Abstract
AIM
To evaluate the effects of lentivirus-mediated pigment epithelium-derived factor (PEDF) gene transfer performed in treatment of rats with established choroidal neovascularization (CNV), and investigates the mechanism by which PEDF inhibits CNV in rats.
METHODS
Brown Norway (BN) rats (n=204) were induced by exposure to a laser, and then randomly assigned to 3 groups: no treatment; treatments with intravitreal injection of lentivirus-PEDF-green fluorescent protein (GFP) or lentivirus-control GFP (free fluorescent protein). Following induction and treatment, the CNV tissue was assessed for form, size and vessel leakage by fluorescein fundus angiography (FFA), optical coherence tomography (OCT), histopathology, and examination of choroidal flat mounts. VEGF, Flk-1, and PEDF expression were evaluated by real-time polymerase chain reaction (PCR) and Western blot.
RESULTS
A stable laser-induced rat model of CNV was successfully established, and used to demonstrate lentivirus-mediated PEDG gene transfer by intravitreal injection. Expression of green fluorescence labelled PEDF was observed in the retina up to 28d after injection. An intravitreal injection of lentivirus-PEDF-GFP at 7d led to a significant reduction in the size, thickness and area of CNV showed by FFA, OCT and choroidal flat mounts. PEDF was up-regulated while VEGF and Flk-1 were down-regulated in the lentivirus-PEDF-GFP group. The differences in VEGF and Flk-1 expression in the control and lentivirus-PEDF groups at 7, 14, 21 and 28d after laser induction were all statistically significant.
CONCLUSION
Lentivirus-mediated PEDF gene transfer is effective for use in treatment of laser-induced CNV, and PEDF exerts its therapeutic effects by inhibiting expression of VEGF and Flk-1.
Keywords: pigment epithelium-derived factor, choroidal neovascularization, lentivirus, vascular endothelial growth factor, Flk-1
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of vision loss among older adults (aged >65y) in populations, and the wet (neovascular or exudative) form of AMD produces severe visual loss. As it has been estimated that 25% of Asians will be > 60 years old by 2050[1], AMD will likely become a major public health problem producing a significant economic burden. While the pathogenesis of AMD is not entirely clear, it appears to involve the complex modulation of several molecular factors, including vascular endothelial growth factor (VEGF) and pigment epithelium-derived factor (PEDF). VEGF is a signalling protein that stimulates vasculogenesis and angiogenesis, and plays a key role in the neovascularization process which occurs in AMD[2]. PEDF is believed to be the most potent natural inhibitor of angiogenesis in the retina and choroid of the eye[3]–[4]. Intravitreal injections of anti-VEGF agents are currently considered an integral part of the standard treatment regimen for neovascular AMD[5]; however, this approach has some disadvantages. The current research on delivery of the PEDF gene using traditional techniques has been limited to experimental studies. No clinical studies have been conducted because of the short duration of gene expression achieved, the difficulty in maintaining the gene in its activated state[6]–[8], and the adverse effects associated with traditional gene delivery techniques. As a result, there is great demand for developing a method which allows the maintenance of high PEDF tissue levels for an extended period of time. A previous study showed that lentiviral vectors are safe and effective for use in transfections designed to maintain the long-term and stable expression of a gene, while not stimulating a significant immune response[9].
In this study, we evaluated the effect of lentivirus-mediated PEDF gene transfer in treating a rat model of established choroidal neovascularization (CNV), and also explored the mechanism for the effect. Our results were encouraging, and suggest a new, innovative strategy for treating CNV.
MATERIALS AND METHODS
Animal Model of Choroidal Neovascularization
All animal studies were conducted in compliance with guidelines provided in the Association for Research in Vision and Ophthalmology (ARVO) Statement. Adult male Brown Norway (BN) rats (200-250 g) were obtained from the animal center of Capital Medical University, and housed in the University's Animal care Facility, food and water were available ad libitum. The rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (4 mg/kg body weight), and their pupils were dilated with topical tropicamide (0.5%). Only right eyes were used in the study. CNV was induced with an argon laser[10] (Coherent; Santa Clara, CA, USA) that had a wave length of 520 nm and a spot size of 100 µm at a power of 180 mW. The exposure time was 0.05s. The laser-induced lesions (8 dots) were located at 2 disc diameters from the head of the optic nerve. The presence of a bubble at the time of lasering was taken as an indication that Bruch′s membrane had ruptured. This injury was sufficient to induce CNV. Twenty-seven spots showed hemorrhage after photocoagulation; however, they were not used in the experiment.
