Abstract
Rapid advances in nanomedicine have significantly changed many aspects of nanoparticle application to the eye including areas of diagnosis, imaging and more importantly drug delivery. The nanoparticle-based drug delivery systems has provided a solution to various drug solubility-related problems in ophthalmology treatment. Nanostructured compounds could be used to achieve local ocular delivery with minimal unwanted systematic side effects produced by taking advantage of the phagocyte system. In addition, the in vivo control release by nanomaterials encapsulated drugs provides prolong exposure of the compound in the body. Furthermore, certain nanoparticles can overcome important body barriers including the blood-retinal barrier as well as the corneal-retinal barrier of the eye for effective delivery of the drug. In summary, the nanotechnology based drug delivery system may serve as an important tool for uveal melanoma treatment.
Keywords: nanomedicine, nanoparticles, gene delivery, drug delivery, uveal melanoma
INTRODUCTION OF UVEAL MELANOMA
Uveal melanoma is the most common adult primary tumor of the eye. The pace of incidence increase of melanoma as a whole group in the USA has surpassed all other types of tumors within the last 20y[1]. The liver is the sole or initial site of tumor spread in more than 80% patients with uveal melanoma; once liver metastases have been diagnosed, the mean survival time drops to only 6-9mo, with a 5-year survival rate <40%[2]–[3].
Although traditionally, eye removal and plaque radiotherapy have been standard approaches to control the primary uveal melanoma, these procedures lead to cosmetic defects and eventual loss of vision. Chemotherapeutic agents have been widely studied for treating the liver metastases of uveal melanoma, yet they are ineffective and does not prolong the survival rate. Overall, the mortality rate of uveal melanoma remains unchanged over the past decades in request of improving therapeutic outcome for patients[4]–[9].
The ineffectiveness of therapeutic agents may be associated with the incapability of delivering a large enough concentration of the compound into the local tumor area in the eye. Studies have found that most systemically administered drugs are consumed by other organs/tissues prior to accumulation in cancer tissues[10]. Such deficiency cannot be resolved by dramatically increasing the amounts of drug administered, as it would likely cause severe systemic side-effects[11]. Instead, local delivery and specifically targeting the tumor cells at the ocular site may achieve improved efficacy along with reduced systemic toxicity.
However, effective drug delivery remains to be a big obstacle of treating uveal melanoma and other intraocular diseases[12]–[15]. The blood-retinal barrier along with aqueous and corneal barriers can restrict the access of drug in the eyes. Due to the structural peculiarities of the posterior segment, it remains as one of the most challenging barrier to overcome by drug delivery[14]. Interestingly, recent progress in nanotechnology provided novel inspirations to the drug delivery in uveal melanoma and other ophthalmological diseases.
THERAPEUTIC NANOPARTICLES
Nanoparticle delivery of drugs can achieve the following objectives[16]: 1) enhances drug permeability; 2) controls the drug stability and release rate; and 3) target delivery of drug into specific tissues[17]–[19]. The main nanoparticles used for therapeutic drug delivery includes: lipid-based nanopartic1les, polymer-based nanoparticles, inorganic nanoparticles, polyion complex micelles, hybrid nanoparticles, polylactic-co-glycolic acid (PLGA) based nanoparticles[20], as well as peptide based nanoparticles. Lipid-based nanoparticles are comprised of cationic lipid nanoparticles, cationic liposomes, and cationic emulsions. Polymer-based nanoparticles can be made up from poly-L-lysine, polyethylenimine (PEI), chitosan, dendrimers, or cyclodextrin. Hybrid nanoparticles include multilayered nanoparticles and also liposome-polycation-DNA nanoparticles while quantum dots and magnetic nanoparticles are in the category of Inorganic nanoparticles. Several studies have applied nanomaterials for uveal melanoma chemotherapy, gene therapy, radiotherapy, and photodynamic therapy (PDT). The outcomes of these works are summarized below.
Nanoparticalized Chemotherapeutic Agents
Researchers have developed and are in the process of developing a variety of nanoparticles which can be structurally customized to deliver chemotherapeutic agents to treat a variety of cancers[18]–[19],[21]–[26]. Compared to conventional delivery of chemotherapy, nanoparticles can transport larger amounts of a payload as they contain high surface area compared to their volume. Solid tumors show much higher uptake and prolonged retention of nanoparticles in contrast to normal tissues, known as the enhanced permeation and retention effect (EPR)[27]. This is due to the cancerous lesions have leaky and deficient vasculatures with large pores which result in higher uptake of the drug[28]–[31]. In addition, the reduced blood flow through the neoplasm and an impaired lymphatic system within the tumor leads to very little clearance of the nanoparticles accumulated in the tumor[32]–[34]. In the last ten years, favorable ocular distribution of nanoparticle packaged drugs led to encouraging progress been made in the field of ocular drug delivery. In particular, dendrimers and cyclodextrins have been used for anterior chamber drug-delivery[35]–[38]. Dendrimers can easily get in or go out from a cell as they are 2 to 20 nm in size with characteristics of narrow polydispersity. It has an advantage of a higher drug payload when comparing to linear polymers as their highly tailorable surface functional groups allows multiple attachment of the compounds[39]. Enhanced ocular bioavailability was observed when polymer nanoparticles were applied[40]. Intravitreal injection of polylactic acid (PLA) and PLGA nanoparticles can leak to the retinal pigment epithelium[41]–[42] as the particles can go through the retinal layers[43]. A strategy of using PLGA nanoparticles to encapsulate dexamethasone acetate was effective for treating choroidal neovascularization (CNV)[44]–[46]. Albumin nanoparticles can be used to carry both positive and negative charge drugs such as ganciclovir or oligonucleotides due to their abundance of charged amino acids[47]. This characteristic make albumin nanoparticles ideal for delivering drugs to the posterior part of the eye, sutable for treating diseasing such as cytomegalovirus retinitis. Elbialy et al[48] have found an important factors to improve the efficiency of ocular drug delivery for prednisolone acetate is to induce the positive charge. With good affinity to the conjunctival surface and corneal, chitosan along with other natural polymer nanoparticles are also efficient to penetrate into the eye[49]. The electrostatic interactions between the positive amine groups of chitosan and negative sialic acid groups of mucins present in the tear film, which is intact with the corneal epithelium, enabled an increase in corneal residence time, and increased penetration of drug-loaded nanoparticles into the intact corneal epithelium[50]–[51]. Adhesion properties of the nanoparticles can be improved with the help of coating various polymers. When we further coat chitosan on the poly epsilon-caprolactone (PECL) encapsulated indomethacin nanoparticle, we observe a significant increase in bioavailability[52]–[53]. If we further coat polyethylene glycol (PEG) onto PECL particles, we could also increase the compound's ability to penetrate the corneal[54]. Recently, hydrogel nanoparticles have been investigated local delivery of chemotherapy compounds to treat uveal melanoma[55]. High concentration of poly N-isopropylacrylamide (PNIPAM) was detected in the uveal tissue after systemic injection of fluorescein-isothiocyanate (FITC) labeled PNIPAM nanoparticle. These findings demonstrated that nanoparticles as a novel carrier has strong potentials to be utilized in the treatment of uveal melanoma. Furthermore, it is possible to deliver more than one chemotherapy drugs at the same time using advanced nanoparticle delivery system to simultaneously target several important tumor signaling pathways. This would result in better treatment efficacy while lowering the drug cytotoxicity in cancer patients[56]–[58]. Currently, several combo drug formulations using such technology are under clinical trials testing. Acute leukemia patients whom received a formulation called CPX-351 which is a cytarabine and daunorubicin molar mixture of 5 to 1 formulated in liposome responded well as the formulation produced a nice synergistic effect[59]–[61]. Another combo nanoparticle drug CPX-1 which is a mixture of irinotecan HCl and floxuridine formulated in loposome also demonstrated synergistic anti-tumor effect againsted late stage solid tumors in a Phase 1 clinical trail[62]. Dendrimers, polymer-drug conjugates, iron oxide particles, nanoemulsions as well as silica particles have all been tested as an attempt to formulate and carry out combination drug delivery to improve therapeutic efficacy[63]–[68]. These particles are promising to be decorated with multiple chemotherapeutic drugs, and may be applied in ocular melanoma to achieve better cytotoxic effects.
