Abstract
AIM
To determine real life clinical outcomes in poorly responsive and treatment-naïve neovascular age related macular degeneration (nvAMD) patients using bimonthly fixed dosing aflibercept regimen.
METHODS
This was a retrospective study of 165 eyes with nvAMD started on aflibercept at Southampton Eye Unit between June 2013 and June 2014. Patients were either switched from pro re nata (PRN) ranibizumab/bevacizumab due to poor response (107 eyes), or treatment-naïve (58 eyes). Patients initially received 3-monthly intravitreal aflibercept injections followed by 2-monthly fixed doses. Clinic visits were scheduled at month 0, 4, 10 and 12. Mean change in best-corrected visual acuity (BCVA) and central retinal thickness (CRT) from baseline were assessed using the Wilcoxon signed-rank test. The proportion of patients maintaining BCVA (<15 letters loss) at 12mo was also evaluated.
RESULTS
Mean BCVA change at month 12 was +3.29 and +4.67 letters in the switched and naïve aflibercept groups respectively (P<0.01). BCVA was maintained in 95.3% of switched and 96.6% of naïve patients. CRT at month 12 showed a decrease of -6.16 µm in the switched group and -35.36 µm in the naïve group (P<0.01). Patients previously treated with ranibizumab/bevacizumab had on average received 7.4 ranibizumab/bevacizumab injections over 12.6mo, attending 10 clinic visits. The fixed dosing aflibercept regimen required an average of 7.1 injections (naïve group), 7.5 injections (switched group) and 4 clinic visits per year.
CONCLUSION
Fixed bimonthly aflibercept is effective in both treatment-naïve and poorly responsive nvAMD patients. Adopting a fixed dosing regimen can reduce patient burden without compromising on outcomes.
Keywords: age-related macular degeneration, ranibizumab, aflibercept, anti-vascular endothelial growth factor
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of blindness in the developed world[1]. Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis in neovascular age-related macular degeneration (nvAMD) and monthly intravitreal injections of the anti-VEGF therapies ranibizumab and bevacizumab have shown improved visual outcomes with similar efficacy[2]–[3]. Studies on aflibercept (a recombinant fusion protein which binds all VEGF-A isoforms and placental growth factors 1 and 2) suggest a higher binding affinity to VEGF with non-inferior clinical outcomes when administered in 2 monthly doses to treatment-naïve nvAMD patients[4]. New evidence suggests that the most favourable long-term visual outcomes are seen when proactive continuous anti-VEGF treatments are given as compared to sporadic or as-needed therapy[5].
Improved visual and anatomical outcomes have also been reported in patients with poorly responsive choroidal neovascularisation (CNV) after switching to aflibercept treatment[6]–[17]. However unlike the fixed dosing regimen used in the seminal VIEW studies[4], these aflibercept studies and case series mostly had short follow-up and followed pro re nata (PRN), variable dosing or treat and extend regimens[6]–[17]. Data from real life bimonthly fixed dosing studies of aflibercept are lacking in current literature. Furthermore, results from major clinical trials have overall shown better visual improvement using fixed proactive dosing regimens[4],[18]–[19] compared with variable dosing anti-VEGF regimens[20]–[24].
There is also currently little data regarding how different CNV subtypes respond to aflibercept therapy. While the VIEW studies reported a higher proportion of minimally and predominantly classic CNV compared to occult CNV, no subtype analysis was performed[4]. Further study is therefore required to determine whether CNV subtypes influence aflibercept treatment outcomes.
The primary aim of this study was to evaluate visual and anatomical outcomes in both poorly responsive and treatment-naïve nvAMD patients started on a bimonthly fixed dosing regimen of aflibercept treatment. Additionally, CNV subtypes were evaluated as possible baseline predictors of treatment response in these patients.
