Abstract
Background
After hematopoietic cell transplantation (HCT), polyoma-BK virus is associated with hemorrhagic cystitis and also with polyomavirus nephropathy (PVN). However, the true burden of post-HCT PVN is unknown because kidney biopsies are avoided due to their bleeding risk. The novel, non-invasive urinary PV-Haufen test detects PVN in kidney transplant recipients with >95% positive/negative predictive values. We hypothesized that the detection of PV-Haufen in voided urine samples–a positive PV-Haufen test–was also clinically significant after HCT.
Methods
We examined 21 suitable urine samples from 14 patients (median age 15 years, 71.4% male) who were selected from repositories for having varying degrees of BK-viremia (range 0-1.0×108 copies/mL), hemorrhagic cystitis (present/absent) and data on kidney function. Urine samples were obtained a median of 88 days post-HCT.
Results
PV-Haufen were detected in 5/14 patient (35.7%) and 7/21 (33.3%) urine samples, with histologic confirmation of PVN in one autopsy specimen. After a median of 285 days post-HCT, patients with PV-Haufen had an increased risk of dialysis-dependent renal failure (p<0.05). All three dialysis-dependent patients had PV-Haufen and died. The presence of urinary PV-Haufen was not significantly correlated with hemorrhagic cystitis. From the 16 urines collected during BK-viremia, 43.8% were PV-Haufen positive and 56.2% negative. PV-Haufen were not present in the five urines from patients without concomitant BK-viremia.
Conclusions
In this proof-of-concept study, a positive PV-Haufen test was only seen in some patients with BK-viremia, and was not associated with hemorrhagic cystitis. The detection of PV-Haufen suggests underlying PVN with an increased risk of kidney failure and dialysis.
INTRODUCTION
Chronic kidney disease (CKD) occurs in 20% of children after hematopoietic cell transplantation (HCT), and in most cases, an underlying cause is not apparent.1 Understanding the mechanisms for CKD post-HCT could establish therapeutic targets and improve survival.2
One potential cause of CKD is polyomavirus nephropathy (PVN). After kidney transplantation, PVN is seen in approximately 5% of patients and can lead to chronic allograft failure and graft loss in up to 50% of cases.3 After HCT, polyoma-BK virus is often associated with hemorrhagic cystitis4, 5 and in sporadic cases with PVN, as documented in a few case reports.6-8 However, the true incidence of PVN post-HCT and its impact on CKD is unknown because kidney biopsies, the traditional method for establishing a definitive diagnosis,3, 9-11 are often avoided due to the high risk of bleeding.12 Thus, the question remains, does PVN contribute to CKD after HCT?
Laboratory tests, especially quantitative polymerase chain reaction (PCR) assays in the blood (viremia) or urine (viruria), are commonly used to assess immunosuppressed patients for the activation and replication of BK virus and their risk for viral disease. These PCR-based screening protocols are well established in kidney transplant recipients in the context of PVN.13-15 However, similar to other latency-establishing DNA viruses such as CMV, the PCR-based detection of viral replication is insufficient for diagnosing disease, including PVN. For example, BK-viremia occurs in approximately 30% of kidney transplant recipients but only a subgroup of these viremic individuals develop PVN, which is why diagnostic confirmation of “definitive” PVN is traditionally established by renal biopsy.3, 9-11, 16 After HCT, BK-viremia is common and associated with hemorrhagic cystitis,4, 17 but in this patient population, the association between viremia and PVN is undetermined.
Recently, a novel, non-invasive urine-based test has been described in kidney transplant recipients to diagnose PVN accurately with >95% positive and negative predictive values. This assay was named the urinary PV-Haufen test.18 The rationale and significance of urinary PV-Haufen as biomarkers for PVN differs from conventional approaches. The PV-Haufen test uses electron microscopy (EM) to detect dense, cast-like PV-aggregates in voided urine samples. PV-Haufen form in virally injured renal tubules in PVN, are flushed out of the kidneys, and appear in the urine as direct markers of intra-renal viral disease.18-21 Thus, the urinary PV-Haufen test offers a more specific approach to diagnose intra-renal PVN that appears to be superior to conventional PCR-based assays. Our objective in this small, observational, non-intervention pilot study was to determine the clinical significance of urinary PV-Haufen shedding post-HCT: Is PVN based on a positive urinary PV-Haufen test seen in these patients and is PVN a potential cause for kidney failure and morbidity?