Gene Delivery with Nanoparticles
Nanomedicine has also been recognized by the National Institutes of Health (NIH) as it released a roadmap for it. Nanoparticles are ideal tools for gene therapy into a single delivery system as it is possible to engineer the desired characteristics to be not susceptible to degradation, not mount an immune response, gain prolonged circulation time, exhibit increased specificity to target tissue, and ultimately deliver the genetic material into the target cells. A variety of cancers has been treated with experiments of nanoparticle gene delivery. Major focuses are in using gold, magnetic nanoparticles, liposome and carbon nanotubes for delivery[69]–[77]. Cationic polymer has already found to be a promising reduction-responsive gene carrier with low cytotoxicity and high transfection ability in melanoma cells[78]. Magnetofection, a method without using virus for transfection has shown to be effective in vitro for treating the B16F1 melanoma cells[79]. The eyes are special sites where foreign antigens are tolerated instead of rejected, making it a great place for taking advantage of gene therapy. The other advantage for gene therapy for the eye is that the eye is a closed organ with limited space. This would limit the local delivered drug diffusing into the body blood circulation because of the physical barrier structures. Therefore, more and more experiments with nanoparticle gene therapy focusing on treating eye diseases are conducted[80]–[82]. As an example, Farjo et al[83] performed subretinal or intravitreal injections delivering CMV-EGFP DNA nanoparticles into the mice eye. Farjo et al[83] showed that the transfection efficiency of the nanoparticles was very high along with nice target gene expression. Vectosome formulation of antisense oligonucleotides were used to study melanoma using a light induced system. In rat experiments, intravitreal injection of vectosomes resulted in a fast transetinal migration followed by uptake from the melanoma cells[84]. The vectosome treatment potently inhibited the cell growth of OCM-1 melanoma cells by 60% compared to the control group. More recently, Wang et al[85] has reported that after transfection with recombinant DNA plasmids such as pEgr1-TNFα, and pEgr1-TNFα-TK with dendrimer nanoparticles as vectors, and then combined with iodine-125 (125I) radiation, the gene expression and protein level of TNFα and HSV1-TK in OCM-1 melanoma cells was increased, cell proliferation was significantly decreased, and the cellular morphology altering, apoptosis and necrosis was observed. The current study suggests that combining gene therapy with nanoparticles may provide a new way of treating uveal melanoma.
Nanoparticles for Brachytherapy
Brachytherapy, where localized radiotherapy is delivered directly to the tumor, is currently a commonly used treatment method for uveal melanoma therapy. However, as the energy absorption dose of normal and tumor tissue are quite similar, the maximum radiation dose is limited to the normal tissue which surrounds the tumor. The use of radiosensitizing agents may address this problem and overcome hypoxia mediated heterogeneity response of the tumor[86]. Fullerene and lipid nanoparticles have been explored as strategies to deliver effective brachytherapy and longitudinal imaging in brain tumors[87]–[89]. More commonly, gold nanoparticles (GNanoparticles, AuNanoparticles) have been applied as radiosensitizing agents due to their high atomic number and strong photoelectric absorption coefficient[90]–[96]. Studies have shown by combining brachytherapy with gold nanoparticles, it could induce apoptosis of melanoma in vitro and in vivo[86],[97]–[99]. Chang et al[97] revealed that gold nanoparticles can sensitize melanoma B16F10 cells to radiation and showed that the nanoparticles can accumulate within the tumor cells. The radiation and nanoparticle combo treatment strategy has also significantly prolonged mice survival while potently inhibiting tumor growth in a B16F10 mice model. Further evidence of apoptosis induction was found in the combination group vs control[97]. Also, gold nanoparticles has additional vasculature disruption properties with combined with brachytherapy[100]–[103]. Berbeco et al[101] found that even low concentrations of AuNanoparticle has vasculature disruption effects to the tumor endothelial cells. As the nanoparticles can target both induce apoptosis of the tumor cells and disrupt its supporting vasculature when combined with radiation, it could potentially be used to support brachytherapy for treating uveal melanoma.
Nanoparticles for Phototherapy
Photodynamic therapy, also known as phototherapy is a treatment method of using special light which are called photosensitizers to activate the drug for cancer therapy. The compound to be delivered is active only after light activation. In tumors, photosensitizers may have multiple effects including direct killing of tumor cells, suppressing tumor vasculature and also activating the body's immune system[104]. As visible light is the most common activator of conventional photosensitizers, it could not infiltrate through very thick tissues. As a result, tissue depth remains one of the major limiting factor for effective photodynamic therapy. In the case of uveal melanoma, the quantity of melanin in the tumor could also affect light absorption and treatment efficacy[105]–[108]. Nanoparticles has unique advantages for phototherapy as it can act as a transducer for converting light with deep penetration ability into light within visible wavelength. It can also be used to carry photosensitizers for treating tumors[73],[109]–[112]. It has been reported that the use photosensitizer loaded-magnetic nanoparticles (MNPs) and/or polyethyleneimine (PEI) are novel attempts to improve phototherapy to treat melanoma[113]–[116]. As single excitation wavelength has multicolor-emission capability, multifunctional nanoplatform as potential dual carrier system has been developed[109],[117]. Makky et al[118] have developed prophyrin-based glycodendrimers with the mannose-specific ligand protein concanavalin A conjugated on to their surface, to specifically target the tumor cells in the retina. These hybrid dendrimers are designed as photosensitizers for preferential accumulation in malignant ocular tissue, for enhancing the effectiveness of PDT. The mannosylated dendrimers demonstrated specific interactions with the receptors in the lipid bilayer inducing protein channel rearrangement favoring the entry of the dendrimers into the cell. Wang et al[119] synthesized α-mannosyl dendrimeric porphyrins, which exhibited good photo efficiency, superior cellular uptake, and significant photo toxicity in retinoblastoma cells.
NANOPARTICLES FOR IMAGING OF UVEAL MELANOMA
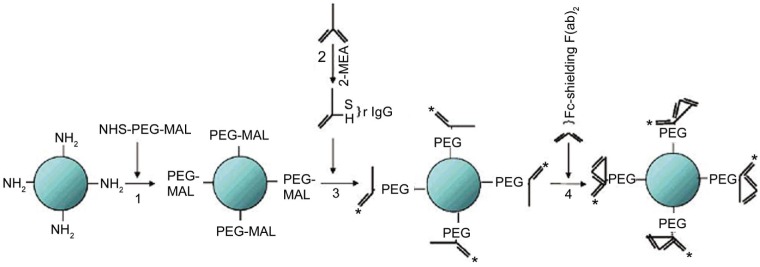
Nanoparticles and nanotechnology has great potential in the field of eye disease and uveal melanoma imaging for early detection and diagnosis[120]. They can be developed into non-invasive biomarkers for detection of the disease[14],[121]. Tari et al[122] invented a nanoparticle which can distinguish early and late stage of retinal vascular diseases. Their proposal is based on a simple matter of particle size. Bigger particles would stay in the blood circulation while smaller particles may manage to get out of the circulation in the early stages of the disease. By labeling the small and big size particles with two separate color dye, we gain the ability to track and monitor the diseases status. Currently, a variety of imaging modalities can reach the eye vasculature. So developing nanoparticle contrast agent to identify different tissues are possible. By the help of nanoparticles as contrast agents, the imaging sensitivity and quality can be improved[123]. For ocular vascular imaging, nanotechnology is mainly used in ophthalmoscopy as well as optical coherence tomography. Both of the application takes advantage of the optical contrast property of the light based imaging modalities. Interestingly, progress has also been made in developing contrast agents in the application of ultrasound, MRI to better monitor the structure of the retinal vasculature. The use of highly specific and highly sensitive quantum dots label is one of promising approaches. Anti-GFAP functionalized quantum dots (QDs) have been optimized for specific labeling and robust imaging of intermediate filaments in astrocyte and Müller glial cells in rat neural sensory retina[124]. Yamamoto et al[125] used a new quantum dot based method to detect vitreous lesions and even demonstrated that this nanotechnology method to stain the vitreous can guild surgeons to do vitrectomy surgeries. Another study conducted by Takeda et al[126] showed that in vivo quantum dot imaging can be used to detect spontaneous CNV of age-related macular degeneration (AMD) before it invades the retina. As CNV can lead to vision loss in AMD patients, the significance of this study is that it brings hope to early detect and diagnose AMD and save the patient's vision. QDs can also be applied into the ocular lymphatic pathway for imaging and may be useful in glaucoma to monitor eye pressure[127]. Recently, antibody-conjugated QDs have also found to be a high-throughput screening system and effective strategy for detection of melanoma[128]. In a melanoma-melanocyte co-culture in vitro model, QDs were constructed and conjugated with antibodies that could recognize melanoma cells. Data shown that these QDs can specifically detect melanoma cells only. Furthermore, PEG can be used to decrease nonspecific binding and decrease the clearance rate of the QDs product (Figure 1)[129]. Chemotherapeutic agents formulated with QDs and liposomes are also used for treating melanoma. Up until now, QDs have been used extensively in vivo to detect tumor vasculature[130]–[132]. Gold nanocages and nanoshells have been used in optical coherence tomography and other imaging approaches[133]–[139]. Iron oxide nanoparticles can be used to target certain cell surface receptors as well as macrophages[56]–[58]. Iron oxide particles with some modifications in size can also be used to detect the integrity of the retinal vessel structure. Manganese oxide particles were developed into the first T1 nanoparticulate contrast agent which is biocompatible[140]. Also, iron oxide particles are developed into novel MRI contrast agent for detecting uveal melanoma in rabbit models[141]. The nanoparticle contrast agent increased the ratio of the T1 to T2 signal intensity in all of the ocular tissue. These findings indicate that nanoparticles are promising to provide noninvasive technique for the diagnosis of ocular melanoma and evaluation of tumor viability following treatments.
Figure 1. Steps of making an antibody labeled quantum dots (QD).
PEG is used to decrease nonspecific binding and decrease the clearance rate while attaching the IgG fragments can lead to increase in affinity[129].