SUBJECTS AND METHODS
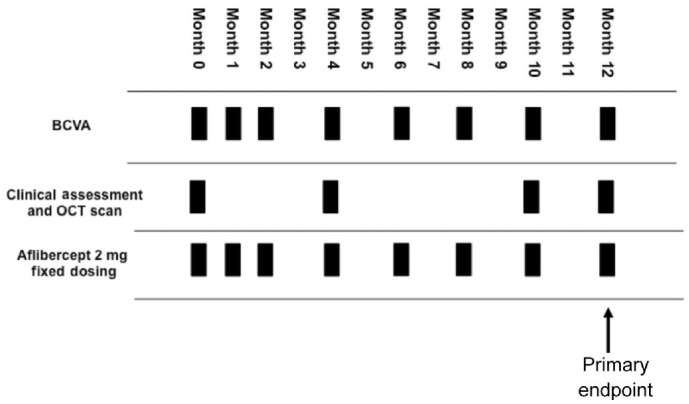
This was a retrospective consecutive review of 145 nvAMD patients (172 eyes) who were started on a fixed bimonthly dosing regimen of 2 mg aflibercept treatment from June 2013 to June 2014 at Southampton Eye Unit. Following 3 monthly loading doses of aflibercept 2 mg, treatment was then administered every 2mo. Patients were reviewed in clinic with visual acuity, optical coherence tomography (OCT) scanning (Topcon 3D OCT-2000) and slit lamp examination at months 0, 4, 10 and 12. At intervening injection visits, only visual acuity was recorded with no assessment in clinic. The OCT clinic assessment visit at month 8 was omitted as per new local aflibercept clinical protocol partly due to pressure on AMD clinic appointments and patients only had visual acuity recorded before aflibercept injection at month 8. At any visit if acuity dropped by 5 letters in either eye, this prompted further OCT patient assessment in clinic. A further aflibercept injection was given at month 12 if there were persistent signs of active nvAMD, as guided by OCT findings. Patients therefore attended 4 clinic/OCT visits and received a total of 7 to 8 injections over the 12mo (Figure 1). Overall, 139 patients (165 eyes) were eligible for inclusion in the study after 4 patients were lost to follow-up and 2 patients who deceased early in the study were excluded. Institutional review board approval was obtained prospectively from the University Hospital Southampton National Health Service Foundation Trust. This study followed the tenets of the Declaration of Helsinki. Data was extracted from the Medisoft electronic patient database (Medisoft, Leeds, UK) and patient records.
Figure 1. Fixed dosing and reduced monitoring year 1 aflibercept treatment protocol used in study.
BCVA: Best-corrected visual acuity; OCT: Optical coherence tomography.
Best corrected visual acuity (BCVA) as Early Treatment Diabetic Retinopathy Study (ETDRS) letters and mean central retinal thickness (CRT) measurements for 12mo after switch to aflibercept treatment in nvAMD patients poorly responsive to treatment with ranibizumab/bevacizumab (switched group; poor responders defined as patients with poor OCT and visual response to at least 3 previous monthly injections) and treatment-naïve nvAMD patients (naïve group) was recorded. Prior to the aflibercept switch, all patients followed the IVAN trial PRN methodology[3]. Number of injections and clinic visits were also recorded. Results were analysed along with data from other aflibercept fixed dosing studies and variable dosing studies such as PRN studies, treat and extend studies and case series reports.
Patient CNV subtypes [predominantly classic (PC), minimally classic (MC), occult, fibro-vascular pigment epithelium detachment (FVPED) and peripapillary CNV (PPCNV)] were determined based on fluorescein angiography (FFA) and OCT features for both switched and naïve aflibercept treatment groups.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism 6 (GraphPad Software, La Jolla California, USA). Data collected was quantitative and could be replicated into GraphPad Prism 6 for analysis. Visual acuity statistical analyses included mean BCVA over time, mean change in BCVA compared to study entry point and proportion of patients maintaining vision (<15 letters lost) at 12mo. The proportion of patients with a gain of ≥5 letters, ≥10letters and ≥15 letters was also ascertained. Anatomical analyses included mean OCT recorded CRT over time and mean change in CRT compared to study entry point.
Non-parametric paired Wilcoxon rank tests (using 2-tailed correction for multiple comparisons) were used to compare the difference in means between study entry point and each follow-up appointment, for both BCVA and CRT values in both groups. Non-parametric unpaired Mann-Whitney U test (2-tailed using 2-tailed correction for multiple comparisons) was used to compare the difference between baseline mean BCVA, CRT and number of injection between switched and naïve treatment groups. Non-parametric Kruskal-Wallis test was used to compare mean BCVA change and mean final BCVA between different CNV subtypes. Unpaired t-test was used to compare baseline age between the two groups. Fisher's exact test was used to compare the proportions of male to female patients and different CNV subtypes between switched and naïve treatment groups. A P value of ≤0.05 was considered to be statistically significant. In cases where monthly BCVA or CRT readings were missing, the last observation carried forward rule was applied for data analysis.
