Abstract
AIM
To establish the role of nitric oxide (NO), ascorbic acid and tumour necrosis factor-α (TNF-α) in the pathogenesis of pseudoexfoliation glaucoma (XFG).
METHODS
Our study included 120 patients who were referred for cataract surgery. All patients were divided into four groups according to clinical findings: XFG, early and late pseudoexfoliation syndrome (XFS), and cataract (without pseudoexfoliation). Serum and aqueous humour levels of the ascorbic acid, NO and TNF-α were measured. The concentrations of the ascorbic acid and NO were measured by an appropriate spectrophotometric method. Enzyme-linked immunosorbent assay (ELISA) was used to determine TNF-α level.
RESULTS
Aqueous humour concentration of ascorbic acid was significantly lower in patients with late XFS (0.61±0.11 mmol/L) and XFG (0.48±0.15 mmol/L) compared to patients with early XFS (0.9±0.15 mmol/L) and cataract (1.16±0.22 mmol/L), while there was no difference in serum concentration in all examined groups. Aqueous humour concentration of NO was significantly higher in patients with XFG (77.7±11.4 µmol/L) compared to patients with early XFS (50.27±9.34 µmol/L) and cataract (49.77±7.1 µmol/L), while serum concentration was increased in the early stage of XFS (73.26±8.29 µmol/L). Aqueous humour level of proinflammatory cytokine TNF-α was increased in patients with XFS (early 460.04±18.32 pg/mL; late 502.42±53.23 pg/mL) and XFG (510.34±43.07 pg/mL), while there was no difference in serum level in all examined groups of patients.
CONCLUSION
Reduced ascorbic acid and elevated NO and inflammation related cytokine TNF-α level in aqueous humour of the patients with developed XFG suggest that oxidative stress induces local inflammation.
Keywords: ascorbic acid, nitric oxide, pseudoexfoliation, tumour necrosis factor alpha
INTRODUCTION
Pseudoexfoliation syndrome (XFS) is an age related disorder of the extracellular matrix (ECM), characterised by an intensive production of abnormal fibrous fibers and their accumulation in the eye[1], but without the development of pseudoexfoliative glaucoma. It is called stress induced elastosis, because the main cause of its production is overproduction of reactive oxygen species[2]. Oxidative stress in the body can be activated by different causes: ultraviolet radiation, ages, infection, etc[3]. Liberated free radicals can activate cytokine secretion from different inflammatory cells. These cytokines can act as pro-inflammatory in this abnormal matrix process in XFS and pseudoexfoliation glaucoma (XFG)[3]. Decreased antioxidative concentration provokes releasing of cytokines and accelerates pseudoexfoliation production[4]. If the pseudoefoliative material is deposited in iridocorneal angle with increased humour aqueous outflow resistance, glaucoma will be developed[3].
Pseudoexfoliation production is in proportion with the age, because older population is more represented in groups with pseudoexfoliation (syndrome or glaucoma), which is in correspondence with the reachable date[5].
Ascorbic acid is one the most important antioxidant and free radical scavenger in the body and can be reduced in patients with cataract, glaucoma, aged related macular disease, etc[6].
Nitric oxide (NO) is short-lived gaseous molecule produced by a group of enzymes called nitric oxide synthases (NOS), using L-arginin to produce NO in the tissue[7]. Inducible nitric oxide synthases (iNOS), one of the three isoforms, is not primarily expressed, but can be induced in the macrophages by bacterial lipopolysaccharide or with secreted cytokines[8]. NO as a potent vasodilatator relaxes smooth muscles and it is a part of different pathological processes in the eye: cataract, uveitis, and glaucoma[9].
TNF-α is a proinflammatory cytokine which plays a role in different physiological and immunological process[10]. It can activate inflammation in the tissue, apoptosis mediated by mitochondria[11], and, contrary to this, it can have neuroprotective role[12]. Its role in the process of glaucoma development is still not clear.
In this study we have indicated the importance of oxidative stress in the development of XFG as well as the potential protective role of NO in this process.