TOXICITY AND BIOCOMPATIBILITY
Toxicology analysis of nanoparticles are similar to the pharmacokinetics concept in pharmacology. Four key components for toxicity analysis of nanoparticles are looking at their adsorption, distribution, metabolism, and excretion inside the body. The toxicity of nanoparticles can be affected by many factors. The particle shape, charge, solubility, dosing frequency as well as particle size, route of injection and patient individual variation can all play a role in formation of the particle toxicity[142]–[150]. For nanoparticles to be used in human eyes, the toxicity of particles particularly need to be tested because if the particles damage the ocular barriers it would likely affect patient vision[43],[151]–[152]. Unfortunately, the journey of using nanoparticles to treat eye diseases has just began. Therefore we currently lack the important local and systematic toxicity data for the particles used for eye imaging, diagnosis and also drug delivery. A few recent studies of toxicity and biocompatibility of nanoparticles in ophthalmology are briefly discussed below. Allergic or hypersensitivity reactions have been reported from the use of titanium dioxide, dendrimers and polystyrene nanoparticles in certain animal models and even in humans[153]–[155]. Carbon nanotubes have the potential of disruping and altering cell membrane[156]–[157]. Made out of the natural lipid, liposome nanoparticles are considered nontoxic and biodegradable. Intravitreal injection of liposome-loaded tacrolimus for suppressing experimental autoimmune uveoretinitis (EAU) caused no side effects on retinal function or systemic cellular immunity[158]. There is also data showing intravenously administered gold nanoparticles can penetrate the blood-retinal barrier without damaging the retina tissue[159]. The potential toxicity of cationic lipids, PEI and polyamidoamine dendrimers was also evaluated by experiments[160]. The results showed that PEI were the least toxic compared to the other two. Silicon QD by intravitreal injection was shown to be well tolerated by rats. No toxicity was observed in the experiment yet the therapeutic effect was obvious for treating retinal degeneration[161]. No toxicity to the retina was observed in another CCR3-targeting QD imaging mice experiment[126]. A detailed review paper summarizing approximately 30 types of nanoparticles was published by Dr. Prow[162].
EYE DRUG DELIVERY ROUTES
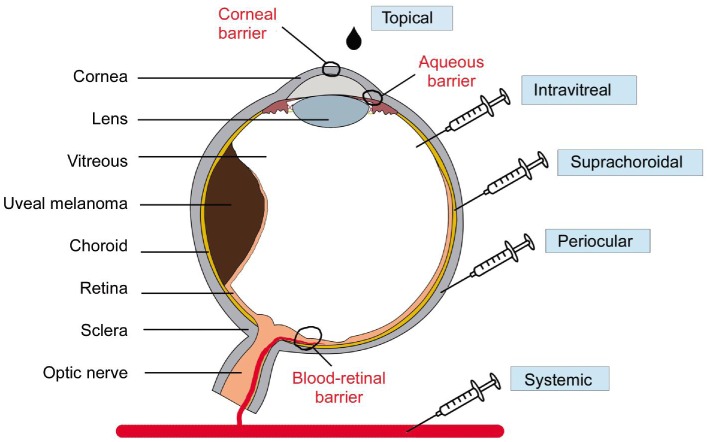
There are five main routes to deliver drugs into the eye, including systemic, periocular, suprachoroidal, intravitreal and topical injection. Figure 2 summarizes the above delivery methods which can also be applied to inject nanoparticles. Topical application is useful for treating disorders affecting the anterior segment of the eye. However, it has a big disadvantage of reaching a high ocular bioavailability as a large portion of the compound applied would be lost due to dilution of tears and lacrimation. Usually, the amount of drug that reaches aqueous humor is <5%. Systemic injection also has disadvantages as the amount of drug that would get to the vitreous cavity is quite low[163]–[166]. Lipophilic drugs are more in favor of going through the blood-retinal barrier and reaching the posterior part of the eye. If the compound is less lipophilic, then more frequent systemic injection is needed to reach and maintain an effective dose. This would very likely lead to higher incidence of systemic side effects to the body. The periocular injection route could have multiple meanings. It may refer to posterior juxtascleral, subconjunctival, retrobulbar, peribulbar or subtenon injection. There are associated risks for periocular injections as well. Common complications include hyphema, increase in intraocular pressure, corneal decompensation and even strabismus[167]–[169]. Intravitreal injections are becoming more popular choice for ocular drug delivery. By micro-needle injection of the compound directly into the vitreous, intravitreal injection could offer a higher drug load in the retina and vitreous compared to other delivery methods. The drug molecular weight is the major factor to affecting drug elimination for intravitreal injection. Disadvantages of intravitreal injection includes development of certain complications such as intravitreal hemorrhages, endophthalmitis and retinal detachment[170]–[177]. Also, patients with the diseases affecting posterior segment usually need multiple intravitreal injections and following careful monitor.
Figure 2. Diagram of the eye with uveal melanoma and routes of drug delivery to the eye (systemic, periocular, suprachoroidal, intravitreal and topical).
MAJOR CHALLENGES AND FUTURE DIRECTIONS
Although significant progress has been made in the application of nanotechnology-based diagnostics and therapy for ocular tumors, formal approved particles for clinical application remains low in numbers. Currently, a solo nanoparticle delivery method cannot provide a conclusion to all the challenges we face in ocular drug delivery. In order to overcome such challenges, we need to invent and discover novel routes for delivering the drug and also creating novel delivery systems. Our aim is to create an efficient and non-invasive way to better delivery drugs to the patients to improve therapeutic efficacy. Recent discovery of the possibility of injecting drugs into the suprachoroidal space provides a new way of treating posterior eye diseases[16],[178]–[182]. By using micro-needle injection into the space between the choroid and the sclera, suprachoroidal space injection can bypass the optical pathway while effectively delivery drugs into the choroid and ciliary body. Therefore, this injection technique may provide new possibilities to ocular melanoma treatment. Another major challenge in the treatment of uveal melanoma is that primary ocular tumor may be radiated or surgically removed, but the liver metastases is hard to treat. Nanoparticles may be useful to early detect and diagnosis ocular melanoma before the liver metastasis develops. Nanoparticles can be also applied for detection of circulating tumor cells, which play vital roles in the uveal melanoma metastasis to the liver.
CONCLUSION
The recent advance in nanotechnology has provided new opportunities in uveal melanoma and other ocular diseases treatment and diagnosis. By utilizing nanotechnology drug delivery systems, we can achieve higher efficacy, less toxicity, prolonged activity and less invasive administration of the drug for treating uveal melanoma. Challenges remains warranting further scientific research.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No. 81201808, No. 81502544); the American Fight For Sight Postdoctoral Award; Central South University Lieying grant; Emory University Melanoma Prevention and Research Discovery Fund; an unrestricted grant from Research to Prevent Blindness, Inc.
Conflicts of Interest: You S, None; Luo J, None; Grossniklaus HE, None; Gou ML, None; Meng K, None; Zhang Q, None.