RESULTS
Patient Demographics and Baseline Characteristics
Eighty-eight patients (107 eyes) were eligible to be included in the switched aflibercept group, while the treatment-naïve aflibercept group had 51 participants (58 eyes). Study entry point baseline characteristics were similar between the switched and naïve aflibercept groups (Table 1). The mean patient age was younger in the switched group (80.0y) compared with the naïve group (82.8y) (P=0.01). In both the treatment groups there was a higher proportion of female patients. There was no significant difference in the male to female ratio between the two groups (P=0.07). Overall, there were fewer PC (7%) and MC (5%), compared to occult CNV lesions (87%, including occult and FVPED). There were significantly fewer FVPED lesions in the naïve group (29%) compared with switched patients (48%), but no significant differences were noted in the prevalence of other CNV subtypes. No adverse events (such as a retinal pigment epithelium rip or endophthalmitis) occurred in any patients during the study period.
Table 1. Patient demographics and baseline characteristics.
| Parameters | Switched aflibercept (n=107) | Naïve aflibercept (n=58) | P |
| Age (a, range) | 80.0±7.4 (62-96) | 82.8±7.4 (60-96) | 0.01a |
| Sex (n, %) | 0.07 | ||
| F | 60 (56) | 41 (70) | |
| M | 47 (44) | 17 (30) | |
| CNV subtypes (n, %) | |||
| PC | 7 (7) | 4 (7) | 1.0 |
| MC | 3 (3) | 6 (10) | 0.07 |
| Occult | 44 (41) | 31 (53) | 0.14 |
| FVPED | 51 (48) | 17 (29) | 0.03a |
| PPCNV | 2 (2) | 0 (0) | 0.54 |
| BCVA at study entry point (ETDRS letters, 95% CI) | 56.3±14.8 (53.5-59.1) | 54.1±13.0 (50.6-57.5) | 0.33 |
| Baseline CRT at entry point (µm, 95% CI) | 254.4±34.5 (247.8-261.0) | 284.2±55.1 (269.7-298.7) | <0.0001a |
| Follow-up (mo) | 12 | 12 | - |
| Mean No. of injections | 7.5±0.9 | 7.1±0.8 | 0.003a |
aSignificant, 14 of the 107 eyes had received a combination of ranibizumab and bevacizumab therapy. PC: Predominantly classic; MC: Minimally classic; FVPED: Fibrovascular pigment epithelial detachment; PPCNV: Peri-papillary choroidal neovascular membrane; SD: Standard deviation; CI: Confidence interval; ETDRS: Early Treatment Diabetic Retinopathy Study.
x±s
Mean BCVA at baseline was 56.3 letters (20/75) in the switched group and 54.1 letters (20/83) in the naïve group (P=0.33). Mean baseline CRT was significantly less in the switched group, 254.4 µm, compared with the naïve group, 284.2 µm (P<0.0001).
Within the aflibercept switched group, the mean follow-up period before switch to aflibercept was 12.6mo with patients receiving on average 7.4 injections (all had received ranibizumab, except for 14 of 107 patients who had received a combination of ranibizumab and bevacizumab) and attending on average 10 clinic appointments with OCT scans per year. The mean time between last ranibizumab/bevacizumab injection and first aflibercept injection was 9.2wk. Following the switch to fixed dosing aflibercept, patients received on average 7.5 injections compared with 7.1 injections in the naïve group over 12mo follow-up (P=0.003). All patients treated with the aflibercept regimen attended 4 clinic visits over 12mo.
Visual Acuity
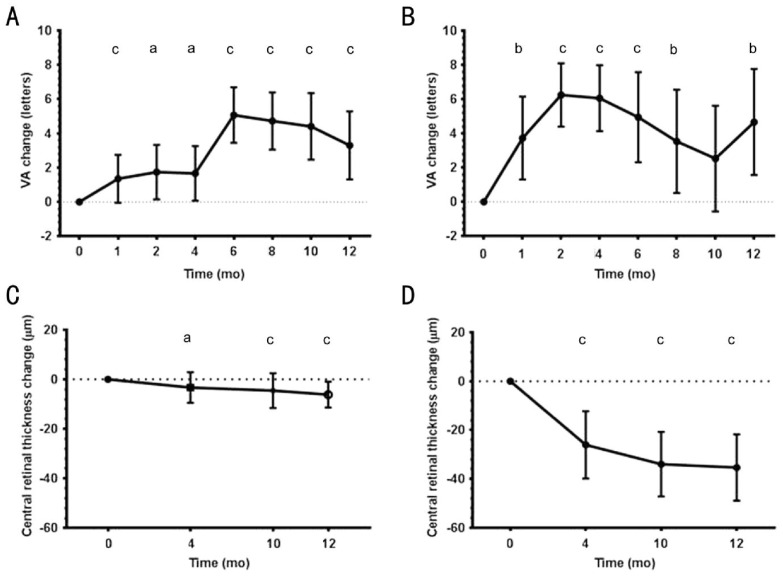
After switching to aflibercept, BCVA improved significantly at all follow-up visits compared with baseline (P<0.02) (Figure 2A). A mean gain of +3.28 (±10.53 standard deviation) letters was seen at month 12 (P=0.0005). There appeared to be a steep gain of 3.4 letters from months 4 to 6. At the end of month 12, 47% had gained ≥5 letters, 30% gained ≥10 letters and 16% gained ≥15 letters; 95% of patients maintained BCVA at 12mo (Table 2).
