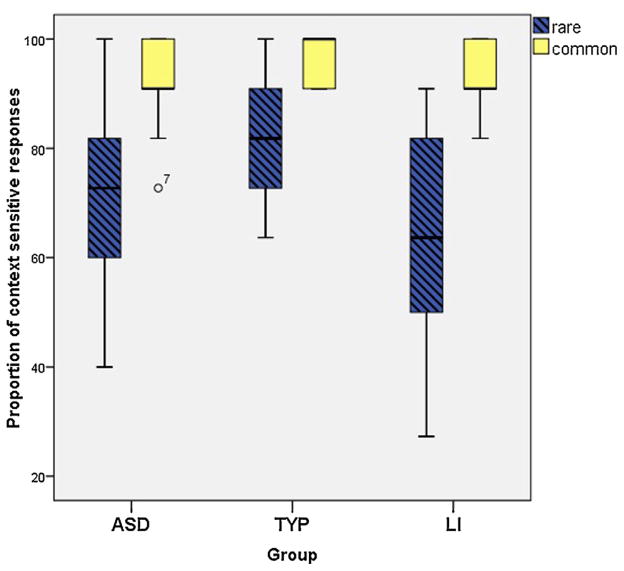
A mixed ANOVA with homonym type (common, rare) as a within-subjects factor and group (ASD, TYP, LI) as a between-subjects factor was conducted. There was a main effect of homonym type, F (1, 81) = 132.92, p < .001, ηp2 = .621 and a main effect of group, F (1, 81) = 14.04, p < .001, ηp2 = .257. Finally, there was a significant interaction between group and homonym type, F (2, 81) = 7.81, p < .005, ηp2 < .162.
To further investigate the significant effect of group, post hoc tests (Tukey) were conducted. There was a significant difference between the ASD and TYP group, p < .005, and between the LI and TYP group, p < .001. However the ASD and LI groups did not differ significantly, p = .130.
As seen in Fig. 5, all groups showed more context sensitive responses in the common condition than in the rare homonym condition. The figure also illustrates the interaction effect, as homonym frequency had a greater impact on the ASD and LI groups than on the TYP group.
Fig. 5.
Boxplot of proportion of context sensitive responses by group and homonym type.
3.2.2. Regression analysis
As for Experiment 1, we ran a regression model to identify which factors predict sensitivity to context in language processing, specifically for context sensitive responses for rare versions of homonyms which cannot be obtained without integrating context. The model included the three groups of participants, and nonverbal IQ (measured by Raven SPM), structural language ability (TROG-D), and ASD diagnosis (yes or no) were entered as predictors of context sensitive responses for rare versions of homonyms. The TROG-D was chosen here as the most accurate measurement of structural language abilities. Using the enter method it was found that full model explained a significant amount of the variance in context sensitive responses (F (3, 80) = 13.59, p < .001, R2 = .338, R2Adjusted = .313). The analysis demonstrated that nonverbal IQ (Beta = −.108, t (80) = −1.043, p = .300) and ASD diagnosis (Beta = −.172, t (80) = −1.822, p = .072) were not significant predictors. However, structural language ability did significantly predict context sensitive responses (Beta = .630, t (80) = 6.098, p < .001). This result is depicted in Fig. 6.
Fig. 6.
Partial regression plot of the residuals of the outcome variable (context sensitive responses) and the predictor variable structural language showing that structural language was a significant positive predictor of context sensitive responses.
3.3. Discussion
The children with ASD and varied language abilities tested in Experiment 2 showed reduced context sensitivity in the sentence completion task, giving fewer context sensitive completions than the TYP group. On the surface this finding would seem to support the Weak Central Coherence account; however, the LI group provided even fewer context sensitive responses, performing significantly below TYP controls as well. Thus these findings extend the implications of Experiment 1 by demonstrating that reduced context sensitivity is not unique to ASD. Context sensitive responses in all groups followed a pattern of lexical frequency as predicted, with fewer context sensitive answers provided for items with the rare versus the common version of the homonym. The pattern of results obtained across groups suggests that general structural language abilities rather ASD diagnosis leads to reduced context sensitivity in language processing.
The regression analysis strongly corroborates this claim: Structural language abilities, measured by the TROG-D, rather than nonverbal IQ or ASD diagnosis predicted context sensitive responses for rare versions of homonyms, as was found in Experiment 1.