REFERENCES
- 1.Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther. 2010;10(11):1811–1823. doi: 10.1586/era.10.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yonekawa Y, Kim IK. Epidemiology and management of uveal melanoma. Hematol Oncol Clin North Am. 2012;26(6):1169–1184. doi: 10.1016/j.hoc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Richtig E, Langmann G, Mullner K, Smolle J. Ocular melanoma: epidemiology, clinical presentation and relationship with dysplastic nevi. Ophthalmologica. 2004;218(2):111–114. doi: 10.1159/000076146. [DOI] [PubMed] [Google Scholar]
- 4.Damato B. Does ocular treatment of uveal melanoma influence survival? Br J Cancer. 2010;103(3):285–290. doi: 10.1038/sj.bjc.6605765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damato B, Coupland SE. Managing patients with ocular melanoma: state of the art. Clin Experiment Ophthalmol. 2008;36(7):589–590. doi: 10.1111/j.1442-9071.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 6.Pons F, Plana M, Caminal JM, Pera J, Fernandes I, Perez J, Garcia-Del-Muro X, Marcoval J, Penin R, Fabra A, Piulats JM. Metastatic uveal melanoma: is there a role for conventional chemotherapy? -A single center study based on 58 patients. Melanoma Res. 2011;21(3):217–222. doi: 10.1097/CMR.0b013e3283457726. [DOI] [PubMed] [Google Scholar]
- 7.Atzpodien J, Terfloth K, Fluck M, Reitz M. Cisplatin, gemcitabine and treosulfan is effective in chemotherapy-pretreated relapsed stage IV uveal melanoma patients. Cancer Chemother Pharmacol. 2008;62(4):685–688. doi: 10.1007/s00280-007-0655-9. [DOI] [PubMed] [Google Scholar]
- 8.Kalirai H, Damato BE, Coupland SE. Uveal melanoma cell lines contain stem-like cells that self-renew, produce differentiated progeny, and survive chemotherapy. Invest Ophthalmol Vis Sci. 2011;52(11):8458–8466. doi: 10.1167/iovs.11-7379. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Hieber M, Schmittel A, Thiel E, Keilholz U. A phase II study of bendamustine chemotherapy as second-line treatment in metastatic uveal melanoma. Melanoma Res. 2004;14(6):439–442. doi: 10.1097/00008390-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Wilson MW, Czechonska G, Finger PT, Rausen A, Hooper ME, Haik BG. Chemotherapy for eye cancer. Surv Ophthalmol. 2001;45(5):416–444. doi: 10.1016/s0039-6257(00)00210-1. [DOI] [PubMed] [Google Scholar]
- 11.Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51(1):19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Swaminathan S, Vavia PR, Trotta F, Cavalli R. Nanosponges encapsulating dexamethasone for ocular delivery: formulation design, physicochemical characterization, safety and corneal permeability assessment. J Biomed Nanotechnol. 2013;9(6):998–1007. doi: 10.1166/jbn.2013.1594. [DOI] [PubMed] [Google Scholar]
- 13.Kompella UB, Kadam RS, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010;1(3):435–456. doi: 10.4155/TDE.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kompella UB, Amrite AC, Pacha Ravi R, Durazo SA. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog Retin Eye Res. 2013;36:172–198. doi: 10.1016/j.preteyeres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe-Rendleman CL, Durazo SA, Kompella UB, Rittenhouse KD, Di Polo A, Weiner AL, Grossniklaus HE, Naash MI, Lewin AS, Horsager A, Edelhauser HF. Drug and gene delivery to the back of the eye: from bench to bedside. Invest Ophthalmol Vis Sci. 2014;55(4):2714–2730. doi: 10.1167/iovs.13-13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abarca EM, Salmon JH, Gilger BC. Effect of choroidal perfusion on ocular tissue distribution after intravitreal or suprachoroidal injection in an arterially perfused ex vivo pig eye model. J Ocul Pharmacol Ther. 2013;29(8):715–722. doi: 10.1089/jop.2013.0063. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger JG, Cesarano J. Robotic deposition of model hydroxyapatite scaffolds with multiple architectures and multiscale porosity for bone tissue engineering. J Biomed Mater Res A. 2007;82(2):383–394. doi: 10.1002/jbm.a.31072. [DOI] [PubMed] [Google Scholar]
- 18.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caron WP, Morgan KP, Zamboni BA, Zamboni WC. A review of study designs and outcomes of phase I clinical studies of nanoparticle agents compared with small-molecule anticancer agents. Clin Cancer Res. 2013;19(12):3309–3315. doi: 10.1158/1078-0432.CCR-12-3649. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Diaz M, Foged C, Nielsen HM. Improved insulin loading in poly(lactic-co-glycolic) acid (PLGA) nanoparticles upon self-assembly with lipids. Int J Pharm. 2015;482(1–2):84–91. doi: 10.1016/j.ijpharm.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Roy A, Singh MS, Upadhyay P, Bhaskar S. Combined chemo-immunotherapy as a prospective strategy to combat cancer: a nanoparticle based approach. Mol Pharm. 2010;7(5):1778–1788. doi: 10.1021/mp100153r. [DOI] [PubMed] [Google Scholar]
- 22.Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, Zhang T, Wang AZ. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32(33):8548–8554. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao X, Zhang Z, Guo L, Zhu J, Peng M, Vermorken AJ, Van de Ven WJ, Chen C, Cui Y. A novel magnetic nanoparticle drug carrier for enhanced cancer chemotherapy. PLoS One. 2012;7(10):e40388. doi: 10.1371/journal.pone.0040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seidman AD, Conlin AK, Bach A, Moynahan ME, Lake D, Forero A, Wright GS, Hackney MH, Clawson A, Norton L, Hudis CA. Randomized phase II trial of weekly vs. every 2 weeks vs. every 3 weeks nanoparticle albumin-bound paclitaxel with bevacizumab as first-line chemotherapy for metastatic breast cancer. Clin Breast Cancer. 2013;13(4):239–246.e1. doi: 10.1016/j.clbc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Petryk AA, Giustini AJ, Gottesman RE, Kaufman PA, Hoopes PJ. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int J Hyperthermia. 2013;29(8):845–851. doi: 10.3109/02656736.2013.825014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Porter AL, Aminabhavi TM, Zhu D. Nano-enabled drug delivery systems for brain cancer and Alzheimer's disease: research patterns and opportunities. Nanomedicine. 2015;11(7):1763–1771. doi: 10.1016/j.nano.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura H, Jun F, Maeda H. Development of next-generation macromolecular drugs based on the EPR effect: challenges and pitfalls. Expert Opin Drug Deliv. 2015;12(1):53–64. doi: 10.1517/17425247.2014.955011. [DOI] [PubMed] [Google Scholar]
- 28.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188(6):759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee D, Sengupta S. Nanoparticles in cancer chemotherapy. Prog Mol Biol Transl Sci. 2011;104:489–507. doi: 10.1016/B978-0-12-416020-0.00012-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 33.Alexis F, Pridgen EM, Langer R, Farokhzad OC. Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol. 2010;(197):55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 34.Fox ME, Szoka FC, Frechet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42(8):1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spataro G, Malecaze F, Turrin CO, Soler V, Duhayon C, Elena PP, Majoral JP, Caminade AM. Designing dendrimers for ocular drug delivery. Eur J Med Chem. 2010;45(1):326–334. doi: 10.1016/j.ejmech.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Yao C, Wang W, Zhou X, Qu T, Mu H, Liang R, Wang A, Sun K. Effects of poly (amidoamine) dendrimers on ocular absorption of puerarin using microdialysis. J Ocul Pharmacol Ther. 2011;27(6):565–569. doi: 10.1089/jop.2010.0196. [DOI] [PubMed] [Google Scholar]
- 37.Siefert B, Pleyer U, Muller M, Hartmann C, Keipert S. Influence of cyclodextrins on the in vitro corneal permeability and in vivo ocular distribution of thalidomide. J Ocul Pharmacol Ther. 1999;15(5):429–438. doi: 10.1089/jop.1999.15.429. [DOI] [PubMed] [Google Scholar]
- 38.Puglia C, Offerta A, Carbone C, Bonina F, Pignatello R, Puglisi G. Lipid nanocarriers (LNC) and their applications in ocular drug delivery. Curr Med Chem. 2015;22(13):1589–1602. doi: 10.2174/0929867322666150209152259. [DOI] [PubMed] [Google Scholar]
- 39.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5–6):171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Gupta P, Kumar M. Recent advance technique for ocular drug delivery by Gupta et al.: Nanoparticle laden in situ gel. J Pharm Bioallied Sci. 2013;5(3):175. [PMC free article] [PubMed] [Google Scholar]
- 41.Bazhan SI, Belavin PA, Seregin SV, Danilyuk NK, Babkina IN, Karpenko LI, Nekrasova NA, Lebedev LR, Ignatyev GM, Agafonov AP, Poryvaeva VA, Aborneva IV, Ilyichev AA. Designing and engineering of DNA-vaccine construction encoding multiple CTL-epitopes of major HIV-1 antigens. Vaccine. 2004;22(13–14):1672–1682. doi: 10.1016/j.vaccine.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 42.Pescina S, Sonvico F, Santi P, Nicoli S. Therapeutics and carriers: the dual role of proteins in nanoparticles for ocular delivery. Curr Top Med Chem. 2015;15(4):369–385. doi: 10.2174/1568026615666150108150217. [DOI] [PubMed] [Google Scholar]