SUBJECTS AND METHODS
Our study includes 120 patients, recruited for the cataract surgery. All patients were divided into four groups: XFS-early (diffuse precipitated white flakes on anteriol lens capsule or on pupillary margin, mild pupillary dilatation followed by pigment dispersion and its deposition on anterior lens capsule and chambre angle, intial pupillary ruff atrophy) and late stage (massive pseudoexfoliation deposition on pupillary margin and anterior lens capsule with well developed clear zone without pseudoexfoliation material, heavy pigment deposition on lens capsule, chamber angle-specially Sampaolesi line with high restricted mydriasis) XFG and control group (with no other eye disease). Serum samples were collected before cataract surgery. After paracenthesis was done, the samples of aqueous humour were collected during cataract surgery using 27 gauge needles. The samples of humour and sera were stored at -80°C until biochemical analysis. All patients underwent a complete ophthalmological examination. Pseudoexfoliation material can be found on corneal endothelial cells, iridocorneal angle, lens, pupilar margin, cilliar zonulas, vitreous, etc. and it was detected during detailed slit lampe examination in mydriasis. Aplanation tonometry was used for intraocular pressure measurements. Direct fundus examination was conducted for all patients to detect optic head changes. Based on intraocular pressure levels (IOP>22 mm Hg), optic head changes (disturbed C/D ratio) and reproducible visual field defects in computed perimetry, patients with pseudoexfoliation can be divided into two groups: XFS and XFG. Patients with the history of previous trauma, intraocular inflammation, diabetes mellitus, myopia, earlier laser photocoagulation, cryo therapy or intraocular surgery were excluded from the study. Control group was selected in terms of age and sex.
The regional Ethics Committee approved the study protocol and prior to the initiation a written informed consent was obtained from all subjects according to the Declaration of Helsinki.
Measurement of Ascorbic Acid Concentration in Aqueous Humour and Serum
The determination of vitamin C in the samples was done by technique described by Rutkowski and Grzegorczyk[13]. In the centrifugal test-tube we measured 0.5 mL of analysed liquid (aqueous humour-diluted 1/10 by distilled water), with 0.5 mL of the phosphotungstate reagent (PR). It was mixed thoroughly and left in a room temperature for 30min. The tubes were centrifuged (7000 rpm, 10min), and the whole of the separated supernatant was collected with a pipette. The supernatant is a test sample for spectrophotometric measurements. Standard sample was prepared as above (1 mL of standard solution was used instead of analysed solution), but with no centrifugation. We measured the absorbance of the test sample and of the standard sample at 700 nm against the mixture PR: 50 mmol/L solution of oxalic acid=1:1 (v/v) as a reference sample. Using the precise formula, we calculated the concentration of ascorbic acid in the analysed solution.
Measurement of Nitric Oxide Concentration in Aqueous Humour and Serum
The NO concentration in the samples was measured using the technique described by Green et al[14]. We pippeted 0.1 mL 3 mol/L perchloric acid-PCA, 0.4 mL 20 mmol/L ethylendiaminetetraacetic-EDTA and 0.2 mL of analysed solution into the Eppendorf tube (aqueous humour-diluted 1/10 by distilled water). The samples were incubated on -4°C for 10min, centrifuged for 4min on 1500 rpm. Supernatant of the analysed solution was dropped out, and the precipitate was mixed in Green's reagent. Green's reagent was prepared directly prior to the measurements. Distilled water was used for control measurements. In the test tube we put 0.1 mL of extracted plasma, 250 µL of Green's reagent and 125 µL of ammoniac buffer (pH 9)- mixed solutions of ammonia (1 mol/L) and ammonium chloride (1 mol/L). After stabilisation of the mixture, concentration of NO was measured by spectrophotometric method on 550 nm; and was compared to NO standards.
Enzyme-linked Immunosorbent Assay
Sera (undiluted) and aqueous humour (diluted 1/10 by distilled water) were collected by single needle stick from cubital vein and from anterior chamber of the eye from selected patients; and stored at -80°C until thawed for assay. Serum and humour levels of cytokines were measured in a sample with high sensitivity enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, Minn, USA) specific for human cytokines. Labelling procedure was conducted according to R&D Systems instructions manual. All measurements were performed in triplicate.
Statistical Analysis
The one-way ANOVA or Kruskal-Wallis tests were performed using SPSS 19.0 statistical software package (SPSS Inc., Chicago, IL, USA). The results were expressed as the mean±SE. All P values were 2-sided and a P value <0.05 was considered statistically significant (significance levels as indicated in figure legends). Spearman's correlation coefficients were calculated to assess the relationships between TNF-α, and NO. Exact P values and the number (n) of patients are given in the results section and figure legends of the respective study.