Lentivirus-pigment Epithelium-derived Factor and Treatment Groups
A total of 204 CNV BN rats were randomly assigned to three groups (68 rats in each group): 1) a no treatment group; 2) a group injected with lentivirus-PEDF-green fluorescent protein (GFP); 3) a group injected with lentivirus-control GFP. The lentivirus-PEDF vector and control vector were purchased from GeneChem Company (Shanghai, China). The lentivirus vector system comprised GV208, pHelper 1 vector, and pHelper 2 vector. The mouse PEDF gene-expression sequence was subcloned to generate an entry vector, and the target gene fragment was obtained after AgeI/AgeI enzyme digestion. The sequences of the forward and reverse primers were 5′-GAGGATCCCCGGGTACCGGTCGCCACCATGCAGACCCTGGTGCTACTC-3′ and 5′-TCACCATGGCGACCGGAGTGCTGCTGGGGTCCAGGATTC-3′, respectively, and the polymerase chain reaction (PCR) product size was 1300 bp. PEDF over-expression plasmids and the RNAi vector PEDF-RNAi-LV for the PEDF gene that produces PEDF shRNA were successfully constructed, and packaged into 293T cells. A control vector was constructed using identical methods, except that the coding region for GFP was substituted for the PEDF coding region. When the 293T cells reached 90% to 95% confluency, the complete culture medium was removed, and the cells were exposed to medium containing either the packaging mix, expression plasmid DNA, or control plasmid DNA with lipofectamine. After incubation for 24h, the infection medium was replaced with complete culture medium. Lentivirus-containing supernatant fractions were harvested at 72h after transfection; after which, they were centrifuged to remove pelleted debris and stored at -80°C. The final concentration of the virus suspension was 2×1012 TU/L. Intravitreal injections were performed using a Harvard pump microinjection apparatus (Hamilton Company, USA) and pulled glass micropipets. Each micropipet was calibrated to deliver 5 uL of vehicle upon depression of a foot switch. When the foot switch was depressed, the sharpened tip of the micropipet was passed through the sclera just behind the limbus into the vitreous cavity.
Observations of Green Fluorescent Protein Expression
The eyes were enucleated at 7, 14, 21 and 28d after laser treatment (two eyes at each time point), and immediately embedded in Optimum Cutting Temperature compound (Tissue-Tek, Sakura Finetek Inc; Tokyo, Japan). The embedded eyes were then frozen in a cryostat (Leica CM1900; Berlin, Germany) maintained at -20°C and 6 µm serial frozen sections of each eye were cut along the vertical meridian plane and air-dried. The sections were then mounted onto slides and fixed in formalin solution for 30min; after which, they were examined by fluorescence microscopy (emission wavelength=490 nm) to observe expression of GFP fluorescence.
Fluorescein Fundus Angiography and Optical Coherence Tomography
The dilated pupils of anesthetized rats were examined by fluorescein fundus angiography (FFA) performed using a digital fundus camera (Model TRC 50 IA; Topcon, Paramus, Japan). Optical coherence tomography (OCT) was performed using a Cirrus HD-OCT device (Carl Zeiss Meditec; Dublin, CA, USA). Fluorescein sodium was injected intraperitoneally (0.5 mL/kg of 10% fluorescein sodium), and the timer was started as soon as the injection was administered. FFA and OCT were performed for each animal at 7, 14, 21 and 28d after laser treatment. The OCT images were used to evaluate the maximum thickness of CNV, defined as the highly reflective layer above the retinal pigment epithelium (RPE). To minimize observer variations, all scans were performed by the same experienced physician. The thickness of CNV was quantified using proprietary software designed for use in Cirrus HD-OCT. Fluorescein angiograms were qualitatively and quantitatively evaluated by a blinded observer, and the degree of laser-induced CNV was estimated by angiographic leakage. The laser-induced lesions were graded based on the observed fluorescein leakage, and then divided into the following four categories as described by Semkova et al[11]: (+) no leakage and little damage; slight hyperfluorescence without leakage or increased fluorescence size and intensity; (++) leakage and moderate damage; points of increased hyperfluorescence intensity, but no increase in size; (+++) leakage and severe damage; points of hyperfluorescence that show increased size and intensity; (++++) leakage and very severe damage. Confluent points of increased hyperfluorescence, with the retinas almost completely occupied by the leakage; sometimes difficult to distinguish the different laser-induced burns.
Choroidal Flat Mounts
Briefly, eyes were enucleated at 7, 14, 21 and 28d after laser treatment (five eyes at each time point), and immediately fixed for 1h in a solution of 4% paraformaldehyde (EM Grade; Polysciences, Inc.; Warrington, PA, USA) in phosphate-buffered saline (PBS; 9 g/L NaCl, 0.232 g/L KH2PO4, and 0.703 g/L Na2HPO4; pH 7.3). Under a dissecting microscope, the anterior segment and crystalline lens were removed, and the retinas were detached and separated from the optic nerve head with a pair of fine curved scissors. The remaining eye cups were washed with cold ICC buffer (0.5% BSA, 0.2% Tween 20, and 0.05% sodium azide) in PBS. Next, a 1:100 dilution of rhodamine Ricinus communis agglutinin I was incubated at 4°C with gentle rotation for 4h and then washed with cold immunocytochemical (ICC) buffer. A 1:1000 dilution of a 10 mg/mL solution of 4′,6-diamidino-2-phenylindole (DAPI) was incubated for 15min, and then washed with cold ICC buffer. Radial cuts were made toward the optic nerve head, and the sclera-choroid/RPE complexes were flat mounted, covered and sealed. The confocal images obtained were evaluated with commercial image-analysis software, and the results were compared with those obtained using our fluorescent labeling technique. All images were collected by confocal microscopy.
Measurements of Pigment Epithelium-derived Factor and Vascular Endothelial Growth Factor Activity by Western Blot
Eyes were enucleated at 7, 14, 21 and 28d after laser treatment (five eyes at each time point). Each eye was bisected posterior to the iris; after which, the lens was lifted out, and the vitreous removed. After a careful examination and dissection to remove any contaminating material, the vitreous was placed into tubes surrounded by ice. The rat vitreous was then pooled, and the total volume was measured by pipette aspiration. Next, the vitreous was quickly homogenized in ice-cold lysis buffer (pH 7.5), and an aliquot of the homogenate was assayed for protein content using the bicinchoninic acid protein assay. The remaining supernatant was stored at -70°C for future analysis of its PEDF and VEGF contents.