Figure 2. Mean ETDRS letter visual acuity changes.
Acuity changes shown over 12mo from time of first aflibercept treatment for A) poorly responsive patients switched to aflibercept, B) treatment-naïve patients. Corresponding change in central retinal thickness over 12mo from time of first aflibercept treatment for C) poorly responsive patients switched to aflibercept, D) treatment-naïve patients. VA: Visual acuity; SD: Standard deviation; CI: Confidence intervals. Significance are shown (aP<0.05, bP<0.01, cP<0.001).
Table 2. Mean ETDRS letter gain and proportion of ETDRS letters gained for each treatment group.
| Study groups | Follow-up (mo) | Mean BCVA change (ETDRS) | ≥5 letters gain | ≥10 letters gain | ≥15 letters gain | <15 letter loss |
| Switched aflibercept (n=107) | 12 | +3.28 | 50 (47) | 31 (30) | 17 (16) | 102 (95) |
| Naïve aflibercept (n=58) | 12 | +4.67 | 30 (52) | 21 (36) | 10 (17) | 56 (97) |
ETDRS: Early treatment diabetic retinopathy study.
n (%)
Significant improvements in BCVA were also seen in the treatment naïve patients compared to baseline (P<0.01), with the exception of month 10 (P=0.145) (Figure 2B). A mean gain of +4.67 (±11.81 standard deviation) letters was seen over the 12-month period (P=0.0033). Fifty-two percent of patients gained ≥5 ETRS letters, 36% gained ≥10 letters and 17% gained ≥15 letters; 97% maintained BCVA at 12mo (Table 2).
Central Retinal Thickness
CRT showed little variation within the switched aflibercept group, with a maximum mean decrease of -6.16 µm at month 12. This decrease in CRT was small and reached significance at months 4 to 12 as compared to baseline (Figure 2C).
In the treatment-naïve aflibercept group, changes in CRT compared to entry point were significantly reduced at all observed time points, with a maximum mean decrease of -35.36 µm at month 12 (P=0.0001) (Figure 2D).
Choroidal Neovascularisation Subtypes
While variations in visual outcomes were observed when comparing CNV subtypes, patient numbers for many CNV sub-groups were small and these did not reach statistical significance (Table 3). In the total cohort, visual improvement was greater for PC CNV type compared with MC, occult and FVPED CNV types (P=0.53). Both eyes with PPCNV had poor visual outcomes despite aflibercept treatment. In the switched group, eyes with FVPED and PC CNV showed the greatest visual improvements of +5.12 and +4.79 letters respectively. In the naïve group, PC CNV showed the most improvement of +10.25 letters. Across the entire aflibercept cohort, at 12mo the mean final BCVA was 58.68 letters (20/67) for PC CNV, 48.5 letters (20/107) for MC and 60.02 letters (20/63) for occult CNV types (occult, and FVPED); this difference between CNV types did not reach significance (P=0.13).
Table 3. Mean change in ETDRS letters with aflibercept treatment over 12mo for different choroidal neovascularisation subtypes across aflibercept groups.
| BCVA change in groups | Predominantly classic | Minimally classic | Occult | FVPED | PPCNV |
| Entire cohort (n=165)1 | 11 (+6.77±11.4) | 9 (+0.28±12.2) | 75 (+3.67±10.4) | 68 (+4.57±10.7) | 2 (-21.0±12.7) |
| Switched group (n=107)2 | 7 (+4.79±11.7) | 3 (-3.5±2.78) | 44 (+2.47±9.64) | 49 (+5.12±10.22) | 2 (-21±12.7) |
| Naïve group (n=58)3 | 4 (+10.25±11.53) | 6 (+2.17±14.9) | 31 (+5.39±11.3) | 17 (+2.94±12.2) | 0 (-) |
Non-parametric Kruskal-Wallis test comparison of mean BCVA change across all CNV subtypes: 1Entire cohort P=0.13; 2Switched group P=0.05; 3Naïve group P=0.58. ETDRS: Early Treatment Diabetic Retinopathy Study; CNV: Choroidal neovascularisation; BCVA: Best-corrected visual acuity; SD: Standard deviation; FVPED: Fibrovascular pigment epithelial defect; PED: Pigment epithelial defect; PPCNV: Peripapillary choroidal neovascularisation.