RESULTS
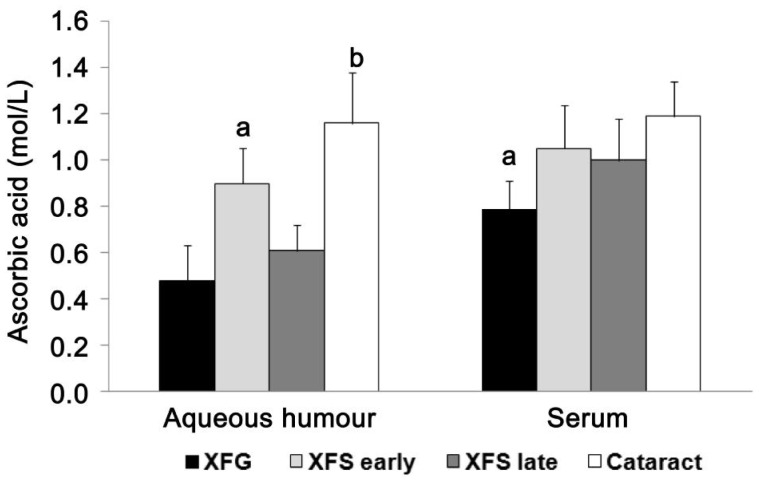
Aqueous humour concentration of ascorbic acid is significantly lower in patients with late XFS and XFG compared to patients with early XFS and cataract, while there was no difference in serum concentration in all examined groups Ascorbic acid concentration in aqueous humour and serum in the control group (cataract), as well as in patients with XFG and XFS, are presented in Figure 1. The concentrations of ascorbic acid in the aqueous humour from patients with developed XFG (0.48±0.15 mmol/L) and late stage of XFS (0.61±0.11 mmol/L) were significantly lower (P<0.05; P<0.001) compared to patients with early stage (0.9±0.15 mmol/L) of XFS and cataract (1.16±0.22 mmol/L). When we analysed serum concentrations of ascorbic acid in patients with XFG (0.79±0.12 mmol/L), XFS-early (1.05±0.19 mmol/L) and late stage (1.0±0.18 mmol/L) and cataract (1.19±0.15 mmol/L), there was statistically significant decreased concentrations of ascorbic acid in patients with XFG compared with other groups of patients (XFS-early and late stage, and control group), P<0.05 (Figure 1).
Figure 1. Aqueous humour and serum concentrations of ascorbic acid (n=30 patients per group).
Measurements of concentrations of ascorbic acid in aqueous humour indicated significantly decreased (P<0.001) level in patients with developed XFG and late XFS compared to patients of other two groups. There was no statistical significant difference in serum concentration of ascorbic acid in all examined groups. aP<0.05, bP<0.001.
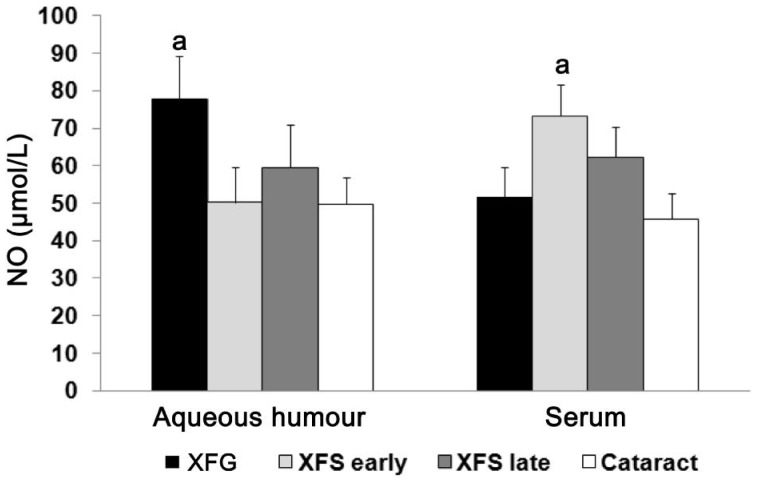
Aqueous humour concentration of NO is significantly higher in patients with XFG compared to patients with early XFS and cataract, while serum concentration was increased in the early stage of XFS. NO concentration in aqueous humour and serum in the control group (cataract), as well as in patients with XFG and XFS (early and late stage), are shown in Figure 2. We found that the concentration of NO in the aqueous humour from patients with developed XFG (77.7±11.4 µmol/L) was significantly increased (P<0.05) in comparison to early XFS (50.27±9.34 µmol/L) and cataract (49.77±7.1 µmol/L) (Figure 2). In our study we demonstrated that there was a significant difference (P<0.05) in NO serum concentrations between patients with early XFS (73.26±8.29 µmol/L) and cataract (45.73±6.98 µmol/L) and XFG (51.54±8.23 µmol/L) (Figure 2).
