The mechanisms underlying recovery of language after left hemisphere stroke are incompletely understood. Xing et al. report increases in grey matter volumes in the right temporoparietal cortex in stroke survivors relative to healthy controls, and a positive correlation with spontaneous speech, naming, and repetition scores.
Keywords: aphasia, stroke, grey matter, outcome, right hemisphere, voxel-based morphometry
The mechanisms underlying recovery of language after left hemisphere stroke are incompletely understood. Xing et al. report increases in grey matter volumes in the right temporoparietal cortex in stroke survivors relative to healthy controls, and a positive correlation with spontaneous speech, naming, and repetition scores.
Abstract
The neural mechanisms underlying recovery of language after left hemisphere stroke remain elusive. Although older evidence suggested that right hemisphere language homologues compensate for damage in left hemisphere language areas, the current prevailing theory suggests that right hemisphere engagement is ineffective or even maladaptive. Using a novel combination of support vector regression-based lesion-symptom mapping and voxel-based morphometry, we aimed to determine whether local grey matter volume in the right hemisphere independently contributes to aphasia outcomes after chronic left hemisphere stroke. Thirty-two left hemisphere stroke survivors with aphasia underwent language assessment with the Western Aphasia Battery-Revised and tests of other cognitive domains. High-resolution T1-weighted images were obtained in aphasia patients and 30 demographically matched healthy controls. Support vector regression-based multivariate lesion-symptom mapping was used to identify critical language areas in the left hemisphere and then to quantify each stroke survivor’s lesion burden in these areas. After controlling for these direct effects of the stroke on language, voxel-based morphometry was then used to determine whether local grey matter volumes in the right hemisphere explained additional variance in language outcomes. In brain areas in which grey matter volumes related to language outcomes, we then compared grey matter volumes in patients and healthy controls to assess post-stroke plasticity. Lesion–symptom mapping showed that specific left hemisphere regions related to different language abilities. After controlling for lesion burden in these areas, lesion size, and demographic factors, grey matter volumes in parts of the right temporoparietal cortex positively related to spontaneous speech, naming, and repetition scores. Examining whether domain general cognitive functions might explain these relationships, partial correlations demonstrated that grey matter volumes in these clusters related to verbal working memory capacity, but not other cognitive functions. Further, grey matter volumes in these areas were greater in stroke survivors than healthy control subjects. To confirm this result, 10 chronic left hemisphere stroke survivors with no history of aphasia were identified. Grey matter volumes in right temporoparietal clusters were greater in stroke survivors with aphasia compared to those without history of aphasia. These findings suggest that the grey matter structure of right hemisphere posterior dorsal stream language homologues independently contributes to language production abilities in chronic left hemisphere stroke, and that these areas may undergo hypertrophy after a stroke causing aphasia.
Introduction
Approximately one-third of stroke survivors have aphasia, a loss of language ability, as one of their presenting symptoms. Two-thirds of these individuals never regain their prior level of language functioning (Gottesman and Hillis, 2010). Thus, ∼20% of stroke survivors are left with chronic aphasia, which causes difficulty communicating in daily life, decreases quality of life and causes substantial long-term disability (Gottesman and Hillis, 2010; Berthier and Pulvermuller, 2011; Gialanella et al., 2011; Gonzalez-Fernandez et al., 2013).
Engagement of preserved left hemisphere language areas and recruitment of nearby perilesional tissue is thought to support aphasia recovery after stroke (Karbe et al., 1995; Heiss et al., 2003; Saur et al., 2006; Winhuisen et al., 2007; Meinzer and Breitenstein, 2008; Martin et al., 2009; Fridriksson, 2010; Fridriksson et al., 2012). The role of the right hemisphere in aphasia recovery has been debated since the late 19th century and remains controversial. Evidence of right hemisphere involvement in aphasia recovery begins with Barlow’s 1877 case of a boy who recovered from aphasia after a left inferior frontal gyrus stroke and later worsened again after another stroke to the same location in the right hemisphere (Barlow, 1877). In addition to more recent cases of sequential left and right hemisphere strokes in adults (Basso et al., 1989; Turkeltaub et al., 2012), other lines of evidence suggest right hemisphere compensation in aphasia: a relationship between poor aphasia outcomes and ‘clinically silent’ right hemisphere strokes (Yarnell et al., 1976), worsening of language in aphasic patients after right carotid anaesthesia (Kinsbourne, 1971), and left visual field and left ear advantages in people with aphasia (Moore and Weidner, 1974, 1975; Johnson et al., 1977; Moore and Papanicolaou, 1988). These sources lack the spatial resolution to implicate specific parts of the right hemisphere in aphasia recovery, but suggest that overall the right hemisphere compensates for language deficits after damage to the native left hemisphere network.