The vitreous proteins were separated by electrophoresis on 12% SDS-polyacrylamide gels. Prior to electrophoresis, the protein samples were boiled in a denaturing sample buffer, and an equal amount of protein was loaded onto each lane. Electrophoresis was conducted at 120 V for 1h under reducing conditions at room temperature. The separated proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking the membrane, the proteins were stained with the following antibodies: anti-PEDF monoclonal antibody (1:1000, Biosynthesis Biotech; Beijing, China), anti-VEGF monoclonal antibody (1:1000, Biosynthesis Biotech, China), and anti-β-actin antibody (1:1000, Abcam; Cambridge, MA, USA). The stained protein bands were visualized by incubation with a Horseradish Peroxidase (HRP)-conjugated goat anti-rabbit or anti-mouse secondary antibody (1:10000, ZSGB Biotech; Beijing, China) and chemoluminescent substrates (ECL Plus, PerkinElmer, Inc; Covina, CA, USA). Staining intensity was analyzed with Image J software, and the protein levels were normalized to those of β-actin.
Measurements of Pigment Epithelium-derived Factor, Vascular Endothelial Growth Factor and Flk-1 mRNA by Real-time Polymerase Chain Reaction
PEDF, VEGF and VEGF Receptor-2 (Flk-1) mRNA expression were examined by quantitative real-time PCR. Total tissue RNA was extracted and purified with Trizol reagent. Next, a 5 µL aliquot of purified RNA was used as a template for cDNA synthesis in the presence of 15 µL of 5×RT buffer, 1 µL of random primer, 1 µL of reverse transcriptase, and 8 µL of RNAse-free water. The reverse transcriptase was inactivated at 25°C for 10min, 60°C for 10min, and 70°C for 10min. The PCR reaction mixture (50 µL) consisted of 1 µL of cDNA, 25 µL of 2× PCR buffer, 0.6 µL×2 of primers, and 22.8 µL of RNAse-free water. Next, the newly synthesized cDNA was denatured by heating at 94°C for 3min, and the template was amplified by 35 cycles of an incubation step (94°C for 20s), an annealing (60°C for 20s), and an extension step (70°C for 20s). The sequences of the forward and reverse primers were as follows: 5′-GCAACCCTCGCATAGACCTT-3′ and 5′-CCTGTCCTCGTCCAAGTGAAA-3′, respectively, for the PEDF gene; 5′-ACGAAAGCGCAAGAAATCCC-3′ and 5′-TTAACTCAAGCTGCCTCGCC-3′, respectively, for the VEGF gene; 5′-TAGCACGACAGAGACTGTGAGG-3′ and 5′-TGAGGTGAGAGAGATGGGTAGG-3′, respectively for the Flk-1 gene; 5′-CCCATCTATGAGGGTTACGC-3′ and 5′-TTTAATGTCACGCACGATTTC-3′, respectively for β-actin. A cycle fluorescence intensity was recorded for each sample. The PCR reactions were performed in quintuplicate, and the mean value of the fluorescence intensity was calculated and statistically analyzed.
Statistical Analysis
All data were analyzed using SPSS for Windows, Version 13.0. Chicago, IL: SPSS Inc., and results are presented as the mean±SD. Differences were evaluated using ANOVA, followed by the Student-Newman-Keuls test for multiple comparisons. The Student's t-test was used to analyze pairwise comparisons. A P-value < 0.05 was considered statistically significant.
RESULTS
All rats used in the studies were in good health throughout the experimental procedures, and their eyes showed no obvious complications (e.g. inflammation, corneal opacity, cataract, vitreous opacity, retinal hemorrhage) after intravitreal injection.
Localization of Lentivirus-pigment Epithelium-derived Factor-green Fluorescent Protein Expression
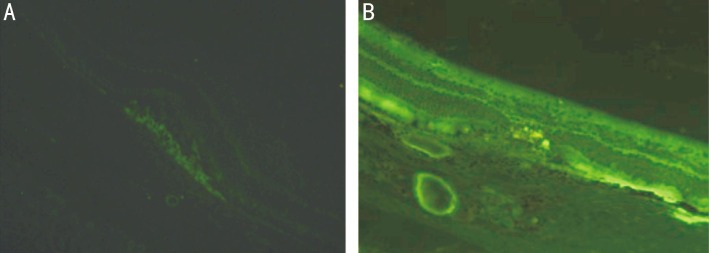
Fluorescence microscopy showed prominent GFP fluorescence in all layers of the retina. This fluorescence was especially prominent in photoreceptors and RPE cells at 7d after intravitreal injection of lentivirus-PEDF-GFP (Figure 1). Green fluorescence was observed until 4wk after injection.
Figure 1. Ocular sections were examined by fluorescence microscopy on day 7 after injection.
A: Representative image of fluorescence microscopy findings in the no treatment group; B: Representative image of fluorescence microscopy findings in the lentivirus-PEDF-GFP group. GFP fluorescence appeared in all retinal layers, and especially in photoreceptors and RPE cells.
