Abstract
Background
The measurement of free thyroid hormone, instead of the total form, is more commonly used in current practice. We aimed to evaluate the usefulness of the ratio of serum free triiodothyronine (FT3, pg/mL) to free thyroxine (FT4, ng/dL) for differentiating Graves' disease from subacute thyroiditis.
Materials and methods
Medical records of thyrotoxic patients aged >15 years who had measurement of FT3, FT4 and thyrotropin on the first diagnosis of thyrotoxicosis before initiating treatment were retrospectively reviewed. Data were collected from all clinics, and were not limited to the endocrine clinic. Pregnant women were excluded.
Results
A total of 548 patients (468 with Graves' disease, 40 with subacute thyroiditis and 40 with toxic adenoma/multinodular goiter) were recruited. Mean age was 43.9 ± 15.4 years. Most were female 434 (79.2%), and goiter was present in 55.3%. Prevalence of T3-toxicosis and T4-toxicosis were 5.6% and 6.6%, respectively. Mean FT3/FT4 ratios were 4.62 ± 2 (10−2 pg/ng) in patients with Graves' disease and 2.73 ± 0.5 in subacute thyroiditis. The area under the ROC curve of the FT3/FT4 ratio for diagnosis of Graves' disease was 0.83 (95%CI, 0.76–0.91). Cutoff level of this ratio >4.4 offered sensitivity of 47.2% and specificity of 92.8%.
Conclusions
FT3/FT4 ratio of >4.4 (10−2 pg/ng) may help in differentiating the cause of thyrotoxicosis.
Keywords: Thyroid function tests, Thyrotoxicosis, Graves' disease, Subacute thyroiditis, Sensitivity and specificity
Abbreviations: Ab, antibody; FT3, free triiodothyronine; TT3, total triiodothyronine; FT4, free thyroxine; TT4, total thyroxine; TFTs, thyroid function tests; Tg, thyroglobulin; TPO, thyroid peroxidase; TRAb, thyrotropin receptor antibody; TSH, thyroid-stimulating hormone
Highlights
-
•
The ideal treatment of thyrotoxicosis should be directed at its etiology.
-
•
Serum total triiodothyronine (T3): thyroxine (T4) ratio (ng/ug) of >20 suggests Graves' disease.
-
•
In the current practice, the measurement of free thyroid hormone is more commonly used.
-
•
Serum free T3/free T4 ratio of >4.4 (10−2 pg/ng) may help in differentiating the cause of thyrotoxicosis.
1. Introduction
Thyrotoxicosis is a common thyroid dysfunction. Common etiologies of hyperthyroidism are Graves' disease, subacute thyroiditis, toxic adenoma, and multinodular goiter. The ratio of total triiodothyronine (T3) to total thyroxine (T4) is a simple and helpful index for the differential diagnosis between Graves' disease and subacute thyroiditis [1], [2]. A total T3 to total T4 ratio less than 20 ng/mg in thyrotoxic patients before therapy is a laboratory signal of destruction-induced thyrotoxicosis [1]. Measurements of free T3 (FT3) and free T4 (FT4), instead of the total form, are more commonly used in current practice because they are less affected by the thyroid hormone-binding proteins. The FT3/FT4 ratio had been reported as a useful indicator for differentiating subacute thyroiditis from Graves' disease [3], [4]. However, these ratios are not widely used because they derived from the small sample studies.
Besides clinical status resulting from thyroid hormone excess, thyrotoxicosis is defined by elevated T3 and/or T4 concentrations. Serum thyrotropin (more frequently referred to as thyroid-stimulating hormone, TSH) level is the most sensitive and specific tool used in the diagnosis of primary thyroid dysfunction. Many organizations currently recommend measurement of both TSH and FT4 at the time of the initial assessment, while the total T3 measurement is only helpful for the diagnosis of T3-toxicosis [5], [6]. T3-toxicosis is a state in which patients have a high level of T3 and low TSH but a normal level of T4. It is caused by iodine deficiency or the earliest stages of disease caused by an autonomously functioning thyroid nodule, multinodular goiter or Graves' disease [7], [8]. Prevalence of T3-toxicosis varies from 1 to 5% [8]. However, data from Thailand reported a higher prevalence of T3-toxicosis compared with T4-toxicosis (16.02% vs 2.94%) [9]. Controversy still exists as to whether T3 or T4 offers better sensitivity for determining a thyrotoxic state, particularly in a limited-resource setting.