However, recent transcranial magnetic stimulation studies of aphasia have shown that inhibiting the right inferior frontal gyrus improves language functions (Naeser et al., 2005; Martin et al., 2009; Barwood et al., 2011; Hamilton et al., 2011). These studies seemingly suggest that right hemisphere recruitment may have a negative impact on aphasia outcome. However, most brain stimulation studies aimed at suppressing right hemisphere processing in chronic aphasia have used protocols thought to inhibit the targeted area immediately after stimulation (Hallett, 2000; Zaghi et al., 2010). There is scarce evidence that the language benefits of these techniques in chronic aphasia are related to long-term right hemisphere inhibition. Moreover, the effects of right hemisphere inhibition in areas outside the right inferior frontal gyrus have not been thoroughly investigated.
Functional imaging findings have also raised questions about whether right hemisphere recruitment may be ineffective or even maladaptive in aphasia recovery (Belin et al., 1996; Cao et al., 1999; Winhuisen et al., 2005; Postman-Caucheteux et al., 2010; Allendorfer et al., 2012). In longitudinal studies, right hemisphere recruitment has often peaked early in recovery and has diminished over time in association with clinical improvements (Fernandez et al., 2004; Saur et al., 2006; Kurland et al., 2008; Breier et al., 2009), seemingly suggesting that disengaging the right hemisphere is beneficial for long-term aphasia recovery.
There is thus a great deal of inconsistency in the literature on right hemisphere contributions to aphasia outcome. This inconsistency may arise in part because of individual differences in recovery. Patients with small left hemisphere lesions and less severe aphasia may be able to recruit residual left hemisphere language-capable areas (Heiss et al., 1999), and thus have little right hemisphere activity. In contrast, individuals with large left hemisphere lesions have more severe aphasia because of the extensive damage to the native left hemisphere language network, and must rely on the right hemisphere more (Anglade et al., 2014). This relationship may lead to the erroneous impression that greater right hemisphere engagement causes worse outcomes, when in fact the causal relationship is reversed. To reveal the true contributions of the right hemisphere to language recovery, it is necessary to first estimate the likely severity of aphasia given the features of the individual and his/her stroke. Only then can one assess whether right hemisphere engagement alters this outcome, either positively or negatively.
Additionally, the impact of performance and effort on task-related activity further complicates the interpretation of functional imaging results. While inverse correlations between activity and performance may suggest maladaptive activity, an alternate interpretation is that individuals with more severe aphasia must exert more effort to perform the task and thus engage the right hemisphere to a greater degree (Just et al., 1996; Fridriksson and Morrow, 2005). Therefore, in addition to task-related functional imaging, measures of the right hemisphere that are independent of effort or difficulty should be examined. Indeed, some recent diffusion tensor imaging studies have suggested that integrity of right hemisphere white matter pathways relates positively to aphasia outcomes (Schlaug et al., 2010; Forkel et al., 2014).
In the present study, we addressed whether language outcomes in chronic aphasia relate to grey matter volume in brain areas not directly impacted by stroke. As noted above, to measure the contributions of the right hemisphere, we first needed to predict the likely language outcomes for each individual based on his/her pattern of left hemisphere damage. To accomplish this, we used support vector regression-based multivariate lesion-symptom mapping (SVR-LSM) to identify critical left hemisphere areas for different language functions, and then calculated the amount of damage in these areas suffered by each stroke survivor. We then used these values along with total lesion size and demographic factors to predict the likely language outcome for each individual based on the direct effects of his/her stroke. Next, we used voxel-based morphometry (VBM) to examine whether local grey matter volume of the uninjured brain tissue contributed to language outcomes independent of these stroke and demographic factors. Finally, for each area in which grey matter volume related to language outcomes, we compared the grey matter volumes in stroke survivors to matched controls to test for differences potentially reflecting plastic changes in grey matter related to post-stroke aphasia.