- 43.Prow TW, Bhutto I, Kim SY, Grebe R, Merges C, McLeod DS, Uno K, Mennon M, Rodriguez L, Leong K, Lutty GA. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine. 2008;4(4):340–349. doi: 10.1016/j.nano.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang C, Jiang L, Bu S, Zhang L, Xie X, Zeng Q, Zhu D, Zheng Y. Intravitreal administration of dexamethasone-loaded PLGA-TPGS nanoparticles for the treatment of posterior segment diseases. J Biomed Nanotechnol. 2013;9(9):1617–1623. doi: 10.1166/jbn.2013.1646. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Li Y, Zhang C, Wang Y, Song C. Pharmacokinetics and tolerance study of intravitreal injection of dexamethasone-loaded nanoparticles in rabbits. Int J Nanomedicine. 2009;4:175–183. doi: 10.2147/ijn.s6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Wang Y, Li Y, Yang X, Zhang P, Hou H, Shi Y, Song C. Inhibitory efficacy of intravitreal dexamethasone acetate-loaded PLGA nanoparticles on choroidal neovascularization in a laser-induced rat model. J Ocul Pharmacol Ther. 2007;23(6):527–540. doi: 10.1089/jop.2007.0002. [DOI] [PubMed] [Google Scholar]
- 47.Merodio M, Irache JM, Valamanesh F, Mirshahi M. Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials. 2002;23(7):1587–1594. doi: 10.1016/s0142-9612(01)00284-8. [DOI] [PubMed] [Google Scholar]
- 48.Elbialy NS, Abdol-Azim BM, Shafaa MW, El Shazly LH, El Shazly AH, Khalil WA. Enhancement of the ocular therapeutic effect of prednisolone acetate by liposomal entrapment. J Biomed Nanotechnol. 2013;9(12):2105–2116. doi: 10.1166/jbn.2013.1711. [DOI] [PubMed] [Google Scholar]
- 49.Na JH, Koo H, Lee S, Min KH, Park K, Yoo H, Lee SH, Park JH, Kwon IC, Jeong SY, Kim K. Real-time and non-invasive optical imaging of tumor-targeting glycol chitosan nanoparticles in various tumor models. Biomaterials. 2011;32(22):5252–5261. doi: 10.1016/j.biomaterials.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 50.Yoncheva K, Vandervoort J, Ludwig A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm Dev Technol. 2011;16(1):29–35. doi: 10.3109/10837450903479954. [DOI] [PubMed] [Google Scholar]
- 51.Paolicelli P, de la Fuente M, Sanchez A, Seijo B, Alonso MJ. Chitosan nanoparticles for drug delivery to the eye. Expert Opin Drug Deliv. 2009;6(3):239–253. doi: 10.1517/17425240902762818. [DOI] [PubMed] [Google Scholar]
- 52.Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H, El dally M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res. 2008;31(8):1040–1049. doi: 10.1007/s12272-001-1266-6. [DOI] [PubMed] [Google Scholar]
- 53.Irache JM, Merodio M, Arnedo A, Camapanero MA, Mirshahi M, Espuelas S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev Med Chem. 2005;5(3):293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- 54.De Campos AM, Sanchez A, Gref R, Calvo P, Alonso MJ. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20(1):73–81. doi: 10.1016/s0928-0987(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Zhou J, Hu Z, Nair A, Tang L. Nanoparticles for Uveal Melanoma Treatment. Proc IEEE Conf Nanotechnol. 2008;2008:822–825. doi: 10.1109/NANO.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen H, Zhao Y, Wang H, Nie G, Nan K. Co-delivery strategies based on multifunctional nanocarriers for cancer therapy. Curr Drug Metab. 2012;13(8):1087–1096. doi: 10.2174/138920012802849995. [DOI] [PubMed] [Google Scholar]
- 57.Esposito C, Crema A, Ponzetto A, Murtas G, Carloni G. Multifunctional anti-cancer nano-platforms are moving to clinical trials. Curr Drug Metab. 2013;14(5):583–604. doi: 10.2174/13892002113149990009. [DOI] [PubMed] [Google Scholar]
- 58.Goncalves G, Vila M, Portoles MT, Vallet-Regi M, Gracio J, Marques PA. Nano-graphene oxide: a potential multifunctional platform for cancer therapy. Adv Healthc Mater. 2013;2(8):1072–1090. doi: 10.1002/adhm.201300023. [DOI] [PubMed] [Google Scholar]
- 59.Gergis U, Roboz G, Shore T, Ritchie E, Mayer S, Wissa U, McKenna M, Christos P, Pearse R, Mark T, Scandura J, van Besien K, Feldman E. A phase I study of CPX-351 in combination with busulfan and fludarabine conditioning and allogeneic stem cell transplantation in adult patients with refractory acute leukemia. Biol Blood Marrow Transplant. 2013;19(7):1040–1045. doi: 10.1016/j.bbmt.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Feldman EJ, Kolitz JE, Trang JM, Liboiron BD, Swenson CE, Chiarella MT, Mayer LD, Louie AC, Lancet JE. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36(10):1283–1289. doi: 10.1016/j.leukres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batist G, Gelmon KA, Chi KN, Miller WH, Jr, Chia SK, Mayer LD, Swenson CE, Janoff AS, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15(2):692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 63.Lee SM, Park H, Yoo KH. Synergistic cancer therapeutic effects of locally delivered drug and heat using multifunctional nanoparticles. Adv Mater Weinheim. 2010;22(36):4049–4053. doi: 10.1002/adma.201001040. [DOI] [PubMed] [Google Scholar]
- 64.Lee AL, Dhillon SH, Wang Y, Pervaiz S, Fan W, Yang YY. Synergistic anti-cancer effects via co-delivery of TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) and doxorubicin using micellar nanoparticles. Mol Biosyst. 2011;7(5):1512–1522. doi: 10.1039/c0mb00266f. [DOI] [PubMed] [Google Scholar]
- 65.Jean M, Alameh M, De Jesus D, Thibault M, Lavertu M, Darras V, Nelea M, Buschmann MD, Merzouki A. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. Eur J Pharm Sci. 2012;45(1–2):138–149. doi: 10.1016/j.ejps.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 66.Blanco E, Sangai T, Hsiao A, Ferrati S, Bai L, Liu X, Meric-Bernstam F, Ferrari M. Multistage delivery of chemotherapeutic nanoparticles for breast cancer treatment. Cancer Lett. 2013;334(2):245–252. doi: 10.1016/j.canlet.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 67.Lin X, Gao R, Zhang Y, Qi N, Zhang Y, Zhang K, He H, Tang X. Lipid nanoparticles for chemotherapeutic applications: strategies to improve anticancer efficacy. Expert Opin Drug Deliv. 2012;9(7):767–781. doi: 10.1517/17425247.2012.685933. [DOI] [PubMed] [Google Scholar]
- 68.Cheng FY, Su CH, Wu PC, Yeh CS. Multifunctional polymeric nanoparticles for combined chemotherapeutic and near-infrared photothermal cancer therapy in vitro and in vivo. Chem Commun (Camb) 2010;46(18):3167–3169. doi: 10.1039/b919172k. [DOI] [PubMed] [Google Scholar]
- 69.Yhee JY, Lee SJ, Lee S, Song S, Min HS, Kang SW, Son S, Jeong SY, Kwon IC, Kim SH, Kim K. Tumor-targeting transferrin nanoparticles for systemic polymerized siRNA delivery in tumor-bearing mice. Bioconjug Chem. 2013;24(11):1850–1860. doi: 10.1021/bc400226b. [DOI] [PubMed] [Google Scholar]
- 70.Lee SJ, Yhee JY, Kim SH, Kwon IC, Kim K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J Control Release. 2013;172(1):358–366. doi: 10.1016/j.jconrel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Hafner AM, Corthesy B, Merkle HP. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv Drug Deliv Rev. 2013;65(10):1386–1399. doi: 10.1016/j.addr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Chen YZ, Yao XL, Ruan GX, Zhao QQ, Tang GP, Tabata Y, Gao JQ. Gene-carried chitosan-linked polyethylenimine induced high gene transfection efficiency on dendritic cells. Biotechnol Appl Biochem. 2012;59(5):346–352. doi: 10.1002/bab.1036. [DOI] [PubMed] [Google Scholar]
- 73.Slastnikova TA, Rosenkranz AA, Lupanova TN, Gulak PV, Gnuchev NV, Sobolev AS. Study of efficiency of the modular nanotransporter for targeted delivery of photosensitizers to melanoma cell nuclei in vivo. Dokl Biochem Biophys. 2012;446:235–237. doi: 10.1134/S1607672912050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi KM, Kim K, Kwon IC, Kim IS, Ahn HJ. Systemic delivery of siRNA by chimeric capsid protein: tumor targeting and RNAi activity in vivo. Mol Pharm. 2013;10(1):18–25. doi: 10.1021/mp300211a. [DOI] [PubMed] [Google Scholar]
- 75.Lemarie F, Croft DR, Tate RJ, Ryan KM, Dufes C. Tumor regression following intravenous administration of a tumor-targeted p73 gene delivery system. Biomaterials. 2012;33(9):2701–2709. doi: 10.1016/j.biomaterials.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 76.Numata K, Mieszawska-Czajkowska AJ, Kvenvold LA, Kaplan DL. Silk-based nanocomplexes with tumor-homing peptides for tumor-specific gene delivery. Macromol Biosci. 2012;12(1):75–82. doi: 10.1002/mabi.201100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saie AA, Ray M, Mahmoudi M, Rotello VM. Engineering the nanoparticle-protein interface for cancer therapeutics. Cancer Treat Res. 2015;166:245–273. doi: 10.1007/978-3-319-16555-4_11. [DOI] [PubMed] [Google Scholar]