MATERIALS and METHODS
Study population and sample selection
We conducted a case-control study to evaluate the presence of urinary PV-Haufen in children and young adults after allogeneic HCT. Patients enrolled in ongoing, prospective specimen repositories at the Children's Hospital of Philadelphia (CHOP) and Cincinnati Children's Hospital Medical Center (CCHMC) had urine stored for later testing. For this pilot study, patients were selected if they had available urine samples fixed in paraformaldehyde and varying degrees of BK-viremia (by PCR), data on renal function and data on the presence or absence of hemorrhagic cystitis. Clinical information was recorded from April 2012-September 2014, representing a time period where subjects had been enrolled into the repository studies. All participants gave written informed consent to use their samples and review their clinical information and the Institutional Review Boards at CHOP and CCHMC approved the research.
Detection of PV-Haufen
Urine samples (10-25 ml) were collected, fixed in a 1:1 ratio with 4% neutral buffered paraformaldehyde, and stored at 4 degrees C. PV-Haufen testing was performed at the University of North Carolina according to detailed previous descriptions using conventional negative staining EM.20 Briefly, PV-Haufen are defined as three-dimensional, dense, cast-like, viral aggregates composed of six or more virions (Figure 1).18-20 To search for PV-Haufen, urine samples were clarified, concentrated, and placed on an EM grid where the PV-Haufen adhere by electrostatic binding.20 One EM grid was prepared for each patient's urine sample and examined by transmission EM (LEO EM-910, LEO Electron Microscopy, Thornwood, NY, accelerating voltage, 90-100 kV at 80,000-100,000x magnification) for a maximum of 30 minutes, according to previous descriptions.18 The examining pathologist was blinded to each patient's clinical information.
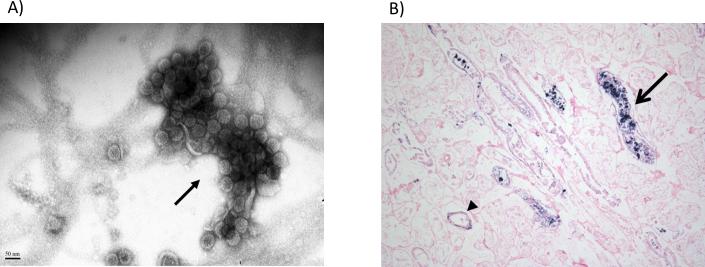
Figure 1. Polyomavirus nephropathy after hematopoietic cell transplant.
A) By routine electron microscopy (negative staining), a large polyomavirus Haufen (resembling a cast, arrow) is detected in a voided urine sample from a hematopoietic cell transplant recipient. B) Kidney autopsy specimen from a hematopoietic cell transplant recipient with detectable urinary polyomavirus Haufen. Tissue stained for BK virus using DNA in situ hybridization. Although the tissue is autolyzed, kidney tubules (arrow head) and tubular lumens (arrow) show positive staining for BK virus infected cells, defining polyomavirus nephropathy.