Quantitative Assessment of Fluorescein Angiographic Features following Lentivirus-mediated Pigment Epithelium-derived Factor Gene Transfer
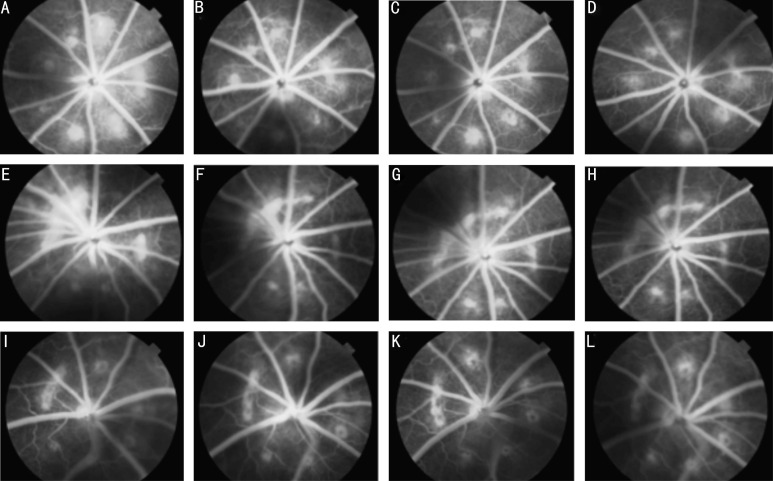
FFA examinations of CNV on day 7 after photocoagulation showed the creation of laser-induced lesions partially resulting from CNV. Moderate-to-severe fluorescein leakage was observed between days 14 and 28 after photocoagulation. FFA showed that intravitreal injection of lentivirus-PEDF on day 7 after laser treatment led to a significant reduction in the size of the CNV area as visualized after fluorescein injection. Large and diffuse areas of leakage were observed in the control rats showing CNV, while CNV lesions in rats which received lentivirus-PEDF-GFP did not exhibit significant leakage (Figure 2).
Figure 2. FFA of CNV lesions.
A-D: Representative late-phase FFA images of untreated laser-induced CNV areas at 7, 14, 21 and 28d after laser photocoagulation. FFA showed that laser-induced lesions partially resulted in emergence of CNV areas on day 7 after photocoagulation. From 14d to 28d, the CNV areas showed moderate-to-severe fluorescein leakage;E-H: Representative late-phase FFA images of control-lentivirus CNV areas at 7, 14, 21 and 28d after laser photocoagulation. The degree of angiographic leakage in this group was the same as that in the untreated laser-induced CNV group; I-L: Representative late-phase FFA images of CVN areas in rats which received lentivirus-PEDF. The images were taken at 7, 14, 21 and 28d after laser photocoagulation. FFA showed that an intravitreal injection of lentivirus-PEDF on day 7 after laser treatment led to a significant reduction in the size of the CNV area, as seen after fluorescein injection. Large and diffuse areas of leakage were not observed.
Optical Coherence Tomography Findings after Choroidal Neovascularization Induction and Lentivirus-mediated Pigment Epithelium-derived Factor Gene Transfer
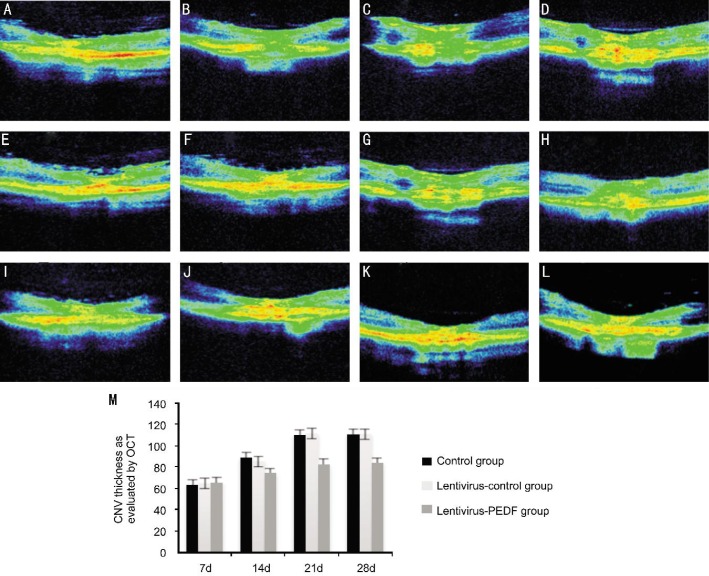
Intraretinal layers were recognizable in high-resolution OCT images, and thus CNV tissue induced by laser photocoagulation was examined in those images. At 7d after photocoagulation, the OCT images showed disruption of a highly reflective layer corresponding to the RPE and choriocapillaris in the lesion. A flat, highly reflective layer resulting from the disruption of the lesions was observed under the sensory retina, and was identified as developmental stage CNV tissue. OCT images obtained between days 14 and 21 showed that the highly reflective layer extended to the subretinal space, and its thickness had increased. On day 28, OCT images showed that the spindle-shaped layer reflected with greater intensity, but its thickness remained similar to that observed on day 21. OCT also showed that an intravitreal injection of lentivirus-PEDF-GFP on day 7 after laser application led to a significant reduction in the thickness of the CNV (Figure 3).
Figure 3. OCT images of CNV lesions.
A-D: Representative OCT images of untreated laser-induced CNV taken at 7, 14, 21 and 28d after laser photocoagulation; E-H: Representative OCT images of control-lentivirus CNV taken at 7, 14, 21 and 28d after laser photocoagulation. The thickness and characteristics of the CNV in this group were the same as in the untreated laser-induced CNV group; I-L: Representative OCT images of lentivirus-PEDF CNV taken at 7, 14, 21 and 28d after laser photocoagulation. In this group, OCT showed significantly reduced thickness of the CNV; M: Comparison of CNV thickness in the three groups.