4. General discussion
The two experiments presented here aimed to further clarify the questions of universality and uniqueness of reduced context sensitivity in language processing in children with ASD, as predicted by the WCC account (Happé & Frith, 2006). In Experiment 1 a group of children with ASD and normal language were tested and did not show any decrement in context sensitive responses on a picture selection task, both in terms of accuracy and response time, questioning the universality of this characteristic in ASD. In Experiment 2 a group of children with ASD and variable language abilities did demonstrate diminished context sensitivity in a sentence completion task. Crucially, this difference in findings between Experiments 1 and 2 was related to the language abilities of the children with ASD tested. Differences in the language abilities of ASD samples tested is also a likely explanation for inconsistent findings in the prior literature. Moreover, Experiment 2 demonstrated that children with LI also showed diminished context sensitivity, to a greater extent than the ASD group, indicating that this characteristic is also not unique to ASD. Finally, for each experiment strikingly similar results were found in regression models that directly examined nonverbal IQ, structural language, and autism diagnosis as predictors of context sensitive responses; structural language was the strongest and only significant predictor in each case. These findings are particularly compelling since they were replicated across two samples tested in different languages (English and German) and on different language processing tasks (picture selection and sentence completion). These results unmistakably challenge the WCC account as an explanation for language problems in ASD and add to growing evidence of this nature (e.g., Brock et al., 2008; Norbury, 2005). As recently discussed for other cognitive accounts, like theory of mind (Gernsbacher & Pripas-Kapit, 2012; Loukusa, Mäkinen, Kuusikko-Gauffin, Ebeling, & Moilanen, 2014), it is increasingly clear that a more accurate and parsimonious account of deceased context sensitivity is that it stems from difficulties with basic aspects of language processing, rather than a cognitive style of decreased context sensitivity specific to ASD.
An additional objective was to test context sensitivity for oral language specifically and to disentangle methodological factors, especially priming effects, which might have impacted previous results. In both studies well-designed experimental tasks were created by using empirically based data, e.g. word frequencies, and a relatively high item number. Furthermore, while many prior studies used scores from verbal scales in larger intelligence assessments or only measures of receptive vocabulary, both experiments here employed comprehensive standardized language measures. Given that both experiments strictly excluded lexical associates from stimulus materials it is unlikely that subtle lexical priming effects, rather than true integration of sentence context, contributed to task success. However, a couple of limitations should be noted. Above all, the two experiments were not completely parallel and were conducted in different countries as parts of different larger investigations. While the first experiment used pictures in a computerized task and gathered response times, the second experiment did not use pictorial stimuli and required both receptive and expressive language abilities, although the language production task demands were low since children could complete in a simple way, even with single words. In addition, the studies investigated a particular age range and used a very structured linguistic task format. Therefore, generalization to other age ranges and more natural communication situations is limited. Having taken these limitations into account, the remarkably consistent findings from two different language samples, investigating almost 50 children with ASD, argue against the assumption of reduced context sensitivity as a universal and unique factor underlying language processing in ASD.
Future studies should build upon the present evidence to further define language phenotypes based on structural language abilities within the autism spectrum. Interestingly, similar to our results, recent outcomes of reading research show that children with ASD who score lower on structural language measures are those who struggle with reading comprehension (e.g., Norbury and Nation, 2011). The evidence from oral and written language processing should be linked to neuroscientific findings. Sharda, Khundrakpam, Evans and Singh (2014) investigated connections between brain regions in the fronto-temporal regions of children with ASD and controls by using a new approach called structural covariance networks (SCN). Their results indicated that disruptions were modulated by verbal abilities rather than by ASD diagnosis per se, further substantiating the pattern of the behavioral findings discussed here.
Furthermore, longitudinal studies would shed light on developmental language trajectories of different language phenotypes. Lombardo et al. (2015) recently investigated neurobiological factors underlying different early language trajectories in ASD. They tested 1- to 4-year-olds with ASD, with non-ASD language or developmental delay, or who were developing typically and identified atypical activation in pre-diagnosis fMRI response to speech, specifically in children with ASD who went on to have poor language outcomes (defined as −1 SD in the receptive and expressive language scores on the Mullen Scale of Early Learning). Thus, early neural markers allow for ASD subgroup prognosis. Further studies like these with different age ranges are highly important for more homogenous samples in research as well as clinical questions of treatment success (see also Grzadzinski, Huerta, & Lord, 2013).
Additional work should also investigate potential relations between language and cognitive factors, like central coherence in auditory processing and theory of mind, as well as between different cognitive factors in children with ASD with and without LI as well as children with primary LI (e.g. Taylor, Maybery, Grayndler, & Whitehouse, 2014).
Although delays in language development are no longer part of ASD diagnosis according to the DSM-5 (APA, 2013), our finding that structural language ability is the strongest predictor of children’s ability to process language with respect to semantic context highlights the need for comprehensive assessment of language in research as well as clinical settings.