- 78.Cheng G, He Y, Xie L, Nie Y, He B, Zhang Z, Gu Z. Development of a reduction-sensitive diselenide-conjugated oligoethylenimine nanoparticulate system as a gene carrier. Int J Nanomedicine. 2012;7:3991–4006. doi: 10.2147/IJN.S32961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prosen L, Prijic S, Music B, Lavrencak J, Cemazar M, Sersa G. Magnetofection: a reproducible method for gene delivery to melanoma cells. Biomed Res Int. 2013;2013:209452. doi: 10.1155/2013/209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhatia S, Menezes ME, Das SK, Emdad L, Dasgupta S, Wang XY, Sarkar D, Fisher PB. Innovative approaches for enhancing cancer gene therapy. Discov Med. 2013;15(84):309–317. [PubMed] [Google Scholar]
- 81.Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res. 2008;48(3):319–324. doi: 10.1016/j.visres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zorzi GK, Parraga JE, Seijo B, Sanchez A. Hybrid nanoparticle design based on cationized gelatin and the polyanions dextran sulfate and chondroitin sulfate for ocular gene therapy. Macromol Biosci. 2011;11(7):905–913. doi: 10.1002/mabi.201100005. [DOI] [PubMed] [Google Scholar]
- 83.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Normand N, Valamanesh F, Savoldelli M, Mascarelli F, BenEzra D, Courtois Y, Behar-Cohen F. VP22 light controlled delivery of oligonucleotides to ocular cells in vitro and in vivo. Mol Vis. 2005;11:184–191. [PubMed] [Google Scholar]
- 85.Wang Y, Mo L, Wei W, Shi X. Efficacy and safety of dendrimer nanoparticles with coexpression of tumor necrosis factor-α and herpes simplex virus thymidine kinase in gene radiotherapy of the human uveal melanoma OCM-1 cell line. Int J Nanomedicine. 2013;8:3805–3816. doi: 10.2147/IJN.S48950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Butterworth KT, McMahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4(16):4830–4838. doi: 10.1039/c2nr31227a. [DOI] [PubMed] [Google Scholar]
- 87.Shultz MD, Wilson JD, Fuller CE, Zhang J, Dorn HC, Fatouros PP. Metallofullerene-based nanoplatform for brain tumor brachytherapy and longitudinal imaging in a murine orthotopic xenograft model. Radiology. 2011;261(1):136–143. doi: 10.1148/radiol.11102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson JD, Broaddus WC, Dorn HC, Fatouros PP, Chalfant CE, Shultz MD. Metallofullerene-nanoplatform-delivered interstitial brachytherapy improved survival in a murine model of glioblastoma multiforme. Bioconjug Chem. 2012;23(9):1873–1880. doi: 10.1021/bc300206q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Phillips WT, Goins B, Bao A, Vargas D, Guttierez JE, Trevino A, Miller JR, Henry J, Zuniga R, Vecil G, Brenner AJ. Rhenium-186 liposomes as convection-enhanced nanoparticle brachytherapy for treatment of glioblastoma. Neuro-oncology. 2012;14(4):416–425. doi: 10.1093/neuonc/nos060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys Med Biol. 2010;55(11):3045–3059. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 91.Kamiar A, Ghotalou R, Vali Zadeh H. Preparation, physicochemical characterization and performance evaluation of gold nanoparticles in radiotherapy. Adv Pharm Bull. 2013;3(2):425–428. doi: 10.5681/apb.2013.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsiamas P, Liu B, Cifter F, Ngwa WF, Berbeco RI, Kappas C, Theodorou K, Marcus K, Makrigiorgos MG, Sajo E, Zygmanski P. Impact of beam quality on megavoltage radiotherapy treatment techniques utilizing gold nanoparticles for dose enhancement. Phys Med Biol. 2013;58(3):451–464. doi: 10.1088/0031-9155/58/3/451. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin MF, Woodward J, Boll RA, Wall JS, Rondinone AJ, Kennel SJ, Mirzadeh S, Robertson JD. Gold coated lanthanide phosphate nanoparticles for targeted alpha generator radiotherapy. PLoS One. 2013;8(1):e54531. doi: 10.1371/journal.pone.0054531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker CR, Pushpavanam K, Nair DG, Potta T, Sutiyoso C, Kodibagkar VD, Sapareto S, Chang J, Rege K. Generation of polypeptide-templated gold nanoparticles using ionizing radiation. Langmuir. 2013;29(32):10166–10173. doi: 10.1021/la400567d. [DOI] [PubMed] [Google Scholar]
- 95.Chattopadhyay N, Cai Z, Kwon YL, Lechtman E, Pignol JP, Reilly RM. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Research and Treatment. 2013;137:81–91. doi: 10.1007/s10549-012-2338-4. [DOI] [PubMed] [Google Scholar]
- 96.Zhang XD, Wu D, Shen X, Chen J, Sun YM, Liu PX, Liang XJ. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012;33(27):6408–6419. doi: 10.1016/j.biomaterials.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 97.Chang MY, Shiau AL, Chen YH, Chang CJ, Chen HH, Wu CL. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99(7):1479–1484. doi: 10.1111/j.1349-7006.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hebert EM, Debouttiere PJ, Lepage M, Sanche L, Hunting DJ. Preferential tumour accumulation of gold nanoparticles, visualised by Magnetic Resonance Imaging: radiosensitisation studies in vivo and in vitro. Int J Radiat Biol. 2010;86(8):692–700. doi: 10.3109/09553001003746067. [DOI] [PubMed] [Google Scholar]
- 99.Khan MK, Minc LD, Nigavekar SS, Kariapper MS, Nair BM, Schipper M, Cook AC, Lesniak WG, Balogh LP. Fabrication of {198Au0} radioactive composite nanodevices and their use for nanobrachytherapy. Nanomedicine. 2008;4(1):57–69. doi: 10.1016/j.nano.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ngwa W, Makrigiorgos GM, Berbeco RI. Gold nanoparticle-aided brachytherapy with vascular dose painting: estimation of dose enhancement to the tumor endothelial cell nucleus. Med Phys. 2012;39(1):392–398. doi: 10.1118/1.3671905. [DOI] [PubMed] [Google Scholar]
- 101.Berbeco RI, Ngwa W, Makrigiorgos GM. Localized dose enhancement to tumor blood vessel endothelial cells via megavoltage X-rays and targeted gold nanoparticles: new potential for external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(1):270–276. doi: 10.1016/j.ijrobp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 102.Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources. Phys Med Biol. 2009;54(16):4889–4905. doi: 10.1088/0031-9155/54/16/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ngwa W, Makrigiorgos GM, Berbeco RI. Applying gold nanoparticles as tumor-vascular disrupting agents during brachytherapy: estimation of endothelial dose enhancement. Phys Med Biol. 2010;55(21):6533–6548. doi: 10.1088/0031-9155/55/21/013. [DOI] [PubMed] [Google Scholar]
- 104.Nowis D, Makowski M, Stoklosa T, Legat M, Issat T, Golab J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim Pol. 2005;52(2):339–352. [PubMed] [Google Scholar]
- 105.Young LH, Howard MA, Hu LK, Kim RY, Gragoudas ES. Photodynamic therapy of pigmented choroidal melanomas using a liposomal preparation of benzoporphyrin derivative. Arch Ophthalmol. 1996;114(2):186–192. doi: 10.1001/archopht.1996.01100130180013. [DOI] [PubMed] [Google Scholar]
- 106.Barbazetto IA, Lee TC, Rollins IS, Chang S, Abramson DH. Treatment of choroidal melanoma using photodynamic therapy. Am J Ophthalmol. 2003;135(6):898–899. doi: 10.1016/s0002-9394(02)02222-5. [DOI] [PubMed] [Google Scholar]
- 107.Canal-Fontcuberta I, Salomao DR, Robertson D, Cantrill HL, Koozekanani D, Rath PP, Pulido JS. Clinical and histopathologic findings after photodynamic therapy of choroidal melanoma. Retina. 2012;32(5):942–948. doi: 10.1097/IAE.0b013e31825097c1. [DOI] [PubMed] [Google Scholar]
- 108.Bolfarini GC, Siqueira-Moura MP, Demets GJ, Morais PC, Tedesco AC. In vitro evaluation of combined hyperthermia and photodynamic effects using magnetoliposomes loaded with cucurbituril zinc phthalocyanine complex on melanoma. J Photochem Photobiol B. 2012;115:1–4. doi: 10.1016/j.jphotobiol.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat Med. 2012;18(10):1580–1585. doi: 10.1038/nm.2933. [DOI] [PubMed] [Google Scholar]
- 110.Brevet D, Gary-Bobo M, Raehm L, Richeter S, Hocine O, Amro K, Loock B, Couleaud P, Frochot C, Morère A, Maillard P, Garcia M, Durand JO. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem Commun (Camb) 2009;(12):1475–1477. doi: 10.1039/b900427k. [DOI] [PubMed] [Google Scholar]