21 urine samples from 14 patients were included into the study; three urine samples with marked degradation were excluded. Two patients underwent autopsy two and five weeks after the last urinary PV-Haufen testing, respectively. No kidney tissue was available from the remaining 12 patients. Study results were qualitatively reported as either PV-Haufen present (one or more) or as PV-Haufen not detected/absent; quantitation of PV-Haufen shedding (number of PV-Haufen/mL of urine) was also reported, as previously described.20
Clinical and laboratory data
We included clinical and laboratory data for each patient from the start of transplant until the end of follow-up (September 30, 2014) or patient death, whichever came first. Clinical data included demographics, transplant characteristics, treatment for BK virus infection, concomitant viral infections, development of acute graft versus host disease (GVHD), hemorrhagic cystitis, and kidney failure requiring dialysis. Hemorrhagic cystitis was defined as gross hematuria.17
Laboratory information obtained as part of clinical care (serum creatinine and BK virus PCR plasma testing) was recorded. At CCHMC, BK virus PCR screening was performed routinely in all patients, while at CHOP, BK virus PCR testing was done only for clinical indication. Creatinine-estimated glomerular filtration rate (eGFR) was calculated with the bedside Chronic Kidney Disease in Children (CKID) formula where eGFR (ml/min/1.73m2) = (0.413*baseline height (cm)/serum creatinine).22
Outcomes and statistical analyses
Continuous data were reported as medians (interquartile ranges (IQR) and categorical data as proportions. Clinical outcomes between patients with and without detectable PV-Haufen were compared using Wilcoxon rank-sum for continuous variables (eGFR) and Fisher exact test for categorical variables (hemorrhagic cystitis, need for dialysis, or death). Analyses were conducted using STATA software (version 12, College Station, Texas). A two-sided p-value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
Fourteen patients with 21 available urine samples were included into this pilot study. Seven patients had two separate urine samples tested for PV-Haufen and the remaining seven patients had one sample each. The median age of the patients was 15 years (IQR 10.8-25.5 years) and 71.4% were male. Most of the patients underwent HCT for an indication of malignancy and received myeloablative conditioning which included cyclophosphamide. All patients received GVHD prophylaxis with cyclosporine. Patients were followed for a median of 285 days after transplant (IQR 162-396 days, range 87-815 days); clinical characteristics are shown in supplemental Table 1.
Urinary PV-Haufen test results
The PV-Haufen results are shown in Table 1. PV-Haufen were detected in 5/14 patients (35.7%) and 7/21 (33.3%) urine samples with quantitative assays ranging from 56 PV-Haufen/mL to 4233/mL urine. Urine samples had been obtained a median of 88 days (IQR 31-122 days, range 20-799 days) after HCT. In the seven patients with repeat urine testing, two patients had detectable PV-Haufen in both samples (PV-Haufen shedding persisted for 84 and 267 days), two patients had PV-Haufen in only one out of two samples (PV-Haufen appeared 282 days after a previous negative sample in one patient and PV-Haufen were no longer detected after 28 days in the second patient) and three patients were negative for PV-Haufen in two samples tested (separated by 32, 33, and 70 days).
Table 1.
PV-Haufen results and clinical data for 14 children and young adults after allogeneic hematopoietic cell transplant (HCT)
| ID | PV-Haufen testing day post-HCT | PV-Haufen POS/Neg, #Haufen/mL urine | Blood BK PCR at PV-Haufen testing (copies/mL) | Creatinine-eGFR (mL/min/1.73m2) | Hemorrhagic cystitis at time of PV-Haufen testing | Radiographic evidence of obstruction | BK treatment | Status at last follow up | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-HCT | At PV-Haufen testing | ||||||||