Materials and methods
Participants
The study was approved by the Georgetown University Institutional Review Board and written informed consent was obtained from all study participants prior to enrolment in the study.
Patients
Thirty-two chronic left hemisphere stroke survivors with history of aphasia were recruited with inclusion criteria as follows: native English speaker; at least 6 months post-stroke; able to follow testing instructions; no history of other significant neurological illnesses. See Table 1 for characteristics of the group, and Supplementary Table 1 for the characteristics of individual participants. All patients had aphasia at the time of stroke based on medical records and received speech-language therapy. Most of the patients received several different types of speech-language therapy from multiple clinicians targeting various aspects of speech and language at different times in their recovery. Many also used tablet or smartphone apps to augment speech therapy. Thus, the type and total dose of therapy are not easily quantifiable.
Table 1.
Demographic data and language performance tests of aphasic patients and healthy controls
| Patients group (n = 32) | Controls group (n = 30) | Statistics | P-value | |
|---|---|---|---|---|
| Demographic variable | ||||
| Age (years) | 59.13 (9.41) | 60.15 (13.90) | t(60) = −0.34 | 0.74 |
| Gender (M/F) | 22/10 | 18/12 | χ2(1) = 0.52 | 0.47 |
| Education (years) | 16.81 (2.90) | 16.57 (2.54) | t(60) = 0.35 | 0.73 |
| Handedness (R/L) | 26/6 | 27/3 | χ2(1) = 0.96 | 0.33 |
| Time post stroke (months) | 45.46 (35.16) | - | - | - |
| Lesion size (ml) | 116.48 (80.22) | - | - | - |
| Language evaluation | ||||
| Aphasia Quotient | 68.27 (24.12) | - | - | - |
| Naming/Word-Finding | 6.21 (2.88) | - | - | - |
| Auditory-Verbal Comprehension | 7.49 (1.68) | - | - | - |
| Repetition | 6.39 (2.74) | - | - | - |
| Spontaneous Speech | 13.56 (5.76) | - | - | - |
Standard deviations are presented in parenthesis. M = male; F = female; R = right-handed; L = left-handed.
Healthy control subjects
Thirty healthy control subjects without neurological or psychiatric disorder, and matched to the stroke group on age, education, handedness, and gender, were enrolled in the study (Table 1).
Aphasia and cognitive testing
Patients were given the Western Aphasia Battery-Revised (WAB-R) (Kertesz et al., 1982), which includes subtests that provide composite scores of Spontaneous Speech, Repetition, Naming/Word-Finding and Auditory-Verbal Comprehension. Totalling these scores provides the Aphasia Quotient (AQ), a measure of overall aphasia severity on a scale of 0–100.
Other language and cognitive domains were tested with the Cognitive Linguistic Quick Test executive composite, excluding generative naming to provide a pure non-language measure; forward and backward digit span (Wechsler, 1987), with an option to respond by pointing to a number line to prevent motor speech deficits from limiting performance; Corsi Blocks forward and backward spatial span (Corsi, 1972); Apraxia battery for adults-2 subtest 2A (Dabul, 2000) and a 30-item pseudoword repetition task.
Image acquisition
MRI data were acquired on a 3.0 T Siemens Trio scanner at the Georgetown University Medical Center with a 3D T1-weighted sequence (magnetization-prepared rapid-acquisition gradient echo) with the following parameters: repetition time = 1900 ms; echo time = 2.56 ms; flip angle = 9°; 160 contiguous 1 mm sagittal slices; field of view = 250 × 250 mm; matrix size = 246 × 256, voxel size = 1 × 1 × 1 mm; slice thickness = 1 mm.