- 111.Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn Ther. 2011;8(1):14–29. doi: 10.1016/j.pdpdt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Xiao L, Gu L, Howell SB, Sailor MJ. Porous silicon nanoparticle photosensitizers for singlet oxygen and their phototoxicity against cancer cells. ACS Nano. 2011;5(5):3651–3659. doi: 10.1021/nn1035262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balivada S, Rachakatla RS, Wang H, Samarakoon TN, Dani RK, Pyle M, Kroh FO, Walker B, Leaym X, Koper OB, Tamura M, Chikan V, Bossmann SH, Troyer DL. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: a mouse study. BMC Cancer. 2010;10:119. doi: 10.1186/1471-2407-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rachakatla RS, Balivada S, Seo GM, Myers CB, Wang H, Samarakoon TN, Dani R, Pyle M, Kroh FO, Walker B, Leaym X, Koper OB, Chikan V, Bossmann SH, Tamura M, Troyer DL. Attenuation of mouse melanoma by A/C magnetic field after delivery of bi-magnetic nanoparticles by neural progenitor cells. ACS Nano. 2010;4(12):7093–7104. doi: 10.1021/nn100870z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camerin M, Magaraggia M, Soncin M, Jori G, Moreno M, Chambrier I, Cook MJ, Russell DA. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur J Cancer. 2010;46(10):1910–1918. doi: 10.1016/j.ejca.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 116.Mbakidi JP, Drogat N, Granet R, Ouk TS, Ratinaud MH, Riviere E, Rivière E, Verdier M, Sol V. Hydrophilic chlorin-conjugated magnetic nanoparticles--potential anticancer agent for the treatment of melanoma by PDT. Bioorg Med Chem Lett. 2013;23(9):2486–2490. doi: 10.1016/j.bmcl.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 117.Conte C, Ungaro F, Maglio G, Tirino P, Siracusano G, Sciortino MT, Leone N, Palma G, Barbieri A, Arra C, Mazzaglia A, Quaglia F. Biodegradable core-shell nanoassemblies for the delivery of docetaxel and Zn(II)-phthalocyanine inspired by combination therapy for cancer. J Control Release. 2013;167(1):40–52. doi: 10.1016/j.jconrel.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 118.Makky A, Michel JP, Maillard P, Rosilio V. Biomimetic liposomes and planar supported bilayers for the assessment of glycodendrimeric porphyrins interaction with an immobilized lectin. Biochim Biophys Acta. 2011;1808(3):656–666. doi: 10.1016/j.bbamem.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 119.Wang ZJ, Chauvin B, Maillard P, Hammerer F, Carez D, Croisy A, Sandré C, Chollet-Martin S, Prognon P, Paul JL, Blais J, Kasselouri A. Glycodendrimeric phenylporphyrins as new candidates for retinoblastoma PDT: blood carriers and photodynamic activity in cells. J Photoch Photobio B. 2012;115:16–24. doi: 10.1016/j.jphotobiol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Cormode DP, Skajaa GO, Delshad A, Parker N, Jarzyna PA, Calcagno C, Galper MW, Skajaa T, Briley-Saebo KC, Bell HM, Gordon RE, Fayad ZA, Woo SL, Mulder WJ. A versatile and tunable coating strategy allows control of nanocrystal delivery to cell types in the liver. Bioconjug Chem. 2011;22(3):353–361. doi: 10.1021/bc1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson DF, Vinogradov SA, Grosul P, Vaccarezza MN, Kuroki A, Bennett J. Oxygen distribution and vascular injury in the mouse eye measured by phosphorescence-lifetime imaging. Appl Opt. 2005;44(25):5239–5248. doi: 10.1364/ao.44.005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tari SR, Barile GR, Kompella UB. Polychromatic, diversely-sized particles for angiography. Google Patents. 2008 [Google Scholar]
- 123.Hahn MA, Singh AK, Sharma P, Brown SC, Moudgil BM. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal Bioanal Chem. 2011;399(1):3–27. doi: 10.1007/s00216-010-4207-5. [DOI] [PubMed] [Google Scholar]
- 124.Pathak S, Tolentino R, Nguyen K, D'Amico L, Barron E, Cheng L, Freeman WR, Silva GA. Quantum dot labeling and imaging of glial fibrillary acidic protein intermediate filaments and gliosis in the rat neural retina and dissociated astrocytes. J Nanosci Nanotechnol. 2009;9(8):5047–5054. doi: 10.1166/jnn.2009.gr08. [DOI] [PubMed] [Google Scholar]
- 125.Yamamoto S, Manabe N, Fujioka K, Hoshino A, Yamamoto K. Visualizing vitreous using quantum dots as imaging agents. IEEE Trans Nanobioscience. 2007;6(1):94–98. doi: 10.1109/tnb.2007.891883. [DOI] [PubMed] [Google Scholar]
- 126.Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460(7252):225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tam AL, Gupta N, Zhang Z, Yucel YH. Quantum dots trace lymphatic drainage from the mouse eye. Nanotechnology. 2011;22(42):425101. doi: 10.1088/0957-4484/22/42/425101. [DOI] [PubMed] [Google Scholar]
- 128.Kim MJ, Lee JY, Nehrbass U, Song R, Choi Y. Detection of melanoma using antibody-conjugated quantum dots in a coculture model for high-throughput screening system. Analyst. 2012;137(6):1440–1445. doi: 10.1039/c2an16013g. [DOI] [PubMed] [Google Scholar]
- 129.Jayagopal A, Russ PK, Haselton FR. Surface engineering of quantum dots for in vivo vascular imaging. Bioconjug Chem. 2007;18(5):1424–1433. doi: 10.1021/bc070020r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballou B, Ernst LA, Andreko S, Harper T, Fitzpatrick JA, Waggoner AS, Bruchez MP. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug Chem. 2007;18(2):389–396. doi: 10.1021/bc060261j. [DOI] [PubMed] [Google Scholar]
- 131.Ballou B, Lagerholm BC, Ernst LA, Bruchez MP, Waggoner AS. Noninvasive imaging of quantum dots in mice. Bioconjug Chem. 2004;15(1):79–86. doi: 10.1021/bc034153y. [DOI] [PubMed] [Google Scholar]
- 132.Su Y, Peng F, Jiang Z, Zhong Y, Lu Y, Jiang X, Huang Q, Fan C, Lee ST, He Y. In vivo distribution, pharmacokinetics, and toxicity of aqueous synthesized cadmium-containing quantum dots. Biomaterials. 2011;32(25):5855–5862. doi: 10.1016/j.biomaterials.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 133.Loo C, Lin A, Hirsch L, Lee MH, Barton J, Halas N, West J, Drezek R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat. 2004;3(1):33–40. doi: 10.1177/153303460400300104. [DOI] [PubMed] [Google Scholar]
- 134.Coughlin AJ, Ananta JS, Deng N, Larina IV, Decuzzi P, West JL. Gadolinium-conjugated gold nanoshells for multimodal diagnostic imaging and photothermal cancer therapy. Small. 2014;10(3):556–565. doi: 10.1002/smll.201302217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prabhu P, Patravale V. The upcoming field of theranostic nanomedicine: an overview. J Biomed Nanotechnol. 2012;8(6):859–882. doi: 10.1166/jbn.2012.1459. [DOI] [PubMed] [Google Scholar]
- 136.Zhou D, Xiao H, Meng F, Zhou S, Guo J, Li X, Jing X, Huang Y. Layer-by-Layer Assembled Polypeptide Capsules for Platinum-Based Pro-Drug Delivery. Bioconjug Chem. 2012;23(12):2335–2343. doi: 10.1021/bc300144e. [DOI] [PubMed] [Google Scholar]
- 137.Raju HB, Hu Y, Padgett KR, Rodriguez JE, Goldberg JL. Investigation of nanoparticles using magnetic resonance imaging after intravitreal injection. Clin Experiment Ophthalmol. 2012;40(1):100–107. doi: 10.1111/j.1442-9071.2011.02651.x. [DOI] [PubMed] [Google Scholar]
- 138.Gabriele ML, Wollstein G, Ishikawa H, Kagemann L, Xu J, Folio LS, Schuman JS. Optical coherence tomography: history, current status, and laboratory work. Invest Ophthalmol Vis Sci. 2011;52(5):2425–2436. doi: 10.1167/iovs.10-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agrawal A, Connors M, Beylin A, Liang CP, Barton D, Chen Y, Drezek RA, Pfefer TJ. Characterizing the point spread function of retinal OCT devices with a model eye-based phantom. Biomed Opt Express. 2012;3(5):1116–1126. doi: 10.1364/BOE.3.0011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Na HB, Lee JH, An K, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, Hyeon T. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew Chem Int Ed Engl. 2007;46(28):5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 141.Krause M, Kwong KK, Xiong J, Gragoudas ES, Young LH. MRI of blood volume and cellular uptake of superparamagnetic iron in an animal model of choroidal melanoma. Ophthalmic Res. 2002;34(4):241–250. doi: 10.1159/000063883. [DOI] [PubMed] [Google Scholar]
- 142.Nemmar A, Holme JA, Rosas I, Schwarze PE, Alfaro-Moreno E. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. Biomed Res Int. 2013;2013:279371. doi: 10.1155/2013/279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Horvath E, Mate Z, Takacs S, Pusztai P, Sapi A, Konya Z, Nagymajtenyi L, Papp A. General and electrophysiological toxic effects of manganese in rats following subacute administration in dissolved and nanoparticle form. Scientific World Journal. 2012;2012:520632. doi: 10.1100/2012/520632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu L, Wang Z, Shen B. Large-scale gold nanoparticle superlattice and its SERS properties for the quantitative detection of toxic carbaryl. Nanoscale. 2013;5(12):5274–5278. doi: 10.1039/c3nr00571b. [DOI] [PubMed] [Google Scholar]
- 145.Love SA, Maurer-Jones MA, Thompson JW, Lin YS, Haynes CL. Assessing nanoparticle toxicity. Annu Rev Anal Chem (Palo Alto Calif) 2012;5:181–205. doi: 10.1146/annurev-anchem-062011-143134. [DOI] [PubMed] [Google Scholar]
- 146.Joris F, Manshian BB, Peynshaert K, De Smedt SC, Braeckmans K, Soenen SJ. Assessing nanoparticle toxicity in cell-based assays: influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem Soc Rev. 2013;42(21):8339–8359. doi: 10.1039/c3cs60145e. [DOI] [PubMed] [Google Scholar]