| 1 | 126 | POS, 225 | 1,206,325 | 95 | 109 | Yes | Yes, mild | cidof, cipro | Dialysis, then died of acute GVHD/fungal infection |
| 2 | 27 | POS, 507 | 29,620 | 102 | 89 | Yes | n/a | cidof | Dialysis, then died of GVHD/multiple infections†, autopsy without polyomavirus nephropathy |
| 111 | POS, 56 | 245,504 | 89 | Yes | Yes, moderate | ||||
| 3 | 60 | POS, 507 | 43,520 | 131 | 164 | Yes | No | cidof | Alive |
| 88 | Neg | 6,174 | 131 | Yes | |||||
| 4 | 24 | Neg | 26,907 | 121 | 147 | Yes | n/a | cidof | Alive |
| 306 | POS, 733 | 87,759,086 | 56 | No | |||||
| 5 | 56 | Neg | 209,537 | 116 | 69 | Yes | Yes, mild | cidof, cipro | Died, fungal infection |
| 88 | Neg | 1,863,356 | 44 | Yes | |||||
| 6 | 56 | Neg | 1,150,961 | 164 | 24 | Yes | Yes, moderate | cidof, cipro, leflun | Alive |
| 89 | Neg | 2,362 | 48 | Yes | Resolved | ||||
| 7 | 20 | Neg | 1537 | 106 | 123 | Yes | n/a | cidof | Alive |
| 90 | Neg | 0 | 123 | No | No | ||||
| 8 | 117 | Neg | 607,463 | 142 | 122 | Yes | Yes, mild | cidof, cipro | Alive |
| 9 | 122 | Neg | 26,959 | 112 | 72 | Yes | n/a | cidof, cipro, leflun | Alive |
| 10 | 532 | POS, 4233 | 100,405,579 | 117 | 41 | No | n/a | cidof | Dialysis, then died of mycobacterial infection, autopsy with polyomavirus nephropathy |
| 799 | POS, 1128 | 99,389,429 | 13 | No | No | ||||
| 11 | 167 | Neg | 0* | 132 | 44 | No | No | cidof, cipro | Alive |
| 12 | 31 | Neg | 0 | 218 | 144 | No | n/a | none | Died, acute GVHD/bowel perforation |
| 13 | 26 | Neg | 0 | 203 | 196 | No | n/a | none | Alive |
| 14 | 29 | Neg | 0 | 94 | 116 | No | n/a | none | Alive |
PV, polyomavirus; POS, positive, PCR, polymerase chain reaction; eGFR, estimated glomerular filtration rate; GVHD, graft versus host disease n/a, not available; cidof, cidofovir; cipro, ciprofloxacin; leflun, leflunomide.
Patient 11 had 4 months of BK viremia >10,000 copies/mL with concomitant hemorrhagic cystitis, but resolved BK viremia and cystitis at the time of PV-Haufen testing.
Autopsy positive for CMV, HHV-6, and adenovirus (liver) and adenovirus and Candida albicans (lung).
BK-viremia was detected at 16/21 time points when urine was collected (median blood BK PCR of 227,520 copies/mL, IQR 26,933-1,534,841 copies/mL). From these 16 urine samples collected during BK-viremia, 7/16 (43.8%) were PV-Haufen positive (median blood BK PCR of 1,206,325 copies/mL, IQR 43,520-1×108 copies/mL) and the remaining 9/16 (56.2%) were negative for PV-Haufen (median blood BK PCR of 26,959 copies/mL, IQR 6174-607,463 copies/mL). PV-Haufen were not present in the five urine samples obtained from patients without concomitant BK-viremia.
Association between qualitative PV-Haufen test results and clinical outcomes
Of the 14 patients, 10 (71.4%) developed hemorrhagic cystitis at a median of 27.5 days (IQR 20-33 days) after transplant. Among the 10 patients with hemorrhagic cystitis, four (40.0%) had detectable PV-Haufen and six (60.0%) did not (p>0.99) at any point during observation.
All 14 patients had normal kidney function prior to HCT with a median creatinine-eGFR of 118.9 ml/min/1.73m2 (IQR 105.6-142.1 ml/min/1.73m2). At last follow-up, immediately before dialysis initiation, or prior to death, the five patients with detectable PV-Haufen had a median creatinine-eGFR of 58.3 ml/min/1.73m2 (IQR 10.6-82.0 ml/min/1.73m2) and the nine patients without detectable PV-Haufen had a median creatinine-eGFR of 73.9 ml/min/1.73m2 (IQR 59.6-105.6 ml/min/1.73m2, p=0.16).
Three patients (21.4%) developed dialysis-dependent kidney failure at a median of 153 days (IQR 130-796 days) after transplant. All three patients who received dialysis had detectable PV-Haufen while none of the nine patients without PV-Haufen required dialysis (p<0.05). A total of five patients (35.7%) died at a median of 162 days (IQR 150-264 days) after HCT. Among the patients who died, 3/5 (60.0%) had detectable PV-Haufen compared to 2/9 (22.2%) surviving patients with a positive PV-Haufen test (p=0.27).