Areas of CNV in Choroidal Flat Mounts
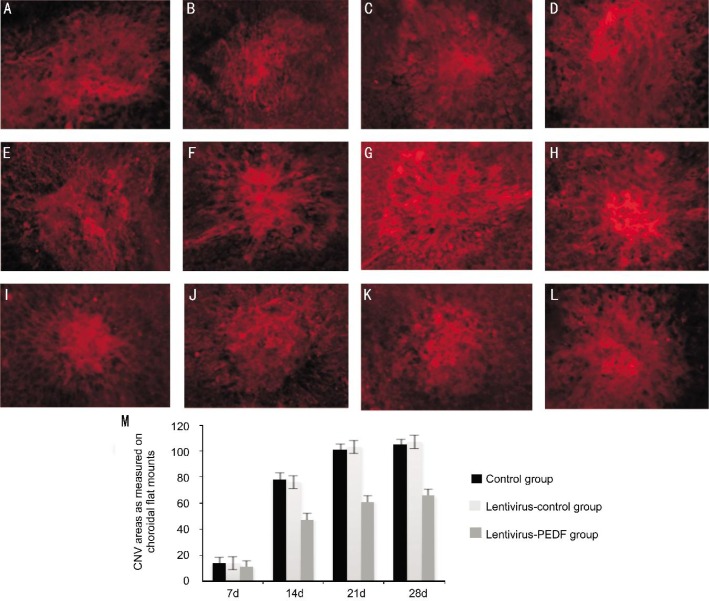
The areas of the induced CNV lesions as measured in choroidal flat mounts were smaller in eyes that received an intravitreal injection of lentivirus-PEDF-GFP on day 7, when compared with those areas in control eyes (10.93±6.02×10−3 mm2 vs 14.16±6.73×10−3 mm2, respectively) (Figure 4). The differences between lesion areas in the control group/lentivirus-control-GFP group and lentivirus-PEDF group remained statistically significant (P<0.05) from day 7 to day 28 after photocoagulation.
Figure 4. Choroidal flat mounts of CNV lesions.
A-D: Representative images of choroidal flat mounts of untreated laser-induced CNV regions at 7, 14, 21 and 28d after laser photocoagulation; E-H: Representative images of choroidal flat mounts of control-lentivirus CNV at 7, 14, 21 and 28d after laser photocoagulation; I-L: Representative images of choroidal flat mounts of lentivirus-PEDF CNV at 7, 14, 21 and 28d after laser photocoagulation. The areas of the induced CNV lesions measured in choroidal flat mounts were smaller in eyes that received an intravitreal injection of lentivirus-PEDF on day 7 when compared to those areas in control eyes. All experiments were repeated 3 times, and data are expressed as the mean±SD (n=5 eyes). The differences between control and lentivirus-PEDF treated eyes remained statistically significant from day 7 to day 28 (P < 0.05).
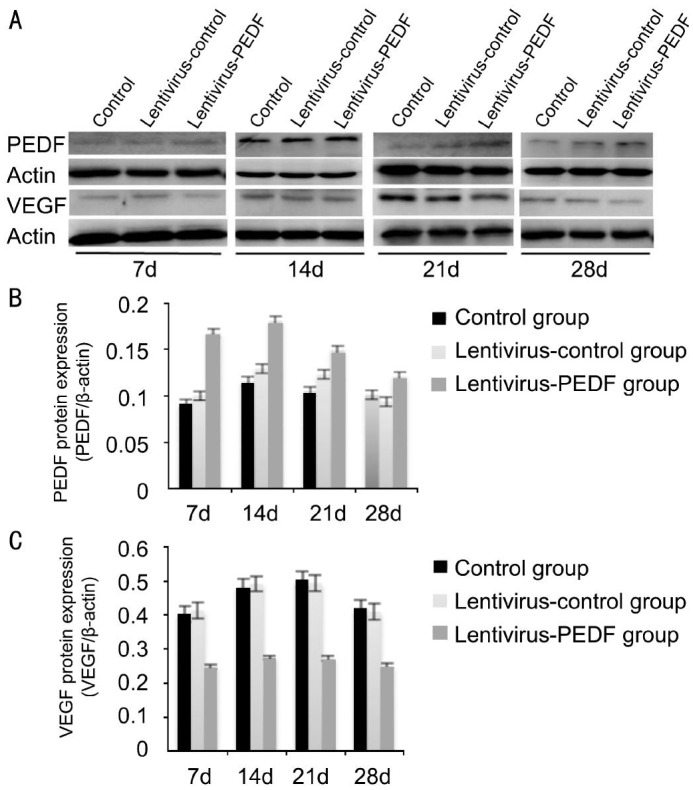
Quantitative Assessment of Western Blot Studies
Western blot analyses were performed to evaluate VEGF and PEDF expression. Each experiment was repeated 3 times. When compared with controls, PEDF levels were up-regulated and VEGF levels were down-regulated in the lentivirus-PEDF-GFP group after day 7. The differences in PEDF and VEGF expression in the control, lentivirus-control-GFP, and lentivirus-PEDF-GFP groups on days 7, 14, 21 and 28 after photocoagulation were all statistically significant (P < 0.05, Figure 5).
Figure 5. Western blot analysis of PEDF and VEGF protein expression in the control group, lentivirus-control group, and lentivirus-PEDF group.