Image data preprocessing
Support vector regression-based lesion-symptom mapping protocol
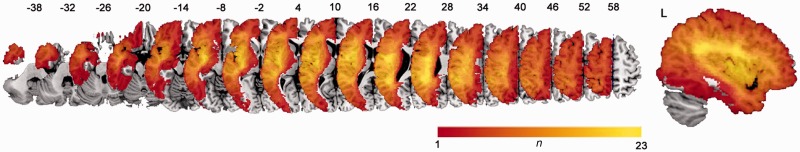
SVR-LSM, a multivariate lesion-symptom mapping approach (Zhang et al., 2014), was used to define critical left hemisphere areas in which damage relates to language impairment on a given WAB-R measure. These results were used to quantify individual differences in left hemisphere stroke locations that relate to the behavioural scores, which is vital to determine if grey matter volume in the right hemisphere contributes additional variance to scores. Lesion masks were created by manually tracing stroke damage on the T1-weighted images in native space in MRIcron (Rorden and Brett, 2000). All lesion masks were checked by two board certified neurologists (S.X. and P.E.T.) and then warped into the Montreal Neurological Institute (MNI) space by applying deformation fields derived from the VBM8 analysis (see below). A lesion overlap map is shown in Fig. 1. Multivariate lesion-symptom mapping is more resistant than voxel-based lesion–symptom mapping to localization errors due to lesion covariance and contributions of multiple brain regions to a given behaviour (Mah et al., 2014; Herbet et al., 2015). Here, multivariate lesion–symptom mapping was carried out using SVR-LSM running under Matlab R2014a (Zhang et al., 2014). SVR-LSM uses a machine learning-based multivariate support vector regression algorithm to find lesion–symptom relationships. SVR-LSM analyses were conducted for the overall severity (WAB-R AQ) as well as for each WAB-R subscore (Spontaneous Speech, Naming/Word-Finding, Repetition and Auditory-Verbal Comprehension). Only voxels damaged in at least 10% of patients were included in the analysis. Probabilistic maps created using 10 000 permutations of the behavioural scores were thresholded at P < 0.001 to define the critical areas of the left hemisphere in which damage causes deficits on a given WAB-R subscore. The Proportion of Critical Area Damaged (PCAD) was then calculated for each patient for each WAB-R score.
Figure 1.
Lesion overlap map of 32 patients with chronic post-stroke aphasia. The n-value denotes the number of patients with a lesion in each voxel (maximum 23 out of 32). L = left.
Voxel-based morphometry protocol
VBM is a whole-brain technique designed to discover subtle, local changes in grey matter density or volume by applying voxel-wise statistics within the context of Gaussian random fields (Ashburner and Friston, 2000). The structural volume preprocessing and analysis were performed using the VBM8 toolbox in Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm) running under Matlab R2014a. Prior to preprocessing, all images were manually realigned to the anterior commissure to reduce between-subject variability and lesion tracings were used to mask out damaged tissue to achieve accurate segmentation and spatial normalization. Data were subsequently processed via a procedure of joint spatial normalization and segmentation using the unified segmentation approach (Ashburner and Friston, 2005). Specifically, images were corrected for bias-field inhomogeneity, and segmented into grey matter, white matter, and CSF maps. The segmented maps were then registered to a standard template in MNI space using 12-parameter affine linear and non-linear warping transformation. The segmentation procedure was refined with a hidden Markov random field model. Grey matter voxel values were multiplied by the Jacobian matrix parameters derived from normalization to preserve actual grey matter values locally (modulated grey matter volumes). The modulated grey matter volumes were then smoothed with a Gaussian kernel of 8 mm full-width at half-maximum to reduce anatomical variability. All voxels were thresholded at 0.2 to avoid possible edge effects between different tissue types.
Statistical analysis
To determine associations between grey matter volume and behavioural measures, volumetric grey matter images were submitted to separate multiple regression analyses with the overall aphasia severity and WAB-R subscores entered as an explanatory factor. We included factors as nuisance variables that could relate to behavioural performance or grey matter volume: age, gender, level of education, handedness, lesion size, PCAD, and total volume of grey and white matter. We maintained a cluster-level corrected P < 0.05 significance threshold by applying height and extent thresholds of P < 0.005 and k = 323, as empirically determined by Monte Carlo simulation (permutations = 5000, with grey matter mask) (Gianaros et al., 2008; Schwartz et al., 2010).
To clarify how the nuisance covariates included in the VBM analysis contributed to language outcomes with and without grey matter volume in the model, the variables were further introduced into a hierarchical linear regression with language measures as the dependent variables. Partial correlation analyses were performed to test the relationships between grey matter volume in right hemisphere clusters identified in the VBM analysis and the cognitive measures. Further separate univariate analyses were performed to address group differences in mean grey matter volume of each identified cluster between aphasia patients and control subjects. These analyses were conducted using SPSS version 22.
Results
Demographic and behavioural results
Participant demographics and WAB-R scores are shown in Table 1. There were no significant differences in age, gender, level of education or handedness between patient and healthy control groups. Values for individual patients are shown in Supplementary Table 1.