Figure 2. Aqueous humour and serum concentrations of NO (n=30 patients per group).
NO concentration in aqueous humour was significantly increased (P<0.05) in patients with developed XFG compared to patients with XFS-early and late stage and cataract. Serum concentration of the NO was significantly increased in early XFS when compared to other examined groups; aP<0.05.
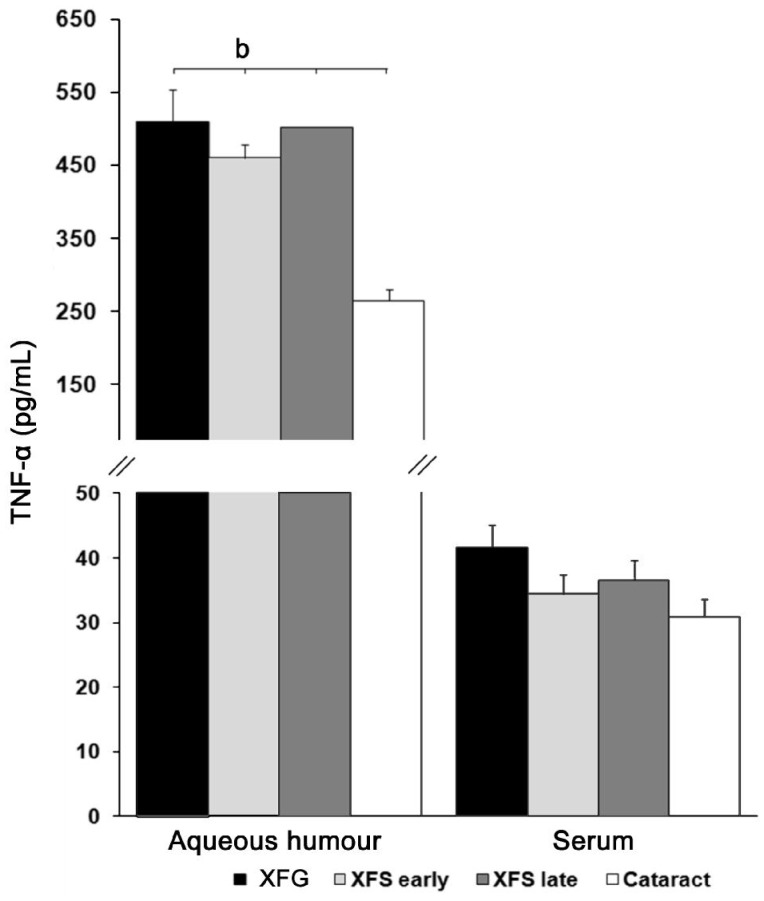
Aqueous humour level of proinflammatory cytokine TNF-α is increased in patients with XFS (early/late) and XFG, while there was no difference in serum level in all examined groups of patients. To study the participation of secreted TNF-α in XFS/XFG, we examined the level of this proinflammatory cytokine in aqueous humour and serum in the patients with cataract, XFG and XFS (Figure 3). Measurements of TNF-α in the aqueous humour indicated significant elevated (P<0.001) level of this proiflammatory cytokine in XFG (510.34±43.07 pg/mL) and XFS (early 460.04±18.32 pg/mL; late 502.42±53.23 pg/mL) in relation to the level in control (264.30±16.4 pg/mL) group. Measurements of TNF-α in the serum of selected patients did not signify statistical differences (P>0.05) between the selected groups. In XFG group the measured level was 41.5±3.55 pg/mL, in early XFS group the level was 34.42±2.98 pg/mL, in late XFS group it was 36.56±3.08 pg/mL, and in the group with cataract 30.95±2.6 pg/mL (Figure 3).