PV-Haufen results in the two patients undergoing autopsy (Figures 1 and 2)
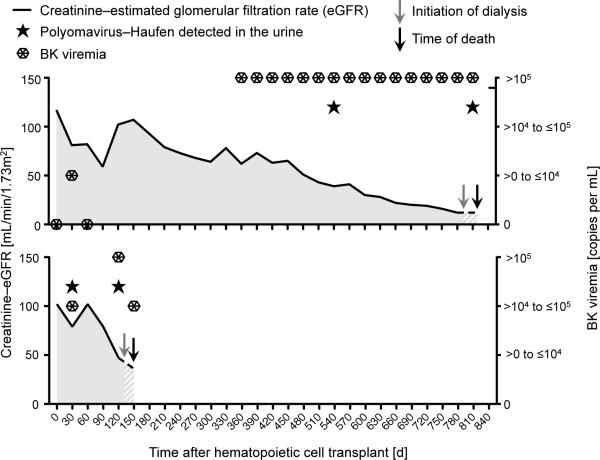
Figure 2. Polyomavirus-Haufen detection in the urine, BK-viremia, and kidney function in the two hematopoietic cell transplant recipients who underwent autopsy.
Both patient number 10 (top panel) and patient number 2 (bottom panel) had persistent BK-viremia and a decreasing creatinine-estimated glomerular filtration rate concomitant with the detection of polyomavirus-Haufen in the urine.
Patient number 10 had abundant PV-Haufen shedding at 532 days after HCT (4233 PV-Haufen/mL of urine) which persisted for 267 days (1128 PV-Haufen/mL urine at the time of second urine testing). The patient had histologic evidence of PVN at autopsy two weeks after last testing (Figure 1). The second patient (patient number 2) had markedly decreasing urinary PV-Haufen numbers during follow-up: first urine testing 27 days post-HCT with 507 PV-Haufen/mL; subsequent urine sampling 84 days later with 56 PV-Haufen/mL urine. About five weeks after the second test, no histologic evidence of PVN was seen at autopsy.
DISCUSSION
Cast-like polyomavirus aggregates in voided urine samples, termed PV-Haufen, have been described as highly specific, non-invasive diagnostic biomarkers for PVN in kidney transplant recipients.10, 18, 20 The underlying concept–the detection of casts in voided urine samples as markers of intra-renal disease–is well established with red blood cell casts, for example, marking glomerulonephritis. Analogously, studies suggest that cast-like PV-Haufen form in PVN in virally injured renal tubules in the presence of high uromodulin concentrations, similar to other casts.21 Singh et al tested for PV-Haufen in urine samples from a large cohort of patients without clinical or biopsy evidence of PVN and 21 primarily kidney transplant recipients with biopsy-proven PVN. The study included one PV-Haufen positive HCT recipient with biopsy-proven PVN and 15 HCT controls that were PV-Haufen negative; seven patients presented with hemorrhagic cystitis. In Singh's study, PV-Haufen as biomarkers for diagnosing PVN had a 97% positive and 100% negative predictive value.18
After kidney transplantation, urinary PV-Haufen shedding follows a dynamic pattern closely reflecting the course of PVN.18 Furthermore, quantitative urinary PV-Haufen testing predicts intra-renal disease severity and the histologic PVN stages.20 Thus, urinary PV-Haufen reflect PVN and not lower urinary tract events. The objective of this pilot analysis was to determine whether urinary PV-Haufen testing could also be of diagnostic value after allogeneic HCT since the incidence of PVN, and its potential impact on renal failure in this patient cohort, is unknown.
In the current proof-of-concept study, urinary PV-Haufen testing post-HCT showed findings very similar to those previously described after renal transplantation.18, 20 The assay was feasible to perform according to established protocols.20 We found that PVN, based on the presence of PV-Haufen in the urine, was more common after HCT than previously thought, affecting one third of our study cohort. Urinary PV-Haufen were present in only a subgroup of patients with BK-viremia and not associated with hemorrhagic cystitis. Patients with detectable PV-Haufen had evidence of CKD and an increased risk for dialysis-dependent renal failure.