Identification of critical left hemisphere areas for language
First, relationships between lesion location in the left hemisphere and language scores on the WAB-R were examined using SVR-LSM. Different, but partially overlapping lesion locations were associated with overall aphasia severity (WAB-R AQ) and each of the WAB-R subscores (Fig. 2A–E). In order to examine the contribution of grey matter volume in the spared brain areas to the WAB-R scores, we first needed to estimate the severity of deficits expected for each individual based on the lesion itself. To accomplish this, we used thresholded SVR-LSM maps for the WAB-R scores as volumes of interest and calculated the proportion of each volume damaged in each subject (termed PCAD). PCADs accounted for a large portion of the variance in all WAB-R scores (AQ: R2 = 0.62, P = 1.0 × 10−7; Naming/Word-Finding R2 = 0.53, P = 2.5 × 10−6; Auditory-Verbal Comprehension R2 = 0.69, P = 5.2 × 10−9; Repetition R2 = 0.62, P = 8.8 × 10−8; and Spontaneous Speech R2 = 0.62, P = 8.6 × 10−8). These strong relationships demonstrate the importance of accounting for the direct effects of the stroke itself on outcomes before considering whether undamaged portions of the brain exert additional influence on outcomes.
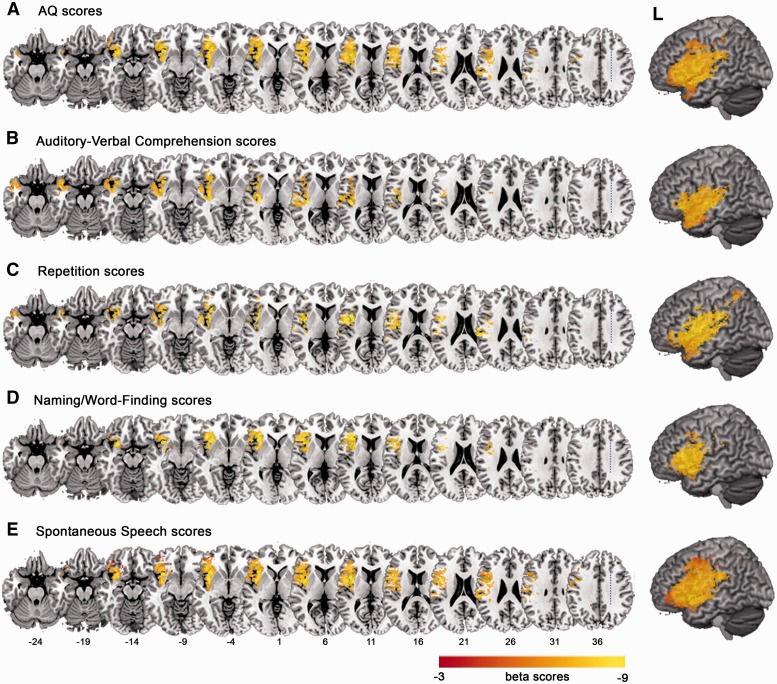
Figure 2.
SVR-LSM beta-maps showing significant voxels associated with WAB-R score. (A–E) WAB-R AQ, Auditory-Verbal Comprehension scores, Repetition scores, Naming/Word-Finding scores and Spontaneous Speech scores. Numbers denote MNI coordinates. Colour bars indicate beta scores. All voxels shown in the results survived threshold at P < 0.001. L = left.
Relationship between grey matter volume and aphasia outcome
To examine whether grey matter volume in areas not directly impacted by stroke influence language outcomes, we performed separate VBM analyses to identify areas of spared cortex related to overall aphasia severity and WAB-R subscores. Critically, to account for interindividual differences that might relate to aphasia outcomes or grey matter volume, we included a number of nuisance covariates in the VBM analyses: PCAD, lesion size, total volumes of grey matter and white matter, age, gender, education and handedness. The VBM analysis on overall aphasia severity (WAB-R AQ) demonstrated a positive relationship between grey matter volume and AQ in the right temporoparietal cortex including the supramarginal gyrus and posterior superior temporal gyrus, as well as in the left cerebellum lobules IV–VI (Fig. 3A and Table 2). For Repetition, a positive relationship between grey matter volume and performance was observed in the right temporoparietal cortex including the supramarginal gyrus, the posterior superior temporal gyrus and the posterior middle temporal gyrus (Fig. 4A and Table 2). For Naming/Word-Finding, grey matter volume in the right supramarginal gyrus and the posterior superior temporal gyrus was also positively related to performance (Fig. 5A and Table 2). For Spontaneous Speech, a small area of the right posterior superior temporal gyrus related positively to the scores, along with bilateral areas of the cerebellum in lobules IV and V (Fig. 6A and Table 2). No negative relationships between grey matter volume and performance were found, and no relationships between grey matter volume and Auditory–Verbal Comprehension were identified.