n (x±s)
DISCUSSION
This study investigated the visual and anatomical outcomes for nvAMD patients treated with a fixed bimonthly dosing regimen of aflibercept. Our data shows significant improvements in mean BCVA and CRT at 12mo for both poorly responsive patients and treatment-naïve patients. Poorly responsive patients switched to aflibercept showed only a small decrease in mean CRT compared to treatment-naïve patients, but both groups had significant visual improvements at 12mo follow-up. Mean BCVA improvement was +3.28 letters in the poorly responsive group and +4.67 letters in the treatment-naïve group, with over 95% of all patients maintaining vision at 12mo. Visual acuity improvement peaked late at month 6 in the switched group, which appears to be a delayed effect after the loading dose. While the frequency of aflibercept injections remained similar to that for prior treatment with ranibizumab, the number of clinic appointments decreased from an average of 10 per year with PRN ranibizumab dosing to 4 per year with fixed dosing aflibercept treatment. Adopting this regimen reduced patient clinic visits by up to 6 per year, this could potentially make savings for stretched AMD clinic resources. For example, in a sample AMD cohort of 1000 patients, switching from monthly PRN dosing to fixed bimonthly aflibercept could save up to 6000 clinic appointments per year.
Furthermore, visual outcomes for our switched group were favourable to those of previous studies on aflibercept in poorly responsive nvAMD with 12mo follow-up (Table 4). While these studies showed anatomical improvements, visual gains were not significant[14]–[16]. Baseline visual acuity and patient demographics in our study were similar to those from previous reports. Our cohort had fewer injections with ranibizumab prior to commencing fixed dosing aflibercept treatment, which may partly account for the superior visual outcomes we observed. Differences in dosing regimen could also be a contributory factor. Of note, the only studies to report comparable BCVA improvements to our study also employed a fixed dosing aflibercept regimen[7],[17]. The mean visual improvements reported by Chang et al[7] and Singh et al[17] were +6.9 letters and +5.9 letters respectively at 6mo, compared with +5.1 letters at 6mo in our switched cohort. Moon da et al[25] reported visual improvements in 29 treatment refractory nvAMD (n=18) and PCV (n=14) patients where VA and OCT improved from 0.58 to 0.55 (P=0.005) and from 404 to 321 µm (P<0.001) respectively after switching to aflibercept. Kawashima et al[26] in a small group of ranibizumab resistant nvAMD (n=15) and PCV patients (n=26) reported 1 line BCVA improvement in PCV but not in AMD patients. In a smaller study by Oishi et al[27] (nvAMD=46 and PCV=42), logMAR BCVA improved from 0.36 to 0.21. Overall, the mean BCVA improvement of +4.67 letters in our naïve nvAMD patient group is comparable to a recently published UK study by Talks et al[28] which showed a +5.1 letter mean improvement.
Table 4. Summary of published studies on 6-12mo visual outcomes in poorly responsive neovascular age-related macular degeneration patients switched to aflibercept (last screened by PubMed on 11th September 2015).