Close associations between urinary PV-Haufen shedding and renal histology were reported previously in kidney transplant recipients;18 such analyses are largely lacking in our HCT cohort due to the nature of the underlying disease preventing invasive diagnostic interventions and the collection of biopsies. Tissue specimens were only available from two patients that support our overall interpretation and analysis. One patient presented with abundant PV-Haufen shedding and histologic evidence of PVN at time of autopsy conducted shortly after testing. The second patient presented with minimal PV-Haufen shedding and no evidence of PVN at time of autopsy four weeks post-testing. We interpret findings made in the second patient not as contradictory but rather as expected and confirmatory since low PV-Haufen counts in the urine suggest low grade PVN with minimal productive intra-renal BK replication that resolved over four weeks until autopsy was conducted.20 Rapid resolution of PVN has been previously noted.18
Some patients in our pilot study demonstrated a mildly positive PV-Haufen test with only low level PV-Haufen shedding. If data collected in renal allograft recipients can be extrapolated to patients post-HCT, then this observation reflects a focal stage of PVN20 that might have good outcome and respond favorably to therapeutic interventions. Future studies are needed to further investigate this aspect.
Conventional PCR tests targeting BK virus gene sequences in urine or blood are often difficult to interpret. Overall, BK-viruria has a low positive predictive value for PVN or bladder injury in both kidney transplant and HCT recipients,17, 18, 23 as recently reviewed.10 The kidney transplant literature suggests that BK-viremia, especially if persistent and/or high grade (>10,000 copies/mL), is “practically” synonymous with intrinsic kidney injury as virally-damaged tubules leak BK viral particles into the blood, where they are detected by PCR.9, 10, 16, 24, 25 However, whether this concept is, indeed, universally true and valid post-HCT is doubtful. For example, BK-viremia is not specific to kidney injury and may originate from extra-renal anatomic sites, such as the bladder (during hemorrhagic cystitis) or even salivary glands.23, 26 Post-kidney transplantation, only a subgroup of patients with BK-viremia will develop definitive, biopsy-proven disease (PVN) while most viremic episodes appear to be self-limiting, without urinary PV-Haufen shedding and without any clinical significance. In cases of PVN the level of BK-viremia by PCR only imperfectly reflects the severity of intra-renal disease.20 Last but not least, clinicians managing patients post-HCT usually interpret BK-viremia as a risk factor for hemorrhagic cystitis4, 5 and less for PVN. Thus BK-viremia does not necessarily equal “intra-renal” PVN.
In contrast to PCR-based BK assays, urinary PV-Haufen testing adds a new perspective to non-invasively and accurately identify patients with PVN, some of them presenting with concurrent hemorrhagic cystitis post-HCT (Table 1 and Figure 3). Since invasive procedures such as renal biopsies are often contraindicated after HCT due to the high bleeding risk, urinary PV-Haufen testing becomes particularly significant. From a clinical perspective, PV-Haufen testing is relatively inexpensive (approximately US$300), has a turnaround time of a few hours, and can be performed in any laboratory with EM capability.18, 19 Accordingly, we hope that other investigators can learn to detect PV-Haufen at their laboratories and confirm our single-center observations.
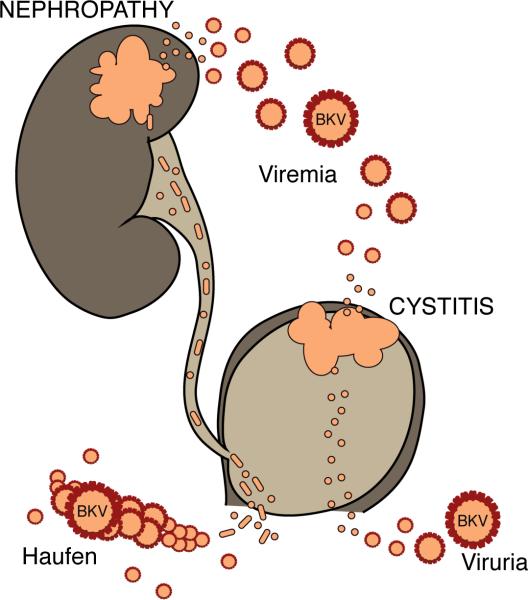
Figure 3. Conceptual model showing anatomical sources of BK virus infection after hematopoietic cell transplantation.