Figure 3. Aqueous humour and serum levels of the TNF-α (n=30 patients per group).
Aqueous humour level of proinflammatory cytokine TNF-α was significantly increased (P<0.001) in patients with XFS (early/late) and XFG compared to patients with cataract. Measurements of TNF-α in the sera of all examined groups did not signify statistical differences. bP<0.001.
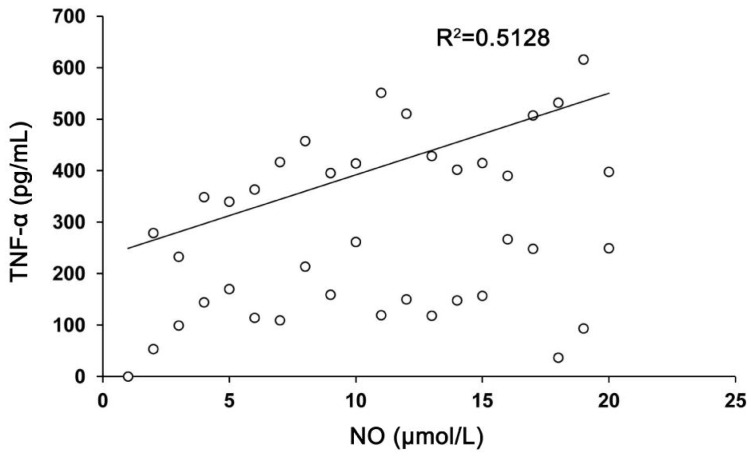
There was a strong positive correlation between aqueous humour levels of TNF-α and NO in XFS and XFG patients. We correlated all results, but we observed only a strong positive correlation between TNF-α and NO in XFS and XFG patients. The results of the relation between aqueous humour level of TNF-α in patients with XFS and the level of NO in patients with XFG (Figure 4) showed a strong positive correlation (r=0.7161; P<0.001). This result can be explained by the fact that TNF-α level was increased in all phases of XFG development, so it can maintain the inflammation in the tissue in the phase of XFG.
Figure 4. Correlation between the aqueous humour levels of TNF-α in patients with XFS and level of NO in patients with XFG signified a strong positive correlation (r=0.7161; P<0.001).
DISCUSSION
Our results indicated statistically significant higher concentration of ascorbic acid in the aqueous humour of patients with cataract in comparison with pseudoexfoliation groups (syndrome-late/early and glaucoma) patients. Koliakos et al[15] also established decreased concentrations of ascorbic acid in XFS, but they did not indicate for its concentrations in XFG. Using those results we have shown the protective role of ascorbic acid in the process of pseudoexfoliation production. Ascorbic acid is important in the proper function of the immune system[12].
Contrary to ascorbic acid, NO concentration was statistically significantly increased in aqueous humour in patients with developed XFG in comparison to patients with early XFS and cataract. These values indicated that in aqueous humour of patients with developed glaucoma ascorbic acid level was decreased with significantly higher level of NO. The endothelium of blood vessels uses NO to signal the surrounding smooth muscle to relax, thus resulting in vasodilatation and increasing blood flow in the tissue by inhibiting vascular smooth muscle contraction and growth, platelet aggregation, and leukocyte adhesion to the endothelium[16]. Phagocytes, as important cells in the immune response, can generate NO[17] by activating NOS using TNF-α as the second signal[18]. Our study indicated significantly higher level of this proinflammatory cytokine in aqueous humour of patients with pseudoexfoliation material, but not in the serum of patients with XFS/XFG. Also, we established a strong positive correlation between aqueous humour levels of TNF-α in XFS patients and NO levels in patients with XFG. All those results explained the fact that TNF-α is the second signal molecule in NO action, and that TNF-α can maintain the inflammation in the tissue in the phase of XFG. Moreover, this is the explanation for increased NO production only locally, in the eye microenvironment, as the final products of inflammatory reaction in the tissue[18].
Many different studies have assessed the systemic and local status of oxidative stress parameters of patients with XFG[5],[19]. Oxidative status in the eye of patients with XFG is furthermore disturbed by increased intraocular pressure owing to accumulated pseudoexfoliation in the iridocorneal angle and decreased blood flow of the retinal blood vessel. Locally disrupted oxidative/antioxidative balance and releasing of its products initiated inflammatory reaction as the first step in pseudoexfoliation production in the tissue[20], with accumulation of different inflammatory cells (phagocytes, lymphocytes, etc.) followed by TNF-α release[21]. Phagocytes in the inflamed tissue can produce NO to generate defense against disturbed homeostasis and to activate vasodilatation of local blood vessel, using TNF-α as a signaling molecule[9]. Dilatation of blood vessels can improve local oxidative status and can control inflammatory reaction[22].