The aim of this study was to evaluate the usefulness of the ratio of serum FT3 to FT4 for differentiating Graves' disease from subacute thyroiditis. We also compared the sensitivity and specificity of T3 and T4 for diagnosis of thyrotoxicosis.
2. Materials and methods
2.1. Study design and participants
This study was approved by the Ethics Committee on Human Experimentation, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok. Medical records of thyrotoxic patients aged more than 15 years who had thyroid function tests, including FT3, FT4 and TSH, on the first date of diagnosis of thyrotoxicosis were retrospectively reviewed. Pregnant women and subjects with isolated TSH levels were excluded from the study. Retrospective data during the study period 2011–2014 were collected from all clinics in Ramathibodi Hospital and not limited to the endocrine clinic. Pregnant women were excluded. Data from Iodine Global Network in 2012, iodine status in Thailand is adequate.
2.2. Definition
Graves' disease was diagnosed by clinical presentations and/or thyroid autoantibody and/or high radioactive iodine uptake (RAIU) tests. Diagnosis of subacute thyroiditis was made on the basis of later development of euthyroid or hypothyroid without receiving any anti-thyroid hormone drug and/or clinical pain and/or low RAIU test. Toxic adenoma/multinodular goiter was diagnosed by the use of RAIU with/without thyroid scan and fine-needle aspiration. Demographic data, diagnosis, clinical presentation, thyroid function tests, thyroid autoantibody and RAIU and thyroid scan results were recorded. Treatment of thyrotoxicosis were based on the decisions of family physicians, internists or endocrinologists. The evaluation of the cause of thyrotoxicosis was reviewed and confirmed by an endocrinologist who specializes in thyroidology. In addition, an endocrinologist was blind to the results of thyroid function tests.
2.3. Serum analysis
The following tests were performed on an ARCHITECT i2000 immunoassay analyzer (Abbott Diagnostics, Lake Forest IL, USA): TSH, FT3, FT4, thyroglobulin autoantibody (TgAb), thyroperoxidase antibody (TPOAb) and TSH receptor antibody (TRAb). All assays were performed using reagents provided by Abbott Diagnostics, according to the instructions on the package insert. The corresponding reference ranges of serum TSH, FT3 and FT4 were 0.35–4.94 mIU/L, 1.71–3.71 pg/mL and 0.70–1.48 ng/dL. The lowest value of serum TSH that could be detected was 0.0038 mIU/L. The highest serum FT3 and FT4 levels that could be measured were 30 pg/mL and 6 ng/dL, respectively.
2.4. Statistical analyses
Statistical analyses were performed using SPSS version 20.0 (IBM Corp, Armonk NY, USA). The Kolmogorov–Smirnov test was used for Gaussian distribution analysis. Data were presented as mean ± SD. A p-value <0.05 was considered statistically significant. Cutoff values of the FT3/FT4 ratio for diagnosis of thyrotoxicosis were calculated using receiver operating characteristic (ROC) curves.
3. Results
3.1. Demographic data
A total of 548 patients with newly-diagnosed of thyrotoxicosis were recruited for the final analysis. The mean age was 43.9 ± 15.4 years (range, 15–86), and most were women 434 (79.2%). Of these patients, 468 (85.4%) were diagnosed with Graves' disease, 40 (7.3%) with subacute thyroiditis, and 40 (7.3%) with toxic adenoma/multinodular goiter (Table 1). Most people lived in Bangkok or the central region of Thailand (53.3%), followed by the northeast (19.1%) and south (8.9.%). Goiter was the most common symptom, found in 55.3%.
Table 1.
Baseline characteristics in the patients with Graves' disease, subacute thyroiditis and toxic adenoma/multinodular goiter.