- 147.Yan QQ, Yang L, Zhao J, Li J, Yang L, Wang ZL. Comparative experiment on nanoparticle-induced toxicity in human vascular endothelial cells. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012;30(11):820–824. [PubMed] [Google Scholar]
- 148.Meng J, Xing J, Ma X, Cao W, Lu J, Wang Y, Gao X, Sun B, Liang X, Zhao Y. Metallofullerol nanoparticles with low toxicity inhibit tumor growth by induction of G0/G1 arrest. Nanomedicine (Lond) 2013;8(2):203–213. doi: 10.2217/nnm.12.95. [DOI] [PubMed] [Google Scholar]
- 149.Muldoon LL, Sandor M, Pinkston KE, Neuwelt EA. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–796. doi: 10.1093/neurosurgery/57.4.785. [DOI] [PubMed] [Google Scholar]
- 150.Glazer ES, Zhu C, Hamir AN, Borne A, Thompson CS, Curley SA. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology. 2011;5(4):459–468. doi: 10.3109/17435390.2010.516026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Raju HB, Hu Y, Vedula A, Dubovy SR, Goldberg JL. Evaluation of magnetic micro- and nanoparticle toxicity to ocular tissues. PLoS One. 2011;6(5):e17452. doi: 10.1371/journal.pone.0017452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bu HZ, Gukasyan HJ, Goulet L, Lou XJ, Xiang C, Koudriakova T. Ocular disposition, pharmacokinetics, efficacy and safety of nanoparticle-formulated ophthalmic drugs. Curr Drug Metab. 2007;8(2):91–107. doi: 10.2174/138920007779815977. [DOI] [PubMed] [Google Scholar]
- 153.Toyama T, Matsuda H, Ishida I, Tani M, Kitaba S, Sano S, Katayama I. A case of toxic epidermal necrolysis-like dermatitis evolving from contact dermatitis of the hands associated with exposure to dendrimers. Contact Derm. 2008;59(2):122–123. doi: 10.1111/j.1600-0536.2008.01340.x. [DOI] [PubMed] [Google Scholar]
- 154.Yanagisawa R, Takano H, Inoue K, Koike E, Kamachi T, Sadakane K, Ichinose T. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp Biol Med (Maywood) 2009;234(3):314–322. doi: 10.3181/0810-RM-304. [DOI] [PubMed] [Google Scholar]
- 155.Yanagisawa R, Takano H, Inoue KI, Koike E, Sadakane K, Ichinose T. Size effects of polystyrene nanoparticles on atopic dermatitislike skin lesions in NC/NGA mice. Int J Immunopathol Pharmacol. 2010;23(1):131–141. doi: 10.1177/039463201002300112. [DOI] [PubMed] [Google Scholar]
- 156.Ali-Boucetta H, Al-Jamal KT, Kostarelos K. Cytotoxic assessment of carbon nanotube interaction with cell cultures. Methods Mol Biol. 2011;726:299–312. doi: 10.1007/978-1-61779-052-2_19. [DOI] [PubMed] [Google Scholar]
- 157.Tsukahara T, Haniu H. Cellular cytotoxic response induced by highly purified multi-wall carbon nanotube in human lung cells. Mol Cell Biochem. 2011;352(1–2):57–63. doi: 10.1007/s11010-011-0739-z. [DOI] [PubMed] [Google Scholar]
- 158.Zhang R, He R, Qian J, Guo J, Xue K, Yuan YF. Treatment of experimental autoimmune uveoretinitis with intravitreal injection of tacrolimus (FK506) encapsulated in liposomes. Invest Ophthalmol Vis Sci. 2010;51(7):3575–3582. doi: 10.1167/iovs.09-4373. [DOI] [PubMed] [Google Scholar]
- 159.Kim JH, Kim JH, Kim KW, Kim MH, Yu YS. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20(50):505101. doi: 10.1088/0957-4484/20/50/505101. [DOI] [PubMed] [Google Scholar]
- 160.Urtti A, Polansky J, Lui GM, Szoka FC. Gene delivery and expression in human retinal pigment epithelial cells: effects of synthetic carriers, serum, extracellular matrix and viral promoters. J Drug Target. 2000;7(6):413–421. doi: 10.3109/10611860009102216. [DOI] [PubMed] [Google Scholar]
- 161.Olson JL, Velez-Montoya R, Mandava N, Stoldt CR. Intravitreal silicon-based quantum dots as neuroprotective factors in a model of retinal photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2012;53(9):5713–5721. doi: 10.1167/iovs.12-9745. [DOI] [PubMed] [Google Scholar]
- 162.Prow TW. Toxicity of nanomaterials to the eye. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):317–333. doi: 10.1002/wnan.65. [DOI] [PubMed] [Google Scholar]
- 163.Rajpal, Srinivas A, Azad RV, Sharma YR, Kumar A, Satpathy G, Velpandian T. Evaluation of vitreous levels of gatifloxacin after systemic administration in inflamed and non-inflamed eyes. Acta ophthalmologica. 2009;87(6):648–652. doi: 10.1111/j.1755-3768.2008.01323.x. [DOI] [PubMed] [Google Scholar]
- 164.Jiang A, Wang J, Joshi M, Christoforidis JB. Systemic treatments for noninfectious vitreous inflammation. Mediators Inflamm. 2013;2013:515312. doi: 10.1155/2013/515312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Horcajada JP, Atienza R, Sarasa M, Soy D, Adan A, Mensa J. Pharmacokinetics of linezolid in human non-inflamed vitreous after systemic administration. J Antimicrob Chemother. 2009;63(3):550–552. doi: 10.1093/jac/dkn516. [DOI] [PubMed] [Google Scholar]
- 166.Byon IS, Jeon HS, Kim HW, Lee SJ, Lee JE, Oum BS. The effect of a systemic angiotensin receptor blocker on vascular endothelial growth factor in the vitreous of patients with proliferative diabetic retinopathy. Curr Eye Res. 2013;38(7):774–780. doi: 10.3109/02713683.2013.772206. [DOI] [PubMed] [Google Scholar]
- 167.Mack WP. Complications in periocular rejuvenation. Facial Plast Surg Clin North Am. 2010;18(3):435–456. doi: 10.1016/j.fsc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 168.Frank RC, Cowan BJ, Harrop AR, Astle WF, McPhalen DF. Visual development in infants: visual complications of periocular haemangiomas. J Plast Reconstr Aesthet Surg. 2010;63(1):1–8. doi: 10.1016/j.bjps.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 169.Castellarin A, Pieramici DJ. Anterior segment complications following periocular and intraocular injections. Ophthalmol Clin North Am. 2004;17(4) doi: 10.1016/j.ohc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 170.Nuzzi R, Tridico F. Local and systemic complications after intravitreal administration of anti-vascular endothelial growth factor agents in the treatment of different ocular diseases: a five-year retrospective study. Semin Ophthalmol. 2015;30(2):129–135. doi: 10.3109/08820538.2013.835833. [DOI] [PubMed] [Google Scholar]
- 171.Pichi F, Nucci P, Ciardella AP. Intravitreal bevacizumab for macular complications from retinal arterial macroaneurysms. Reply. Am J Ophthalmol. 2013;155(4):774–775. doi: 10.1016/j.ajo.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 172.Fasih U, Shaikh N, Rahman A, Sultan S, Fehmi MS, Shaikh A. A one-year follow-up study of ocular and systemic complications of intravitreal injection of bevacizumab (Avastin) J Pak Med Assoc. 2013;63(6):707–710. [PubMed] [Google Scholar]
- 173.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27(7):787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jonas JB. Intravitreal bevacizumab for macular complications from retinal arterial macroaneurysms. Am J Ophthalmol. 2013;155(4):774. doi: 10.1016/j.ajo.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 175.Sigler EJ, Randolph JC, Charles S, Calzada JI. Intravitreal fluorinated gas preference and occurrence of rare ischemic postoperative complications after pars plana vitrectomy: a survey of the american society of retina specialists. J Ophthalmol. 2012;2012:230596. doi: 10.1155/2012/230596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cho HJ, Lee DW, Cho SW, Kim CG, Kim JW. Hemorrhagic complications after intravitreal ranibizumab injection for polypoidal choroidal vasculopathy. Can J Ophthalmol. 2012;47(2):170–175. doi: 10.1016/j.jcjo.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 177.Hashida N, Ohguro N, Nishida K. Efficacy and Complications of Intravitreal Rituximab Injection for Treating Primary Vitreoretinal Lymphoma. Transl Vis Sci Technol. 2012;1(3):1. doi: 10.1167/tvst.1.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gilger BC, Abarca EM, Salmon JH, Patel S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest Ophthalmol Vis Sci. 2013;54(4):2483–2492. doi: 10.1167/iovs.13-11747. [DOI] [PubMed] [Google Scholar]
- 179.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53(8):4433–4441. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Touchard E, Berdugo M, Bigey P, El Sanharawi M, Savoldelli M, Naud MC, Jeanny JC, Behar-Cohen F. Suprachoroidal electrotransfer: a nonviral gene delivery method to transfect the choroid and the retina without detaching the retina. Mol Ther. 2012;20(8):1559–1570. doi: 10.1038/mt.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kadam RS, Williams J, Tyagi P, Edelhauser HF, Kompella UB. Suprachoroidal delivery in a rabbit ex vivo eye model: influence of drug properties, regional differences in delivery, and comparison with intravitreal and intracameral routes. Mol Vis. 2013;19:1198–1210. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 182.Tyagi P, Kadam RS, Kompella UB. Comparison of suprachoroidal drug delivery with subconjunctival and intravitreal routes using noninvasive fluorophotometry. PLoS One. 2012;7(10):e48188. doi: 10.1371/journal.pone.0048188. [DOI] [PMC free article] [PubMed] [Google Scholar]