What this paper adds?
An influential view of cognition in ASD, the weak central coherence account (WCC), proposes a cognitive style focused on details. In the domain of language processing WCC predicts a reduced integration of linguistic and contextual information. This is a commonly held assumption in research as well as in clinical work, although findings are mixed and the possible influence of structural language ability on context sensitivity has been raised. The novel contribution of this paper is to directly test whether reduced sensitivity to context in language processing is universal and unique to children with ASD. This was done in two experiments using different methods of investigating the processing of ambiguous words (homonyms) by relying on sentence context, with distinct samples of participants tested in different languages. The results are remarkably consistent, showing that reduced context sensitivity is neither universal (children with ASD and normal language abilities do not exhibit this, Exp. 1) nor unique (children with Language Impairment do exhibit this characteristic) to children with ASD. Instead, regression models demonstrated that only structural language abilities predicted context sensitivity. These experiments are also novel in controlling for important aspects of stimuli to rule out alternative methods of arriving at context-sensitive responses. Taken together these findings add to growing evidence that reduced context sensitivity in language processing is linked to low structural language abilities across neurodevelopmental disorders, rather than being a universal feature of ASD.
Acknowledgments
This article was supported by an award to M. Eberhardt from the German Academic Exchange Service (DAAD) and was initiated during a postdoctoral fellowship supervised by A. Nadig at McGill University.
Experiment 1 was funded by a United States NRSA Individual postdoctoral award NIDCD F32-DC007297 to A. Nadig, and was conducted with the support of her postdoctoral supervisor Dr. Sally Ozonoff.
Experiment 2 was completed in partial fulfillment of a PhD awarded to M. Eberhardt and was funded by a doctoral grant from the Foundation of German Business. It was conducted with the support of her doctoral supervisor Dr. Susanne Nussbeck. Special thanks also to Ronda Booth for information on coding in their study with a sentence completion task.
We thank all therapy centers, schools, parents and children who participated in this research, as well as the students who assisted with data collection.
Appendix A. Homonym Stimuli
Table A1.
Homonyms Experiment 1.
| Arms | Cell | Hood | Pen | Seal |
| Bank | Chest | Kid | Pitcher | Star |
| Bar | Crane | Letters | Plug | Straw |
| Bat | Diamond | Nut | Pool | Table |
| Bulb | Fan | Organ | Ruler | Tablet |
Table A2.
Homonyms Experiment 2 (original, in German).
| Birne | Karte |
| Bogen | Maus |
| Boxer | Noten |
| Fliege | Schlange |
| Flügel | Schloss |
| Futter | Stück |
Footnotes
A regression model conducted with only the ASD-NL and TYP groups had the same result. As a continuous measure of autism symptoms (the Social Communication Questionnaire, SCQ) was available for all participants in Experiment 1, we also conducted the regression with SCQ scores in place of the dichotomous autism diagnosis variable. Once again the same pattern of results emerged.
Items and examples are translated from German.
References
- APA. Diagnostic and statistical manual of mental disorders: DSM-5. 5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- Beveridge M, Marsh L. The influence of linguistic context on young children’s understanding of homophonic words. Journal of Child Language. 1991;18(02):459–467. doi: 10.1017/s0305000900011168. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Uncommon understanding: Development and disorders of language comprehension in children. Hove: Psychology Press; 1997. [Google Scholar]
- Booth R, Happé F. Hunting with a knife and . . . fork”: Examining central coherence in autism, attention deficit/hyperactivity disorder, and typical development with a linguistic task. Journal of Experimental Child Psychology. 2010;107(4):377–393. doi: 10.1016/j.jecp.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J, Norbury C, Einav S, Nation K. Do individuals with autism process words in context? Evidence from language-mediated eye-movements. Cognition. 2008;108(3):896–904. doi: 10.1016/j.cognition.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Bulheller S, Häcker H. PPVT Peabody Picture Vocabulary Test. Deutschsprachige Fassung des PPVT-III für Jugendliche und Erwachsene von L. M. Dunn & L. M. Dunn: Manual. Frankfurt am Main: Zwets & Zeitlinger; 2003. [Google Scholar]