A: The bands of PEDF and VEGF proteins in the control group, lentivirus-control group and lentivirus-PEDF group on days 7, 14, 21 and 28 after laser photocoagulation; B: Increased expression of PEDF in the lentivirus-PEDF group as compared with expression in the control group and lentivirus-control group from 7 to 28d after laser photocoagulation; C: Decreased VEGF expression in the lentivirus-PEDF group as compared with expression in the control group and lentivirus-control group from 7 to 28d after laser photocoagulation. All experiments were repeated 3 times.
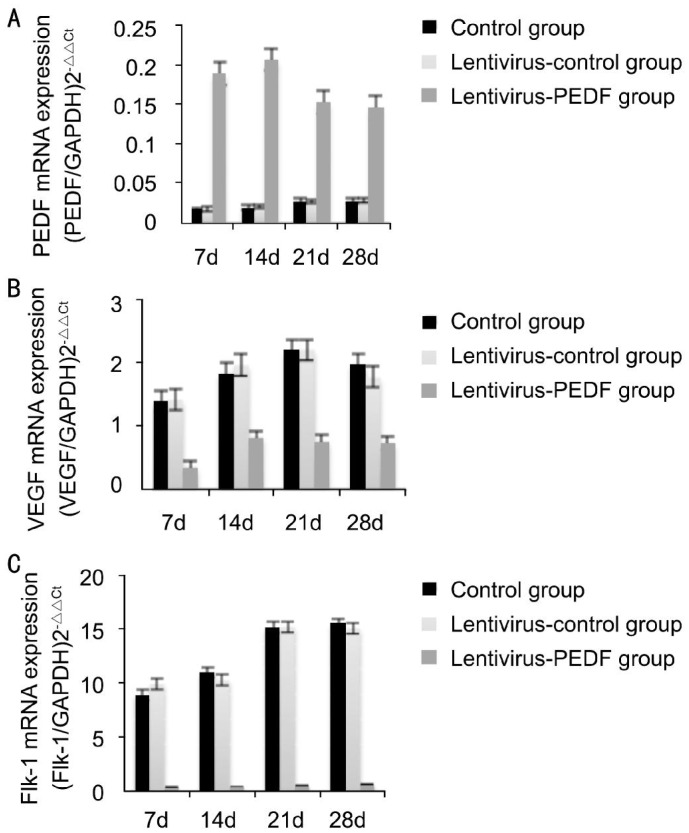
Quantitative Assessment of Real-time Polymerase Chain Reaction
The results of real-time PCR are shown in Figure 6. When compared with the control group, the level of PEDF expression in the lentivirus group began to increase on day 7 after photocoagulation, and was markedly increased on day 14 (Figure 6A). A statistical analysis showed that PEDF expression was significantly higher in the lentivirus-PEDF-GFP group on days 7, 14, 21 and 28 after photocoagulation (P<0.05). In contrast, the levels of VEGF and Flk-1 expression in the lentivirus-PEDF-GFP group were significantly lower than those in the control groups on days 7, 14, 21 and 28 (all P< 0.05) (Figure 6).
Figure 6. Real-time PCR analysis of PEDF, VEGF and Flk-1 mRNA expression in the control-lentivirus group and lentivirus-PEDF group.
A: When compared with the control group and lentivirus-control group, the level of PEDF mRNA expression in the lentivirus-PEDF-GFP group increased after day 7, and was markedly increased on day 14. A statistical analysis showed that PEDF expression in the lentivirus-PEDF-GFP group was significantly higher than that in the control group on days 7, 14, 21 and 28 (all P<0.05); B: Expression of VEGF mRNA in the lentivirus-PEDF group was decreased as compared with expression in the control group and lentivirus-control-GFP group from day 7 to day 28 after laser photocoagulation. The differences in VEGF expression between the control and lentivirus-PEDF-GFP groups on days 7, 14, 21 and 28 were all statistically significant (P<0.05); C: From day 7 to day 28 after laser photocoagulation, there was decreased expression of Flk-1 mRNA in the lentivirus-PEDF-GFP group as compared with expression in the control group and lentivirus-control-GFP group. The differences in Flk-1 mRNA expression between the control group and lentivirus-PEDF-GFP group on days 7, 14, 21 and 28 after photocoagulation were all statistically significant (P<0.05).
DISCUSSION
Antiangiogenic treatment is one of the most effective strategies for managing retinal and CNV diseases, including AMD and diabetic retinopathy. These eye diseases occur worldwide, and can cause irreversible blindness[12]–[13]. It has been suggested that an imbalanced expression of stimulators and inhibitors of blood vessel growth significantly contributes to the pathogenesis of neovascularization, and several therapeutic agents have demonstrated an ability to decrease the amount of neovascularization[13]. VEGF is currently believed to be the most effective factor for promoting vessel growth, whereas PEDF is believed to be the most potent natural inhibitor of retinal and choroidal angiogenesis[4]. The role of VEGF in CNV development has been intensively investigated. For example, VEGF production is upregulated during in both human CNV and laser-induced CNV. Furthermore, administration of exogenous VEGF promotes CNV, while inhibition of VEGF activity leads to CNV suppression. Two receptors for VEGF have been identified, and are referred to as VEGFR-1 and VEGFR-2, respectively. VEGFR-2 is also referred to as Flk-1, and plays a pivotal role in CNV. VEGFR-2 is expressed in all endothelial cells and can be detected on retinal progenitor cells. In contrast to the controversial role of VEGFR-1 in the neovascularization process, the VEGFR-2-mediated signaling pathway is generally considered to promote the formation of new blood vessels[14]–[15]. Intravitreal injections of anti-VEGF agents are currently a part of the standard treatment regimen for neovascular AMD[5]. However, such agents have some disadvantages, such as a short half-time, which leads to the need for repeated injections. A therapeutic strategy that combines an anti-VEGF agent with a second therapeutic modality that has a different mechanism of action would represent an important step forward in the treatment of AMD.