In patients with hemorrhagic cystitis, bladder injury leads to release of viral particles in the urine (viruria) and blood (viremia). In this scenario, kidney function can be decreased if the bladder injury leads to urinary obstruction, but there is no intrinsic renal injury from BK virus/no polyomavirus nephropathy (PVN), and therefore urinary PV-Haufen cannot be detected. In patients with PVN, kidney tubular injury can lead to release of viral particles in the urine (viruria) and blood (viremia). Cast-like PV-Haufen form in virally injured renal tubules and can be detected in voided urine samples as specific biomarkers for “intra-renal” PVN.
Other than lowering immunosuppression, there is limited information on the optimal treatment for BK virus infection,27 and evidence is lacking that fluoroquinolones or leflunomide are effective agents against BK virus.28, 29 Intravenous cidofovir is associated with tubular toxicity and has not been thoroughly tested in PVN. New oral formulations of cidofovir are potentially less nephrotoxic but currently of undetermined effectiveness.27 In this context, urinary PV-Haufen testing may also help in future therapeutic trials, such as reserving potentially toxic treatment (i.e. cidofovir) to those patients with positive PV-Haufen tests suggesting underlying PVN and avoiding it in those with negative test results.
Our observations are limited by the case-control analysis of pre-selected patients with relatively few data points. We did not have longitudinally collected samples in our patients; future research is needed to test for urinary PV-Haufen shedding, other signs of BK virus replication, and kidney function in a consecutive group of patients followed carefully at selected time points after HCT. To fully support the clinical utility of PV-Haufen testing, findings will have to be further confirmed by other investigators in the clinical context of confounding diseases impacting renal function.
In conclusion our results support a novel conceptual model for clinical testing and diagnosing disease associated with BK virus infection in HCT recipients (Figure 3). We believe that PVN is an under-recognized cause of kidney damage and CKD in children after HCT and that urinary PV-Haufen testing can identify patients with viral nephropathy. Larger, prospective studies are underway, systematically collecting urine samples at defined time points to further analyze the clinical significance of urinary PV-Haufen shedding and PVN on renal function and patient management post-HCT.
Supplementary Material
Acknowledgments
Funding
Dr. Laskin was supported by an American Society for Blood and Marrow Transplantation/Genentech New Investigator Award and by National Institute of Diabetes and Digestive and Kidney Diseases grant K23 DK101600. These funding sources did not have any input in the study design, analysis, manuscript preparation, or decision to submit for publication. The project described was also supported by the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS PAGE
- CKD
chronic kidney disease
- EM
electron microscopy
- eGFR
estimated glomerular filtration rate
- GVHD
graft versus host disease
- HCT
hematopoietic cell transplantation
- IQR
interquartile ranges
- PCR
polymerase chain reaction
- PVN
polyomavirus nephropathy
Footnotes
Authorship contributions
B.L., H.K.S., S.D., S.J., and V.N. designed the study, performed research and analyses, and wrote the paper; T.M., S.F., N.B., and C.D. assisted with study patient accrual, data collection, and data analysis. U.B., T.M., S.F., N.B., D.W., J.G., and C.D. provided vital conceptual insights for study design and analysis and edited the manuscript for content.