- Clegg J, Hollis C, Mawhood L, Rutter M. Developmental language disorders—A follow-up in later adult life. Cognitive, language and psychosocial outcomes. Journal of Child Psychology and Psychiatry. 2005;46(2):128–149. doi: 10.1111/j.1469-7610.2004.00342.x. [DOI] [PubMed] [Google Scholar]
- Eberhardt M, Nussbeck S. Timo browses the Internet. He grabs the mouse and . . .?—A Study of Language Comprehension in Children with Autism. Forschung Sprache. 2015;1:3–17. [Google Scholar]
- Eberhardt M. Doctoral dissertation. 1. Marburg: Tectum; 2014. Autismus und Sprache: Wörter, Sätze und Gespräche verstehen. [Google Scholar]
- Fox AV. Das Gesundheitsforum. 4. Idstein: Schulz-Kirchner; 2009. TROG-D Test zur Überprüfung des Grammatikverständnisses: Handbuch. [Google Scholar]
- Frith U. Autism: Explaining the enigma. Oxford: Blackwell; 1989. [Google Scholar]
- Frith U, Snowling M. Reading for meaning and reading for sound in autistic and dyslexic children. British Journal of Developmental Psychology. 1983;(1):329–342. [Google Scholar]
- Gawlick-Grendell LA, Woltz DJ. Meaning dominance norms for 120 homographs. Behavior Research Methods, Instruments, & Computers. 1994;26(1):5–25. [Google Scholar]
- Gernsbacher MA, Pripas-Kapit SR. Who’s Missing the Point?. A Commentary on Claims that Autistic Persons Have a Specific Deficit in Figurative Language Comprehension. Metaphor and Symbol. 2012;27(1):93–105. doi: 10.1080/10926488.2012.656255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm H, Schöler H. HSET Heidelberger Sprachentwicklungstest: Handanweisung. 2. Göttingen: Hogrefe; 1991. [Google Scholar]
- Grzadzinski R, Huerta M, Lord C. DSM-5 and autism spectrum disorders (ASDs): An opportunity for identifying ASD subtypes. Molecular Autism. 2013;4(1):12. doi: 10.1186/2040-2392-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hala S, Pexman PM, Glenwright M. Priming the meaning of homographs in typically developing children and children with autism. Journal of Autism and Developmental Disorders. 2007;37(2):329–340. doi: 10.1007/s10803-006-0162-6. [DOI] [PubMed] [Google Scholar]
- Happé F. Central coherence and theory of mind in autism: Reading homographs in context. British Journal of Developmental Psychology. 1997;15(1):1–12. [Google Scholar]
- Happé F, Booth R. The power of the positive: Revisiting weak coherence in autism spectrum disorders. Quarterly Journal of Experimental Psychology. 2008;61(1):50–63. doi: 10.1080/17470210701508731. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The Weak Coherence Account: Detail-focused Cognitive Style in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Heller KA, Kratzmeier H, Lengfelder A. Matrizen-Test-Manual, Band 1: Ein Handbuch mit deutschen Normen zu den Standard Progressive Matrices von J.C. Raven. Göttingen: Beltz Test; 1998. [Google Scholar]
- Hoy JA, Hatton C, Hare D. Weak central coherence: A cross-domain phenomenon specific to autism? Autism. 2004;8(3):267–281. doi: 10.1177/1362361304045218. [DOI] [PubMed] [Google Scholar]
- Hudry K, Leadbitter K, Temple K, Slonims V, McConachie H, Aldred C, et al. Preschoolers with autism show greater impairment in receptive compared with expressive language abilities. International Journal of Language and Communication Disorders. 2010;45(6):681–690. doi: 10.3109/13682820903461493. [DOI] [PubMed] [Google Scholar]
- Jolliffe T, Baron-Cohen S. A test of central coherence theory: Linguistic processing in high-functioning adults with autism or Asperger syndrome: Is local coherence impaired? Cognition. 1999;71(2):149–185. doi: 10.1016/s0010-0277(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16(2):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellmer L, Hedvall A, Holm A, Fernell E, Gillberg C, Norrelgen F. Language comprehension in preschoolers with autism spectrum disorders without intellectual disability: Use of the Reynell Developmental Language Scales. Research in Autism Spectrum Disorders. 2012;6(3):1119–1125. [Google Scholar]
- Kwok EYL, Brown HM, Smyth RE, Oram CJ. Meta-analysis of receptive and expressive language skills in autism spectrum disorder. Research in Autism Spectrum Disorders. 2015;9:202–222. [Google Scholar]
- Lombardo MV, Pierce K, Eyler LT, Barnes CC, Ahrens-Barbeau C, Solso S, et al. Different functional neural substrates for good and poor language outcome in autism. Neuron. 2015;86(2):567–577. doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López B, Leekam SR. Do children with autism fail to process information in context? Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44(2):285–300. doi: 10.1111/1469-7610.00121. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Pam DiLavore, Susan Risi. Autism diagnostic observation schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Loukusa S, Mäkinen L, Kuusikko-Gauffin S, Ebeling H, Moilanen I. Theory of mind and emotion recognition skills in children with specific language impairment, autism spectrum disorder and typical development: Group differences and connection to knowledge of grammatical morphology, word-finding abilities and verbal working memory. International Journal of Language and Communication Disorders. 2014;49(4):498–507. doi: 10.1111/1460-6984.12091. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Pearlmutter NJ, Seidenberg MS. The lexical nature of syntactic ambiguity resolution. Psychological Review. 1994;101(4):676–703. doi: 10.1037/0033-295x.101.4.676. [DOI] [PubMed] [Google Scholar]