| Study (n, eyes) | Aflibercept dosing regimen | Study design | Mean follow-up (mo) | Average age (a) | Male (%) | Mean baseline BCVA (ETDRS letters)1 |
| Study switched group,107 | Fixed dosing-3 monthly then bimonthly | Retrospective | 12 | 81.6 (range 60-96) | 44 | 56.3 (20/75) |
| Messenger et al[15],109 | Clinician discretion | Retrospective | 12 | 80.3 (range 59-96) | 44 | 59.7 (20/64) |
| Grewal et al[14],21 | 3 loading doses, monthly or bimonthly thereafter | Prospective | 12 | 80.7 (±4.5 SD) | 43 | 64 (20/53) |
| Arcinue et al[16],63 | Bimonthly (no monthly loading). Switched to monthly if persistent fluid | Retrospective | 12 | 81 (IQR 76-87) | 41 | 65 (20/50) |
| Singh et al[17],26 | Fixed dosing-3 monthly, then bimonthly | Prospective | 6 | 78 (range 69-90) | - | 56.4 (20/75) |
| Wykoff et al[12],46 | 3 loading, one at month 5. IPRN at months 4 and 6 | Prospective | 6 | 77.8 (range 55-95) | 48 | 74.2 (20/33) |
| Kumar et al[11],34 | 3 monthly, follow-up at 6 months | Retrospective | 6 | 79 (IQR 72-84) | 29 | 56.5 (20/74) |
| Gharbiya et al[10],31 | 3 monthly, then PRN dosing | Retrospective | 6 | 70.1 (range 60-86) | 30 | 42.5 (20/142) |
| Ferrone et al[9],221 | Clinician discretion (3 injections 4-9wk apart). | Retrospective | 6 | 83 | 33 | 66 (20/48) |
| Cho et al[8],28 | Treat and extend | Retrospective | 6 | 80.68 (range 62-95) | 50 | 59 (20/66) |
| Chang et al[7],49 | Fixed dosing-3 monthly, then bimonthly | Retrospective | 6 | 77.8 (±7.5 SD) | 43 | 60.5 (20/62) |
| Bakall et al[6],36 | Treat and extend after 3 loading doses | Retrospective | 6 | 79 (range 60-88) | 42 | 62.5 (20/56) |
1Where previous studies have reported BCVA in logMAR, this was converted to ETDRS letter equivalents using a published method[24]. BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; ETDRS: Early Treatment Diabetic Retinopathy Study; IQR: Interquartile range; SD: Standard deviation.
Table 4. Summary of published studies on 6-12mo visual outcomes in poorly responsive neovascular age-related macular degeneration patients switched to aflibercept (last screened by PubMed on 11th September 2015) (continued).
| Study (n, eyes) | Mean BCVA change at last follow-up (ETDRS letters) | Proportion gaining ≥5 or ≥10 ETDRS letters | Mean baseline CRT; mean CRT change (µm) | Mean No. of ranibizumab/ bevacizumab (injections) | Treatment duration before switch to aflibercept (mo) | Average No. of aflibercept during study (injections) |
| Study switched group,107 | +3.3 | 47%≥5 letters; 30%≥10 letters | 254.4; -6.2 | 7.4 | 12.6 | 7.5 |
| Messenger et al[15],109 | -2 | 13%≥10 letters | 324; -25 | 21 | - | 7.2 |
| Grewal et al[14],21 | +1 | - | 329.4; -34.7 | 29.8 | 31.6 | 10.2 |
| Arcinue et al[16],63 | 0 | 25%≥5 letters | 355;-107 | 13 | - | - |
| Singh et al[17],26 | +5.9 | 42%≥5 letters | 304.1; -38.6 | 9.6 | 14 | 5 |
| Wykoff et al[12],46 | +0.2 | 9%≥5 letters | 347;-27.3 | 42 | - | 5.6 |
| Kumar et al[11],34 | +5 | - | 415; -67 | 28.6 | - | 5.3 |
| Gharbiya et al[10],31 | +0.3 | 26%≥5 letters | 449; -180 | 34.4 | 41.3 | 4.5 |
| Ferrone et al[9],221 | -2 | - | 300;0 | 14 | - | 3 |
| Cho et al[8],28 | -2.5 | - | 295;-21 | 20.2 | - | 4.4 |
| Chang et al[7],49 | +6.9 | 55%≥5 letters | 448.4; -89.1 | 34.9 | 40.8 | 5 |
| Bakall et al[6],36 | -2.5 | 22%≥5 letters | 358; -60 | 25.6 | - | - |
Variability in loading doses of aflibercept may also affect treatment outcomes. In a study by Arcinue et al[16] treatment-resistant nvAMD patients were switched to 8-weekly aflibercept injections without 3 initial monthly loading doses. Patients were then switched to 4-weekly aflibercept dosing if there was persistent fluid on OCT despite 8-weekly injections. Thirty-three percent of patients required switching to 4-weekly aflibercept injections during the 12mo period and 44% of eyes lost 5 letters or more. By comparison only 19% of our cohort lost 5 or more letters at 12mo, suggesting that 3 monthly loading doses of aflibercept are beneficial and may have a functional effect over and above retinal thickness reduction. This effect is also seen in the study by He et al[29], where logMAR BCVA improved by 0.2 lines after 3 aflibercept injections in switched patients but marginally decreased by 0.45 lines without fixed dosing at 12mo.