| Graves' disease (N = 468) | Subacute thyroiditis (N = 40) | Toxic adenoma/multinodular goiter (N = 40) | |
|---|---|---|---|
| Mean age (years) | 42.35 ± 12.52 | 51.94 ± 20.55 | 44.32 ± 15.02 |
| Female (%) | 79.3% | 75% | 82.5% |
| Signs (%) | |||
| Goiter | 56.2% | 35% | 66.7% |
| Ophthalmopathy | 14.7% | 0% | 0% |
| Acropachy | 0.6% | 0% | 0% |
| Pretibial myxedema | 0.4% | 0% | 0% |
| Thyroid function tests | |||
| Mean FT3 (pg/mL) | 15.72 ± 10.1 | 5.56 ± 2.99 | 5.29 ± 3.81 |
| Mean FT4 (ng/dL) | 3.22 ± 1.31 | 2.00 ± 0.63 | 1.86 ± 0.48 |
| Median TSH (mIU/L, IQR) | 0.0038 (0.0038–0.0038) | 0.01 (0.006–0.028) | 0.0038 (0.0038–0.032) |
| Mean FT3/FT4 (10−2 pg/ng) | 4.62 ± 2 | 2.73 ± 0.5 | 2.67 ± 1.3 |
Abbreviations: FT3, free triiodothyronine; FT4, free thyroxine; IQR, interquartile range; TSH, thyroid-stimulating hormone.
3.2. FT3/FT4 ratio for differentiating between Graves' disease and subacute thyroiditis
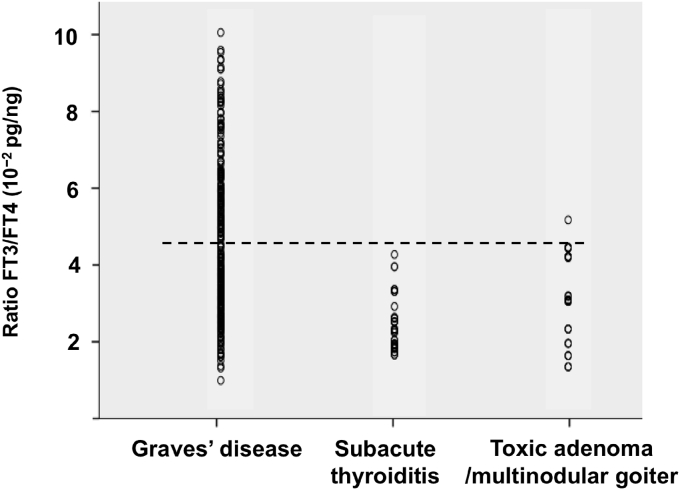
Serum FT3 and FT4 were obtained at the time of diagnosis of thyrotoxicosis. Additional tests to confirmed the etiology of thyrotoxicosis were performed within 2 months after the onset. Mean FT3 to FT4 ratios (10−2 pg/ng) were 4.62 ± 2 in patients with Graves' disease, 2.73 ± 0.5 in cases of subacute thyroiditis, and 2.67 ± 1.3 in toxic adenoma/multinodular goiter (Fig. 1). The area under the ROC curve of the FT3 to FT4 ratio for the diagnosis of Graves' disease was 0.83 (95%CI, 0.76–0.91). A cutoff level of this ratio of more than 4.4 offered sensitivity of 47.2% and specificity of 92.8%. The area under the curve of the FT3 to FT4 ratio for diagnosis of subacute thyroiditis was 0.82 (95%CI, 0.73–0.90). A cutoff level lower than 2.2 offered sensitivity of 44.8% and specificity of 92.8% for diagnosis of subacute thyroiditis.
Fig. 1.
Scatter plot showing the ratio of FT3/FT4 (10−2 pg/ng) for differential diagnosis of thyrotoxicosis. Cutoff level of the FT3/FT4 ratio >4.4 offered sensitivity of 47.2% and specificity of 92.8%.
3.3. Prevalence of T3-and T4-thyrotoxicosis
All patients had low TSH levels, while 481 of 500 (87.8%) had both high T3 and T4. Prevalence of T3-toxicosis and T4-toxicosis was 5.6% and 6.6%, respectively. Graves' disease was found in 88.3% of T3-toxicosis and 81.3% of T4-toxicosis cases. In the group of T3-toxicosis patients, the majority still lived in Bangkok or the central region (62.3%) and had the mean age of 45.3 ± 14.7 years (range, 21–78).
3.4. Sensitivity and specificity of thyroid hormones for diagnosis of thyrotoxicosis
The areas under the ROC curves of FT3 and FT4 for the diagnosis of Graves' disease were 0.84 (95%CI, 0.78–0.89) and 0.82 (95%CI, 0.77–0.87), respectively. Sensitivities of FT3 and FT4 to diagnosis of Graves' disease were 94.1% and 94.4%. Sensitivities of FT3 and FT4 to diagnosis of subacute thyroiditis were 88.2% and 90.2%, respectively.