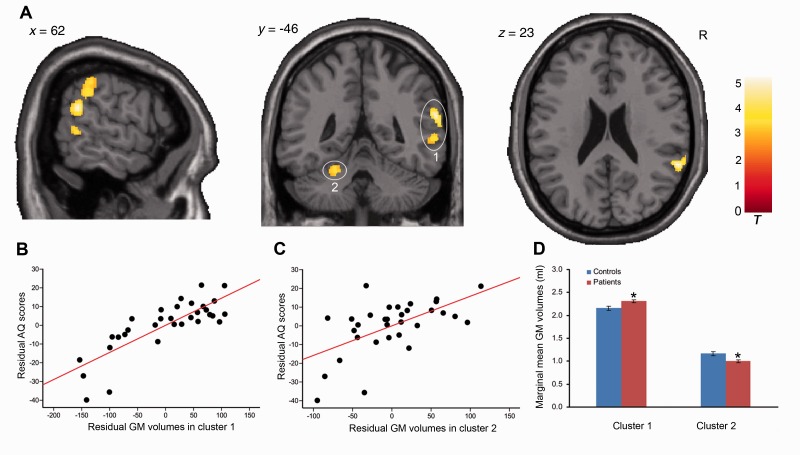
Figure 3.
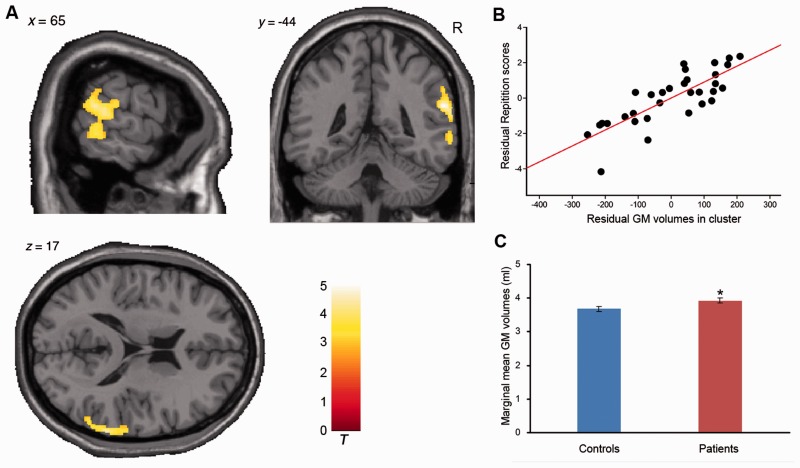
Grey matter structure related to WAB-R AQ. (A) Grey matter volume positively correlated with AQ in the right temporoparietal cortex (cluster 1: size = 930, peak voxel including the right supramarginal gyrus: x = 60, y = −37, z = 35, t = 4.21 and right superior temporal gyrus: x = 60, y = −45, z = 22, t = 5.18, AlphaSim corrected) and left cerebellum lobules IV–VI (cluster 2: size = 342, peak voxel: x = −22, y = −51, z = −27, t = 3.42, AlphaSim corrected), controlling for covariates. Circles and numbers indicate clusters. R = right. (B and C) Scatter plot showing partial regression using AQ as the dependent measure and grey matter extractions of cluster 1 or cluster 2 as the independent, controlling for covariates (cluster 1: P = 8.58 × 10−7; cluster 2: P = 0.002). (D) Difference of marginal mean grey matter extractions between patients and controls with age, gender, level of education and handedness as covariates of no interest. *P < 0.05, compared to controls. GM = grey matter.
Table 2.