As the most effective antiangiogenic factor, PEDF has been the focus of current research on ways to inhibit angiogenesis. Endogenous cellular PEDF was first identified in the conditioned media of cultured human fetal RPE cells by Tombran-Tink and Johnson in 1989[16]. PEDF is a 50 kDa glycoprotein containing eight relatively small exons and seven introns belonging to the noninhibitory serpin family. It is produced by RPE cells, as well as by photoreceptors and ganglion cells, and its highest concentrations are found in the inter-photoreceptor matrix. Previous studies have demonstrated that PEDF is a neuroprotective and neurotrophic factor that promotes neuron differentiation[17]–[18]. In addition to its neurotrophic and neuroprotective functions, PEDF has also been identified as a potent antiangiogenic factor. Presently, research on the delivery of PEDF into cells via traditional gene delivery techniques is limited to experimental in vitro and animal studies, and little clinical trials have been attempted. This is because of the adverse effects associated with traditional gene delivery techniques, and the difficulty of sustaining long-term PEDF expression[6]–[8]. Therefore, it is necessary to develop a new gene vector which demonstrates safe targeting and provides for high transfect efficiency. Lentiviral vectors are efficient and safe when used to transfect a gene that requires long-term stable expression[9]. In the present study, we examined the effect of lentivirus-PEDF gene transfer performed via intravitreal injection in treatment of rats with laser-induced CNV.
Our study showed promising results. We successfully developed a stable laser-induced CNV model, and found that an intravitreal injection of lentivirus-PEDF produced significant inhibition of CNV. We also examined the formation of CNV by several methods, including FFA, OCT and choroidal flat mounts. The results showed that the creation of laser-induced lesions resulted in the emergence of CNV on day 7 after photocoagulation. The induced CNV process was accompanied by moderate-to-severe fluorescein leakage from 14 to 28d after photocoagulation. FFA showed that an intravitreal injection of lentivirus-PEDF on day 7 after laser application led to a significant reduction in the size of the CNV area visualized after fluorescein injection. Large and diffuse areas of leakage were observed in the eyes of the non-treated CNV rats, while the CNV lesions in rats which received lentivirus-mediated transfer of the PEDF gene did not exhibit significant fluorescein leakage. At the same time, choroidal flat mounts showed smaller areas of CNV in the lentivirus-PEDF group. In a previous paper[19], we compared different methods for evaluating laser-induced CNV, including examination of paraffin sections, frozen sections, and the use of high-resolution OCT. The results showed that while the histopathological images obtained from both paraffin sections and frozen sections correlated with the OCT images, only the frozen sections accurately matched the OCT images. Therefore, we selected high resolution OCT as the method to evaluate CNV. The results showed that the CNV regions in the lentivirus-PEDF group were obviously less thick compared to those in the other two groups.
In 2002, Mori et al[8] reported that AAV-mediated gene transfer of PEDF could inhibit CNV When compared with the methods used in that paper, we used more advanced examination methods that allowed us to obtain clearer images and more reliable results. Additionally, we also analyzed the mechanism for CNV inhibition.
Our results showed that in the lentivirus-PEDF group, PEDF was up-regulated, while VEGF and Flk-1 were down-regulated after 7d after photocoagulation. Furthermore, the differences between the control group/lentivirus-control group and lentivirus-PEDF group on days 7, 14, 21 and 28 were all statistically significant (P<0.05). These results strongly suggest that PEDF exerts its antiangiogenic effect by decreasing VEGF expression and down-regulating Flk-1.
We found that lentivirus-PEDF could prevent laser induced-CNV, and the effect could last for up to 28d. However, we did not check for any intraocular inflammatory phenomena. Previously, Mori et al[8] reported that adeno-associated viral (AAV)-mediated transfer of the PEDF gene could inhibit CNV, without producing any significant inflammation. Since that time, adenovirus and adeno-associated virus vector-mediated PEDF gene transfer methods has been used in numerous laboratories[20]–[22]. However, the traditional gene therapy method has some potential disadvantages. These include a short duration of gene expression, the need for repeated intraocular injections, and the possibility of immune reactions[23]; therefore, its application remains limited. In contrast, lentiviral vectors appear to be safe and effective for producing long-term stable gene expression, with little immune response by the recipient. We have not found any previous paper reporting the inhibition of CNV by lentivirus-mediated transfer of PEDF. Our study has some weakness that should be mentioned. First, the observation times used in the study were short, and should be increased to include 2mo or 3mo time points. Second, we did not evaluate immunological reactions. For example, electron microscopy could be used to examine the retina for any toxic effects resulting from the gene transfer process. Third, the effects of lentivirus-PEDF were not compared with the effects other agents such as anti-VEGF agent, AAV-mediated PEDF gene transfer or a combined treatment. In any case, lentivirus-mediated transfer of PEDF was shown to be effective for treating laser-induced CNV, and may also be an innovative approach for treating CNV.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No. 81070735).
Conflicts of Interest: Yu YJ, None; Mo B, None; Liu L, None; Yue YK, None; Yue CL, None; Liu W, None.