4. Discussion
The ratio of total T3 to total T4 is commonly used for differentiating the etiology of thyrotoxicosis between Graves' disease and subacute thyroiditis. However, FT3 and FT4 measurements are frequently ordered in current clinical practice [10]. In this study, the ratio of FT3 to FT4 was useful for distinguishing the cause of thyrotoxicosis. This result is consistent with previous studies [3], [4]. Despite the low sensitivity of the cutoff value of FT3/FT4 ratio, we chose to use high specificity for the diagnosis of Graves' disease. The FT3/FT4 ratio >4.4 (10−2 pg/ng) as to likely confirm the diagnosis of Graves' disease and this approach obviates an unnecessary complex and expensive testing. In the overlapping ratio between 2.2 and 4.4, the authors suggest using a combination of clinical features and investigations to differentiate Graves' disease from subacute thyroiditis, such as clinical follow-up, erythrocyte sedimentation rate (ESR), TRAb, color flow doppler ultrasonography of the thyroid gland, and RAIU. The strength of this study was a higher number of subjects than prior studies. However, this ratio should be validated in other populations. It cannot be applied to hospitalized patients who have the possibility of low T3 levels from non-thyroidal illness. In addition, due to the low sensitivity of the cutoff value of the FT3/FT4 ratio, the use of clinical symptoms to differentiate between Graves' disease and subacute thyroiditis is still important.
Other markers for differentiation between Graves' disease and subacute thyroiditis – basal levels of serum calcitonin [1], peripheral eosinophil/monocyte ratio [3], stem cell factor [11], total alkaline phosphatase activity [12], red blood cell zinc concentration [13], plasma fibrinogen [14], and serial changes in liver function tests [15] – have been reported. However, these parameters are difficult to use in clinical practice.
In this study, the prevalence of T3-toxicosis was not as high as expected in newly diagnosed untreated patients with thyrotoxicosis. Either T3 or T4 can provide diagnostic value for the diagnosis of thyrotoxicosis. T3 is an active form of thyroid hormone that is deiodinated from T4 by type 1 iodothyronine deiodinase (D1) as well as type 2 (D2). In humans, the thyroid expresses both D1 and D2 [16]. High expression of D2 in Graves' thyroid tissue and in some thyroid adenomas has been reported to contribute to relatively high serum T3 levels [17]. T3-toxicosis can be found in subjects with Graves' disease and toxic adenoma/multinodular goiter, especially in the early or relapsed phase of thyrotoxicosis [18]. Also, the frequency of T3-toxicosis was higher in those with iodine deficiency. This study had a lower prevalence of T3-toxicosis than a previous study [9], which might be due to differences in the selected study population. We collected subjects only for newly diagnosed thyrotoxicosis, and included patients from every clinic in the hospital. In contrast, the previous study included only patients from the endocrine clinic, who usually represent only severe cases with high T3 levels [9]. In an era of economic crises, cost-effectiveness is one of the most important health management concerns. More than 90% of patients with thyrotoxicosis can be diagnosed by using only two instead of the three values. This would help reduce the cost by 33%, an estimated 100,000 Thai baht or around $3000 US, in these 500 patients.
In summary, a higher ratio of FT3 to FT4 suggests that the patient may have Graves' disease, and a very low ratio supports a diagnosis of subacute thyroiditis. Use of lab TSH with either FT3 or FT4 will provide high sensitivity and cost-effectiveness.
Ethical approval
This study was approved by the Ethics Committee on Human Experimentation, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok.
Sources of funding
None.
Author contribution
CS: study design, data analysis, writing.
AB: data collection, data analysis.
Conflicts of interest
None.
Guarantor
Chutintorn Sriphrapradang.