In another variable dosing study, Messenger et al[15] investigated outcomes for 109 treatment-resistant nvAMD patients treated with aflibercept, where dosing was based on clinician discretion. While the mean number of injections reported by this group is similar to that of our study, patients lost on average -2 letters at last follow-up. In addition, the mean number of clinic visits over 1y was 8.7 compared with 4 for our fixed dosing regimen.
The pivotal VIEW studies demonstrated non-inferior outcomes with a reduced dosing aflibercept regimen compared with ranibizumab, 94%-96% of treatment-naïve patients maintaining BCVA with mean gains of +8.3 to 9.3 letters across treatment groups at one year[4]. In our analysis of treatment-naïve nvAMD patients, a mean gain of +4.67 letters was observed with 97% of patients maintaining BCVA over the 12mo period. Baseline visual acuity was similar for our treatment-naïve cohort [54.1 letters (20/83)] compared with the VIEW studies [53.8 letters (20/84)]. One possible explanation for the discrepancy in BCVA improvement is the difference in CNV subtypes studied. Eighty-three percent of the treatment-naive patients in our cohort had occult type CNV lesions, compared with 38% occult CNV subtypes in the VIEW studies. In the VIEW study, the baseline proportion of patients showing classic, MC type CNV was 26% and 36% respectively; compared to 7% and 10% respectively in our study. It is well known that classic type CNV's respond well to treatment while occult type CNV less so. This baseline difference in our cohort could likely explain the atypical treatment response in our study with a slight vision drop over 2 to 10mo and recovery thereafter. While the individual numbers of CNV subtypes in our study were too small to draw firm conclusions, our findings bear some resemblance to results from the MARINA[19] and ANCHOR[18] trials on ranibizumab treatment for nvAMD. The MARINA study treated patients with occult and MC CNV lesions, finding visual gains of up to +7.2 letters at 12mo[19]. By comparison the ANCHOR study investigated patients with predominantly classic CNV lesions, showing even greater BCVA improvements of up to +11.3 letters at 12mo[18]. Analysing visual outcomes by CNV subtype in our cohort also revealed greater mean visual improvement overall for eyes with PC CNV compared to occult and MC CNV. Larger studies are needed to evaluate CNV subtype as a prognostic factor in the treatment of nvAMD by anti-VEGF agents.
Strengths of our study include its large sample size and the use of proactive bimonthly fixed dosing treatments. Recent literature shows lack of similar bimonthly aflibercept studies and those reported have very small sample sizes[30]. The main limitation of our study is its retrospective design. Further long-term follow-up is needed to determine whether improvements in BCVA and OCT are maintained. A possible drawback of this dosing regimen is the potential for over-treating some patients, although a recent study by Peden et al[5] published in Ophthalmology reported favourable long-term visual outcomes with continuous anti-VEGF treatment compared with sporadic, as-needed therapy. Contrastingly, bimonthly fixed dosing regimens may have the risk of undertreating some patients for whom monthly aflibercept may be more beneficial. However, this appears to be untrue as seen in a case series by Grewal et al[14] where patients were started on 3 monthly loading doses of aflibercept before either being switched to bimonthly dosing or continued on monthly treatment if there was persistent fluid on OCT. This case series did not report improved visual outcomes when compared to our cohort.
In summary, we investigated the visual and anatomical outcomes of a fixed bimonthly dosing aflibercept regime in poorly responsive and treatment-naïve nvAMD patients. Significant improvements in visual acuity at 12mo noted in the switched group compared better than reports from previously published studies. The number of aflibercept injections was similar to reports with other anti-VEGF dosing regimens, while visual outcomes were better despite considerably fewer monitoring clinic visits used in this study. However, visual improvements for treatment-naïve patients in our cohort did not reach VIEW trial standards, which may be partly due to different proportions of CNV subtypes. Overall, fixed dosing appears to give the best long-term visual outcomes compared with other types of dosing regimens. This statement is supported from both our study and from Peden et al[5]. Future real-life prospective studies with longer follow-up using fixed dosing regimens replicating clinical trials are essential. A formal cost-benefit analysis of different treatment regimens could also help determine the most effective pathway for managing nvAMD.
Acknowledgments
Conflicts of Interest: Alasdair N Warwick has been supported by a travel grant from Bayer. Andrew J Lotery has served on medical advisory boards for Bayer, Roche and Novartis pharmaceuticals. Srini V Goverdhan has been supported by travel grants from Novartis and Bayer. Hannah H Leaver, None.