- Mawhood L, Howlin P. Autism and developmental receptive language disorder—A comparative follow-up in early adult life. I: Cognitive and language outcomes. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2000;41(5):547. doi: 10.1111/1469-7610.00642. [DOI] [PubMed] [Google Scholar]
- Nadig A, Vivanti G, Ozonoff S. Adaptation of object descriptions to a partner under increasing communicative demands: A comparison of children with and without autism. Autism Research. 2009;2(6):334–347. doi: 10.1002/aur.102. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. Retrieved from 〈 http://www.usf.edu/FreeAssociation/〉. [DOI] [PubMed]
- Norbury CF. Barking up the wrong tree? Lexical ambiguity resolution in children with language impairments and autistic spectrum disorders. Journal of Experimental Child Psychology. 2005;90(2):142–171. doi: 10.1016/j.jecp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Norbury CF, Bishop DVM. Inferential processing and story recall in children with communication problems: a comparison of specific language impairment, pragmatic language impairment and high-functioning autism. International Journal of Language and Communication Disorders. 2002;37(3):227–251. doi: 10.1080/13682820210136269. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nation K. Understanding Variability in Reading Comprehension in Adolescents With Autism Spectrum Disorders: Interactions With Language Status and Decoding Skill. Scientific Studies of Reading. 2011;15(3):191–210. [Google Scholar]
- Rajendran G, Mitchell P. Cognitive theories of autism. Developmental Review. 2007;27(2):224–260. [Google Scholar]
- Riches NG, Loucas T, Baird G, Charman T, Simonoff E. Elephants in pyjamas: Testing the weak central coherence account of autism spectrum disorders using a syntactic disambiguation task. Journal of Autism and Developmental Disorders. 2016;46(1):155–163. doi: 10.1007/s10803-015-2560-0. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Berument S, Lord C, Pickles A. Social communication questionnaire (SCQ) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical evaluation of language fundamentals. 4. San Antonio: Psychological Corporation/Harcourt Assessment; 2003. CELF-4. [Google Scholar]
- Sharda M, Khundrakpam B, Evans A, Singh N. Disruption of structural covariance networks for language in autism is modulated by verbal ability. Brain Structure and Function. 2014:1–16. doi: 10.1007/s00429-014-0953-z. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):221–233. doi: 10.1002/mrdd.20046. [DOI] [PubMed] [Google Scholar]
- Simpson GB. Context and the processing of ambiguous words. In: Gernsbacher MA, editor. Handbook of psycholinguistics. San Diego: Academic Press; 1994. pp. 359–374. [Google Scholar]
- Snowling M, Frith U. Comprehension in “hyperlexic” readers. Journal of Experimental Child Psychology. 1986;42(3):392–415. doi: 10.1016/0022-0965(86)90033-0. [DOI] [PubMed] [Google Scholar]
- Swaab T, Brown C, Hagoort P. Understanding words in sentence contexts: The time course of ambiguity resolution: Understanding Language. Brain and Language. 2003;86(2):326–343. doi: 10.1016/s0093-934x(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Taylor L, Maybery M, Grayndler L, Whitehouse AO. Evidence for distinct cognitive profiles in autism spectrum disorders and specific language impairment. Journal of Autism and Developmental Disorders. 2014;44(1):19–30. doi: 10.1007/s10803-013-1847-2. [DOI] [PubMed] [Google Scholar]
- Toppelberg CO, Shapiro T. Language disorders: A 10-year research update review. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(2):143–152. doi: 10.1097/00004583-200002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twilley LC, Dixon P, Taylor D, Clark K. University of Alberta norms of relative meaning frequency for 566 homographs. Memory & Cognition. 1994;22(1):111–126. doi: 10.3758/bf03202766. [DOI] [PubMed] [Google Scholar]
- University Leipzig. Wortschatzportal. 2009 Retrieved from 〈 http://wortschatz.uni-leipzig.de/〉.
- Wechsler D. Wechsler Abbreviated Scale of Intelligence, WASI. San Antonio: Psychological Corporation/Harcourt Assessment; 1999. [Google Scholar]