References
- 1.Amino N., Yabu Y., Miki T. Serum ratio of triiodothyronine to thyroxine, and thyroxine-binding globulin and calcitonin concentrations in Graves' disease and destruction-induced thyrotoxicosis. J. Clin. Endocrinol. Metab. 1981;53(1):113–116. doi: 10.1210/jcem-53-1-113. [DOI] [PubMed] [Google Scholar]
- 2.Shigemasa C., Abe K., Taniguchi S. Lower serum free thyroxine (T4) levels in painless thyroiditis compared with Graves' disease despite similar serum total T4 levels. J. Clin. Endocrinol. Metab. 1987;65(2):359–363. doi: 10.1210/jcem-65-2-359. [DOI] [PubMed] [Google Scholar]
- 3.Izumi Y., Hidaka Y., Tada H. Simple and practical parameters for differentiation between destruction-induced thyrotoxicosis and Graves' thyrotoxicosis. Clin. Endocrinol. Oxf. 2002;57(1):51–58. doi: 10.1046/j.1365-2265.2002.01558.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimura Noh J., Momotani N., Fukada S., Ito K., Miyauchi A., Amino N. Ratio of serum free triiodothyronine to free thyroxine in Graves' hyperthyroidism and thyrotoxicosis caused by painless thyroiditis. Endocr. J. 2005;52(5):537–542. doi: 10.1507/endocrj.52.537. [DOI] [PubMed] [Google Scholar]
- 5.Bahn Chair R.S., Burch H.B., Cooper D.S. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American thyroid association and American association of clinical endocrinologists. Thyroid. 2011;21(6):593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- 6.Moon J.H., Yi K.H. The diagnosis and management of hyperthyroidism in Korea: consensus report of the korean thyroid association. Endocrinol. Metab. Seoul. 2013;28(4):275–279. doi: 10.3803/EnM.2013.28.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitton R.N., Wexler C. Free triiodothyronine toxicosis: a distinct entity. Am. J. Med. 1990;88(5):531–533. doi: 10.1016/0002-9343(90)90435-g. [DOI] [PubMed] [Google Scholar]
- 8.Figge J., Leinung M., Goodman A.D. The clinical evaluation of patients with subclinical hyperthyroidism and free triiodothyronine (free T3) toxicosis. Am. J. Med. 1994;96(3):229–234. doi: 10.1016/0002-9343(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 9.Snabboon T., Sridama V., Sunthornyothin S., Suwanwalaikorn S., Vongthavaravat V. A more appropriate algorithm of thyroid function test in diagnosis of hyperthyroidism for Thai patients. J. Med. Assoc. Thai. 2004;87(Suppl 2):S19–S21. [PubMed] [Google Scholar]
- 10.Thienpont L.M., Van Uytfanghe K., Poppe K., Velkeniers B. Determination of free thyroid hormones. Best. Pract. Res. Clin. Endocrinol. Metab. 2013;27(5):689–700. doi: 10.1016/j.beem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Yamada T., Sato A., Aizawa T. An elevation of stem cell factor in patients with hyperthyroid Graves' disease. Thyroid. 1998;8(6):499–504. doi: 10.1089/thy.1998.8.499. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa T., Sato K., Kato Y., Shimizu S., Takano K. Rapid differential diagnosis of Graves' disease and painless thyroiditis using total T3/T4 ratio, TSH, and total alkaline phosphatase activity. Endocr. J. 2005;52(1):29–36. doi: 10.1507/endocrj.52.29. [DOI] [PubMed] [Google Scholar]
- 13.Sayama N., Yoshida K., Mori K. Measurement of red blood cell zinc concentration with Zn-test kit: discrimination between hyperthyroid Graves' disease and transient thyrotoxicosis. Endocr. J. 1998;45(6):767–772. doi: 10.1507/endocrj.45.767. [DOI] [PubMed] [Google Scholar]
- 14.Ma J., Liu R., Wu D. Utility of plasma fibrinogen in the differential diagnosis of thyrotoxicosis. Int. J. Clin. Exp. Med. 2015;8(1):1220–1226. [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota S., Amino N., Matsumoto Y. Serial changes in liver function tests in patients with thyrotoxicosis induced by Graves' disease and painless thyroiditis. Thyroid. 2008;18(3):283–287. doi: 10.1089/thy.2007.0189. [DOI] [PubMed] [Google Scholar]
- 16.Bianco A.C., Salvatore D., Gereben B., Berry M.J., Larsen P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002;23(1):38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 17.Salvatore D., Tu H., Harney J.W., Larsen P.R. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J. Clin. Invest. 1996;98(4):962–968. doi: 10.1172/JCI118880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shenkman L., Mitsuma T., Blum M., Hollander C.S. Recurrent hyperthyroidism presenting as triiodothyronine toxicosis. Ann. Intern Med. 1972;77(3):410–413. doi: 10.7326/0003-4819-77-3-410. [DOI] [PubMed] [Google Scholar]