REFERENCES
- 1.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC, IVAN study investigators Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122(4):803–808. doi: 10.1016/j.ophtha.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Bakall B, Folk JC, Boldt HC, Sohn EH, Stone EM, Russell SR, Mahajan VB. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156(1):15–22.e1. doi: 10.1016/j.ajo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Chang AA, Li H, Broadhead GK, Hong T, Schlub TE, Wijeyakumar W, Zhu M. Intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Ophthalmology. 2014;121(1):188–192. doi: 10.1016/j.ophtha.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97(8):1032–1035. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 9.Ferrone PJ, Anwar F, Naysan J, Chaudhary K, Fastenberg D, Graham K, Deramo V. Early initial clinical experience with intravitreal aflibercept for wet age-related macular degeneration. Br J Ophthalmol. 2014;98(Suppl. 1):i17–21. doi: 10.1136/bjophthalmol-2013-304474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharbiya M, Iannetti L, Parisi F, De Vico U, Mungo ML, Marenco M. Visual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degeneration. Biomed Res Int. 2014;2014:273754. doi: 10.1155/2014/273754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar N, Marsiglia M, Mrejen S, Fung AT, Slakter J, Sorenson J, Freund KB. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33(8):1605–1612. doi: 10.1097/IAE.0b013e31828e8551. [DOI] [PubMed] [Google Scholar]
- 12.Wykoff CC, Brown DM, Maldonado ME, Croft DE. Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial) Br J Ophthalmol. 2014;98(7):951–955. doi: 10.1136/bjophthalmol-2013-304736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho VY, Yeh S, Olsen TW, Bergstrom CS, Yan J, Cribbs BE, Hubbard GB., 3rd Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013;156(1):23–28.e2. doi: 10.1016/j.ajo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG. Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond) 2014;28(7):895–899. doi: 10.1038/eye.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messenger WB, Campbell JP, Faridi A, Shippey L, Bailey ST, Lauer AK, Flaxel CJ, Hwang TS. Injection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2014;98(9):1205–1207. doi: 10.1136/bjophthalmol-2013-304829. [DOI] [PubMed] [Google Scholar]
- 16.Arcinue CA, Ma F, Barteselli G, Sharpsten L, Gomez ML, Freeman WR. One-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159(3):426–436.e2. doi: 10.1016/j.ajo.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh RP, Srivastava S, Ehlers JP, Bedi R, Schachat AP, Kaiser PK. A single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysis. Br J Ophthalmol. 2014;98(Suppl. 1):i22–27. doi: 10.1136/bjophthalmol-2013-304798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 20.Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, Davis JL, Flynn HW, Jr, Esquiabro M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58.e1. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731–1739. doi: 10.1016/j.ophtha.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831–839. doi: 10.1016/j.ophtha.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Larsen M, Schmidt-Erfurth U, Lanzetta P, Wolf S, Simader C, Tokaji E, Pilz S, Weisberger A, MONT BLANC Study Group Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results. Ophthalmology. 2012;119(5):992–1000. doi: 10.1016/j.ophtha.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Moon da RC, Lee DK, Kim SH, You YS, Kwon OW. Aflibercept Treatment for Neovascular Age-related Macular Degeneration and Polypoidal Choroidal Vasculopathy Refractory to Anti-vascular Endothelial Growth Factor. Korean J Ophthalmol. 2015;29(4):226–232. doi: 10.3341/kjo.2015.29.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawashima Y, Oishi A, Tsujikawa A, Yamashiro K, Miyake M, Ueda-Arakawa N, Yoshikawa M, Takahashi A, Yoshimura N. Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1471–1477. doi: 10.1007/s00417-014-2838-5. [DOI] [PubMed] [Google Scholar]
- 27.Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159(5):853–860e1. doi: 10.1016/j.ajo.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Talks JS, Lotery AJ, Ghanchi F, et al. First-Year Visual Acuity Outcomes Providing Aflibercept According to the VIEW Study Protocol for Age-Related Macular Degeneration. Ophthalmology. 2016;123(2):337–343. doi: 10.1016/j.ophtha.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 29.He L, Silva RA, Ayoub N, Moshfeghi DM, Leng T. Experience With Aflibercept for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmic Surg Lasers Imaging Retina. 2015;46(5):542–549. doi: 10.3928/23258160-20150521-05. [DOI] [PubMed] [Google Scholar]
- 30.Khanani AM. Clinical experience with fixed bimonthly aflibercept dosing in treatment-experienced patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2015;9:1315–1320. doi: 10.2147/OPTH.S88624. [DOI] [PMC free article] [PubMed] [Google Scholar]