Brain grey matter related to language performance
| Tests | Cluster size | t-values | Peak MNI coordinates in mm |
Brain region | Brodmann area | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Multiple regression, Aphasia quotient as predictor−grey matter analysis (controlling for nuisance variablesa) | |||||||
| 930 | 5.18 | 60 | −45 | 22 | Right superior temporal gyrus | BA40, BA22 | |
| 4.21 | 60 | −37 | 35 | Right supramarginal gyrus | BA40, BA42 | ||
| 342 | 3.42 | −22 | −51 | −27 | Left cerebellum lobules IV, V, VI | ||
| Multiple regression, Repetition as predictor–grey matter analysis (controlling for nuisance variablesa) | |||||||
| 1611 | 5.04 | 60 | −45 | 23 | Right superior temporal gyrus | BA40, BA42, BA22 | |
| 4.40 | 60 | −49 | 3 | Right middle temporal gyrus | BA21 | ||
| 3.48 | 66 | −25 | 18 | Right supramarginal gyrus | BA40, BA42 | ||
| Multiple regression, Naming/Word-Finding as predictor–grey matter analysis (controlling for nuisance variablesa) | |||||||
| 449 | 5.40 | 58 | −45 | 22 | Right superior temporal gyrus | BA40, BA42, BA22 | |
| 3.79 | 60 | −36 | 33 | Right supramarginal gyrus | BA40, BA42, BA21 | ||
| Multiple regression, Spontaneous Speech as predictor–grey matter analysis (controlling for nuisance variablesa) | |||||||
| 331 | 4.64 | 66 | −43 | 15 | Right superior temporal gyrus | BA22 | |
| 676 | 3.85 | −20 | −48 | −26 | Left Cerebellum lobules IV, V | ||
| 3.03 | 10 | −52 | −20 | Right Cerebellum lobules IV, V | |||
Clusters showing significant correlations with language performance. We present the size of the clusters (thresholded at P < 0.05, k = 323 voxels, AlphaSim corrected), MNI coordinates of peak voxel within each cluster, brain regions and the corresponding Brodmann area (BA). The x, y, z co−ordinates are according to the MNI atlas. Anatomical location is the peak within a cluster defined as the voxel with the highest t-score. aVariables of no interest include age, gender, level of education, handedness, total volume of grey matter and white matter, lesion size, proportion of critical area damaged (PCAD).
Figure 4.
Grey matter structure related to WAB-R Repetition. (A) Grey matter volume positively correlated with Repetition score in the right temporoparietal cortex (cluster size = 1611, peak voxel including the right superior temporal gyrus: x = 60, y = −45, z = 23, t = 5.04; right middle temporal gyrus: x = 60, y = −49, z = 3, t = 4.40; right supramarginal gyrus: x = 66, y = −25, z = 18, t = 3.48, AlphaSim corrected), controlling for covariates. R = right. (B) Scatter plot showing partial regression using the Repetition score as the dependent measure and grey matter extraction as the independent controlling for covariates (P = 1.20 × 10−6). (C) Difference of marginal mean grey matter extractions between patients and controls with age, gender, level of education and handedness as covariates of no interest. *P < 0.05, compared to controls. GM = grey matter.
Figure 5.
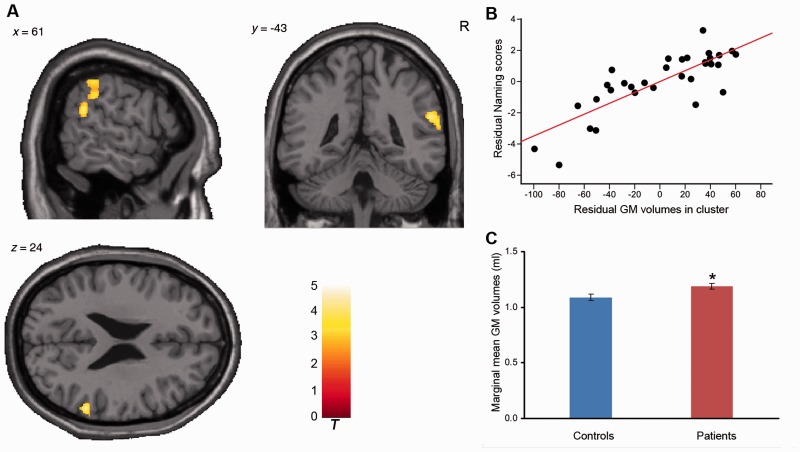
Grey matter structure related to WAB-R Naming/Word-Finding. (A) Grey matter volume positively correlated with Naming/Word-Finding score in the right temporoparietal cortex (cluster size = 449, peak voxel including right superior temporal gyrus: x = 58, y = −45, z = 22, t = 5.40 and right supramarginal gyrus: x = 60, y = −36, z = 33, t = 3.79, AlphaSim corrected), controlling for covariates. R = right. (B) Scatter plot showing partial regression using the Naming/Word-Finding score as the dependent measure and grey matter extraction as the independent, controlling for covariates (P = 1.24 × 10−6). (C) Difference of marginal mean grey matter extractions between patients and controls with age, gender, level of education and handedness as covariates of no interest. *P < 0.05, compared to controls. GM = grey matter.