Disclosures
The authors declare no conflicts of interest
REFERENCES
- 1.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 2.Nieder ML, McDonald GB, Kida A, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biol Blood Marrow Transplant. 2011;17:1573. doi: 10.1016/j.bbmt.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickeleit V, Mengel M, Colvin RB. Renal Transplant Pathology. In: Jennette JC, Olson JL, Silva FG, D'Agati VD, editors. Heptinstall's Pathology of the Kidney. 7th edition Wolters Kluwer; Philadelphia, PA: 2015. pp. 1321–1459. [Google Scholar]
- 4.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41:11. doi: 10.1038/sj.bmt.1705886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood. 2005;106:1130. doi: 10.1182/blood-2004-12-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84:243. doi: 10.1002/ajh.21358. [DOI] [PubMed] [Google Scholar]
- 7.Verghese PS, Finn LS, Englund JA, Sanders JE, Hingorani SR. BK nephropathy in pediatric hematopoietic stem cell transplant recipients. Pediatr Transplant. 2009;13:913. doi: 10.1111/j.1399-3046.2008.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Pinto LN, Laskin BL, Jodele S, Hummel TR, Yin HJ, Goebel J. BK virus nephropathy in a pediatric autologous stem-cell transplant recipient. Pediatr Blood Cancer. 2011;56:495. doi: 10.1002/pbc.22860. [DOI] [PubMed] [Google Scholar]
- 9.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621. doi: 10.1097/TP.0b013e318197c17d. [DOI] [PubMed] [Google Scholar]
- 10.Nickeleit V, Singh HK. Polyomaviruses and disease: is there more to know than viremia and viruria? Curr Opin Organ Transplant. 2015;20:348. doi: 10.1097/MOT.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nickeleit V, Mihatsch MJ. Polyomavirus nephropathy in native kidneys and renal allografts: an update on an escalating threat. Transpl Int. 2006;19:960. doi: 10.1111/j.1432-2277.2006.00360.x. [DOI] [PubMed] [Google Scholar]
- 12.Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol. 2009;4:345. doi: 10.2215/CJN.02070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):179. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277. doi: 10.1097/01.tp.0000156165.83160.09. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 17.Laskin BL, Denburg M, Furth S, et al. BK viremia precedes hemorrhagic cystitis in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:1175. doi: 10.1016/j.bbmt.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh HK, Andreoni KA, Madden V, et al. Presence of urinary Haufen accurately predicts polyomavirus nephropathy. J Am Soc Nephrol. 2009;20:416. doi: 10.1681/ASN.2008010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh HK, Donna Thompson B, Nickeleit V. Viral Haufen are urinary biomarkers of polyomavirus nephropathy: New diagnostic strategies utilizing negative staining electron microscopy. Ultrastruct Pathol. 2009;33:222. doi: 10.3109/01913120903241081. [DOI] [PubMed] [Google Scholar]
- 20.Singh HK, Reisner H, Derebail VK, Kozlowski T, Nickeleit V. Polyomavirus nephropathy: quantitative urinary polyomavirus-haufen testing accurately predicts the degree of intrarenal viral disease. Transplantation. 2015;99:609. doi: 10.1097/TP.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickeleit V, Brylawski B, Singh H, Rivier L. Urinary polyomavirus-Haufen shedding in mouse and man: a proof-of-concept study for a non-invasive urine biomarker for polyomavirus nephropathy. Mod Path. 2013;26:390A. [Google Scholar]
- 22.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1512. doi: 10.1016/j.bbmt.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Elfadawy N, Flechner SM, Schold JD, et al. Transient versus persistent BK viremia and long-term outcomes after kidney and kidney-pancreas transplantation. Clin J Am Soc Nephrol. 2014;9:553. doi: 10.2215/CJN.08420813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickeleit V, Hirsch HH, Zeiler M, et al. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15:324. doi: 10.1093/ndt/15.3.324. [DOI] [PubMed] [Google Scholar]
- 26.Burger-Calderon R, Madden V, Hallett RA, Gingerich AD, Nickeleit V, Webster-Cyriaque J. Replication of oral BK virus in human salivary gland cells. J Virol. 2014;88:559. doi: 10.1128/JVI.02777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dropulic LK, Cohen JI. Update on new antivirals under development for the treatment of double-stranded DNA virus infections. Clin Pharmacol Ther. 2010;88:610. doi: 10.1038/clpt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA. 2014;312:2106. doi: 10.1001/jama.2014.14721. [DOI] [PubMed] [Google Scholar]
- 29.Krisl JC, Taber DJ, Pilch N, et al. Leflunomide efficacy and pharmacodynamics for the treatment of BK viral infection. Clin J Am Soc Nephrol. 2012;7:1003. doi: 10.2215/CJN.12531211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.