REFERENCES
- 1.Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ, Mitchell P, Wong TY. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kwak N, Okamoto B, Wood JM, Campochiaro PA. VEGF is major stimulator in modal of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(10):3158–3164. [PubMed] [Google Scholar]
- 3.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285(5425):245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 4.Renno RZ, Youssri AI, Michaud N, Gragoudas ES, Miller JW. Expression of Pigment Epithelium–Derived Factor in Experimental Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2002;43(5):1574–1580. [PubMed] [Google Scholar]
- 5.Chen G, Li W, Tzekov R, Mao S, Tong Y. Bevacizumab versus ranibizumab for neovascular age-related macular degeneration: a meta-analysis of randomized controlled trials. Retina. 2015;35(2):187–193. doi: 10.1097/IAE.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 6.Yan H, Cui J, Wang Y, Yu Y. Comparison of the effects between intravitreal and periocular injections of adenoviral vectored pigment epithelium-derived factor on suppressing choroidal neovascularization in rats. Ophthalmic Res. 2013;49(2):81–89. doi: 10.1159/000342979. [DOI] [PubMed] [Google Scholar]
- 7.Zhou XY, Liao Q, Pu YM, Tang YQ, Gong X, Li J, Xu Y, Wang ZG. Ultrasound-mediated microbubble delivery of pigment epithelium-derived factor gene into retina inhibits choroidal neovascularization. Chin Med J (Engl) 2009;122(22):2711–2717. [PubMed] [Google Scholar]
- 8.Mori K, Gehlbach P, Yamamoto S, Duh E, Zack DJ, Li Q, Berns KI, Raisler BJ, Hauswirth WW, Campochiaro PA. AAV-mediated gene transfer of pigment epithelium- derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43(6):1994–2000. [PubMed] [Google Scholar]
- 9.Yang H, Grossniklaus HE. Constitutive overexpression of pigment epithelium-derived factor inhibition of ocular melanoma growth and metastasis. Invest Ophthalmol Vis Sci. 2010;51(1):28–34. doi: 10.1167/iovs.09-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29(6):500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semkova I, Fauser S, Lappas A, Smyth N, Kociok N, Kirchhof B, Poulaki V, Joussen AM. Overexpression of FasL in retinal pigment epithelial cells reduces choroidal neovascularization. FASEB J. 2006;20(10):1689–1691. doi: 10.1096/fj.05-5653fje. [DOI] [PubMed] [Google Scholar]
- 12.Drack A. Retinopathy of prematurity. Adv Pediatr. 2006;53:211–226. doi: 10.1016/j.yapd.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Mousa SA, Mousa SS. Current status of vascular endothelial growth factor inhibition in age-related macular degeneration. BioDrugs. 2010;24(3):183–194. doi: 10.2165/11318550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Tamaki Y, Ishii N, Oikawa N, Mizuguchi E, Francis JH, Inoue Y, Iriyama A, Obata R, Yanagi Y. Identification of a novel vascular endothelial growth factor receptor 2 inhibitor and its effect for choroidal neovascularization in vivo. Curr Eye Res. 2008;33(11):1002–1010. doi: 10.1080/02713680802492440. [DOI] [PubMed] [Google Scholar]
- 15.Ohno-Matsui K, Yoshida T, Uetama T, Mochizuki M, Morita I. Vascular endothelial growth factor upregulates pigment epithelium-derived factor expression via VEGFR-1 in human retinal pigment epithelial cells. Biochem Biophys Res Commun. 2003;303(3):962–967. doi: 10.1016/s0006-291x(03)00446-7. [DOI] [PubMed] [Google Scholar]
- 16.Tombran-Tink J, Johnson LV. Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci. 1989;30(8):1700–1707. [PubMed] [Google Scholar]
- 17.Becerra SP. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. Adv Exp Med Biol. 1997;425:223–237. [PubMed] [Google Scholar]
- 18.Araki T, Taniwaki T, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J Neurosci Res. 1998;53(1):7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Jiao J, Mo B, Wei H, Jiang YR. Comparative study of laser-induced choroidal neovascularization in rats by paraffin sections, frozen sections and high-resolution optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):301–307. doi: 10.1007/s00417-012-2204-4. [DOI] [PubMed] [Google Scholar]
- 20.Streck CJ, Zhang Y, Zhou J, Ng C, Nathwani AC, Davidoff AM. Adeno-associated virus vector-mediated delivery of pigment epithelium-derived factor restricts neuroblastoma angiogenesis and growth. J Pediatr Surg. 2005;40(1):236–243. doi: 10.1016/j.jpedsurg.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 21.Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011;178(2):688–698. doi: 10.1016/j.ajpath.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He SS, Shi HS, Yin T, Li YX, Luo ST, Wu QJ, Lu L, Wei YQ, Yang L. AAV-mediated gene transfer of human pigment epithelium-derived factor inhibits lewis lung carcinoma growth in mice. Oncol Rep. 2012;27(4):1142–1148. doi: 10.3892/or.2012.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami Y, Ikeda Y, Yonemitsu Y, Miyazaki M, Inoue M, Hasegawa M, Sueishi K, Ishibashi T. Inhibition of choroidal neovascularization via brief subretinal exposure to a newly developed lentiviral vector pseudotyped with sendai viral envelope proteins. Human Gene Therapy. 2010;21(2):199–209. doi: 10.1089/hum.2009.102. [DOI] [PubMed] [Google Scholar]