Glaucoma is an ocular disease which is characterized by disturbed oxidative/antioxidative status activated by increased intraocular pressure and decreased blood flow in the retinal blood vessel and with the loss of retinal ganglion cells by apoptotic process[23]–[24]. Pressure-loaded glial cells produce TNF-α, as the second signal of NO action; and ascorbic acid is consumed in the struggle for ocular homeostasis[25]. The final result of TNF-α action can be the death of oligodendrocytes and retinal ganglion cells, as part of neurodegeneration[26]–[27].
The applicability of this manuscript is in the fact that disturbed oxidative parameters can provoke pseudoexfoliation production, so their improvement can stop this very complex process which can lead to a serious ocular disease with unknown outcome.
Patients with pseudoexfoliation can have disturbed oxidative status with decreased concentrations of antioxidants and increased levels of oxidants. Ascorbic acid concentrations decrease with the enhance of metabolism activities. There is a higher level of reactive oxygen species production in the eye with inflammation, while ascorbic acid concentrations are reduced as the part of it being used in the defense from inflammation. NO, as an endogenously generated gaseous molecule, has a protective role in the process of XFG development activating local blood vessel dilatation using TNF-α as a secondary signal molecule.
Acknowledgments
We would like to thank Marija Zdravkovic for language revision of the manuscript.
Conflicts of Interest: Sarenac Vulovic T, None; Pavlovic S, None; Jakovljevic V, None; Janicijevic K, None; Zdravkovic N, None.
REFERENCES
- 1.Elhawy E, Kamthan G, Dong CQ, Danias J. Pseudoexfolation syndrome, a systemic disorder with ocular manifestations. Hum Genomics. 2012;6:22. doi: 10.1186/1479-7364-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlötzer-Schrehardt U. Oxidative stress and pseudoexfoliation glaucoma. Klin Monbl Augenheilkd. 2010;227(2):108–113. doi: 10.1055/s-0028-1109977. [DOI] [PubMed] [Google Scholar]
- 3.Zenkel M, Lewczuk P, Jünemann A, Kruse FE, Naumann GO, Schlötzer-Schrehardt U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am J Pathol. 2010;176(6):2868–2879. doi: 10.2353/ajpath.2010.090914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlötzer-Schrehardt U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J Ophthalmol. 2011;18(1):30–36. doi: 10.4103/0974-9233.75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, He M, Zhou M, Zhang X. Ocular pseudoexfoliation syndrome and vascular disease: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92767. doi: 10.1371/journal.pone.0092767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanito M, Kaidzu S, Takai Y, Ohira A. Status of systemic oxidative stresses in patients with primary open-angle glaucoma and pseudoexfoliation syndrome. PLoS One. 2012;7(11):e49680. doi: 10.1371/journal.pone.0049680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin YS, Lin CH, Huang LD, Chao T, Kuo CD, Hung LC, Wong FH, Lin CC, Fu SL. The suppression of thoc1 in cancer cell apoptosis mediated by activated macrophages is nitric oxide-dependent. Biochem Pharmacol. 2013;86(2):242–252. doi: 10.1016/j.bcp.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Babizhayev MA, Deyev AI. Management of the virulent influenza virus infection by oral formulation of nonhydrolized carnosine and isopeptide of carnosine attenuating proinflammatory cytokine-induced nitric oxide production. Am J Ther. 2012;19(1):e25–47. doi: 10.1097/MJT.0b013e3181dcf589. [DOI] [PubMed] [Google Scholar]
- 9.Malaviya R, Gow AJ, Francis M, Abramova EV, Laskin JD, Laskin DL. Radiation-induced lung injury and inflammation in mice: role of inducible nitric oxide synthase and surfactant protein D. Toxicol Sci. 2015;144(1):27–38. doi: 10.1093/toxsci/kfu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorkhabi R, Ghorbanihaghjo A, Ahoor M, Nahaei M, Rashtchizadeh N. High-sensitivity C-reactive protein and tumour necrosis factor alpha in pseudoexfoliation syndrome. Oman Med J. 2013;28(1):16–19. doi: 10.5001/omj.2013.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich M, Diegelmann J, Beigel F, Brand S. IL-17A alone weakly affects the transcriptome of intestinal epithelial cells but strongly modulates the TNF-α-induced expression of inflammatory mediators and inflammatory bowel disease susceptibility genes. Inflamm Bowel Dis. 2014;20(9):1502–1515. doi: 10.1097/MIB.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 12.Fukui H, Iwahashi H, Endoh S, Nishio K, Yoshida Y, Hagihara Y, Horie M. Ascorbic acid attenuates acute pulmonary oxidative stress and inflammation caused by zinc oxide nanoparticles. J Occup Health. 2015;57(2):118–125. doi: 10.1539/joh.14-0161-OA. [DOI] [PubMed] [Google Scholar]
- 13.Rutkowski M, Grzegorczyk K. Modifications of spectrophotometric methods for antioxidative vitamins determination convenient in analytic practice. Acta Sci Pol Technol. Aliment. 2007;6(3):17–28. [Google Scholar]
- 14.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Koliakos GG, Konstas AG, Schlötzer-Schrehardt U, Bufidis T, Georgiadis N, Ringvold A. Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol. 2002;134(6):879–883. doi: 10.1016/s0002-9394(02)01797-x. [DOI] [PubMed] [Google Scholar]
- 16.Quillon A, Fromy B, Debret R. Endothelium microenvironment sensing leading to nitric oxide mediated vasodilation: a review of nervous and biomechanical signals. Nitric Oxide. 2015;45:20–26. doi: 10.1016/j.niox.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Green SJ, Mellouk S, Hoffman SL, Meltzer MS, Nacy CA. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990;25(1-3):15–19. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee MY, Sun KH, Chiang CP, Huang CF, Sun GH, Tsou YC, Liu HY, Tang SJ. Nitric oxide suppresses LPS-induced inflammation in a mouse asthma model by attenuating the interaction of IKK and Hsp90. Exp Biol Med (Maywood) 2015;240(4):498–507. doi: 10.1177/1535370214554880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dursun F, Vural Ozec A, Aydin H, Topalkara A, Dursun A, Toker MI, Erdogan H, Arici MK. Total oxidative stress, paraoxonase and arylesterase levels at patients with pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Int J Ophthalmol. 2015;8(5):985–990. doi: 10.3980/j.issn.2222-3959.2015.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yildirim Z, Yildirim F, Uçgun NI, Sepici-Dinçel A. The role of the cytokines in the pathogenesis of pseudoexfoliation syndrome. Int J Ophthalmol. 2013;6(1):50–53. doi: 10.3980/j.issn.2222-3959.2013.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tezel G, Thornton IL, Tong MG, Luo C, Yang X, Cai J, Powell DW, Soltau JB, Liebmann JM, Ritch R. Immunoproteomic analysis of potential serum biomarker candidates in human glaucoma. Invest Ophthalmol Vis Sci. 2012;53(13):8222–8231. doi: 10.1167/iovs.12-10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Magableh MR, Kemp-Harper BK, Ng HH, Miller AA, Hart JL. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(1):67–74. doi: 10.1007/s00210-013-0920-x. [DOI] [PubMed] [Google Scholar]
- 23.Chang D, Sha Q, Zhang X, Liu P, Rong S, Han T, Liu P, Pan H. The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS One. 2011;6(11):e27218. doi: 10.1371/journal.pone.0027218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslan M, Dogan S, Kucuksayan E. Oxidative stress and potential applications of free radical scavengers in glaucoma. Redox Rep. 2013;18(2):76–87. doi: 10.1179/1351000212Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavet ME, Vittitow JL, Impagnatiello F, Ongini E, Bastia E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest Ophthalmol Vis Sci. 2014;55(8):5005–5015. doi: 10.1167/iovs.14-14515. [DOI] [PubMed] [Google Scholar]
- 26.Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res. 2014;39(2):105–119. doi: 10.3109/02713683.2013.836541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dvoriantchikova G, Ivanov D. Tumor necrosis factor-alpha mediates activation of NF-κB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways. Eur J Neurosci. 2014;40(8):3171–3178. doi: 10.1111/ejn.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]