Figure 6.
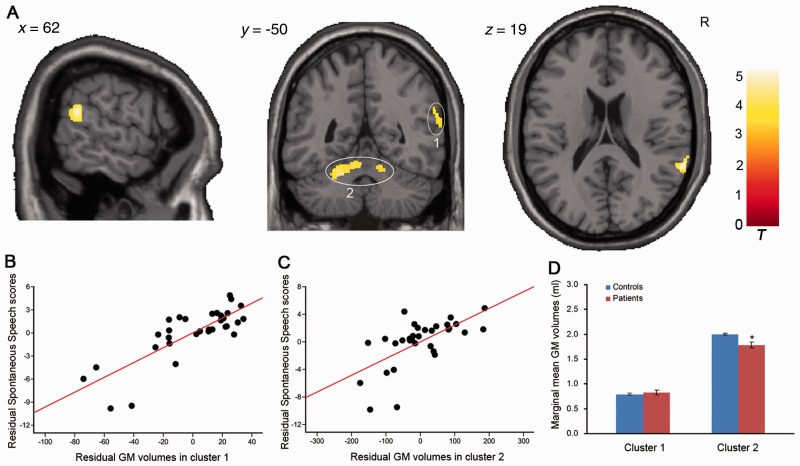
Grey matter structure related to WAB-R Spontaneous Speech. (A) Grey matter volume positively correlated with Spontaneous Speech score in the right superior temporal gyrus (cluster size = 331, peak voxel: x = 66, y = −43, z = 15, t = 4.64, AlphaSim corrected) and left/right cerebellum lobules (cluster size = 676, peak voxel including left cerebellum: x = −20, y = −48, z = −26, t = 3.85 and right cerebellum: x = 10, y = −52, z = −20, t = 3.03, AlphaSim corrected), controlling for covariates. Circles and numbers indicate clusters. R = right. (B and C) Scatter plot showing partial regression using Spontaneous Speech as the dependent measure and grey matter extractions of cluster 1 or cluster 2 as the independent, controlling for covariates (cluster 1: P = 1.04 × 10−6; cluster 2: P = 0.001). (D) Difference of marginal mean grey matter extractions between patients and controls with age, gender, level of education and handedness as covariates of no interest. *P < 0.05, compared to controls. GM = grey matter.
To clarify the relationships between aphasia outcomes, grey matter volume, and covariates observed in the VBM analysis, we next performed separate hierarchical regressions using the WAB-R scores as dependent measures. PCAD, lesion size, total volume of grey matter and white matter, age, gender, level of education and handedness were entered first, followed by grey matter volume. The hierarchical regression analyses showed that PCAD was the only significant predictor of WAB-R scores when excluding grey matter volume. When grey matter volume was added to the models, PCAD, grey matter volume, and age were significant predictors of all language scores. For Repetition only, gender was also a significant predictor. Age emerged as a significant predictor of the WAB-R scores not because of a direct effect on scores, as evidenced by a lack of relationship with scores when grey matter volume was excluded, but rather because grey matter volumes were inversely related to age as expected due to age-related atrophy (AQ right hemisphere cluster R = −0.537, P = 0.002; AQ cerebellum cluster R = −0.467, P = 0.007; Repetition cluster R = −0.452, P = 0.009; Naming/Word-Finding cluster R = −0.488, P = 0.005; Spontaneous Speech right hemisphere cluster R = −0.578, P = 0.001; Spontaneous Speech cerebellum cluster R = −0.426, P = 0.015). For WAB-R AQ and Spontaneous Speech, the grey matter volumes for both right hemisphere and cerebellar clusters were added into the model, and in both cases, only the right hemisphere grey matter volume was a significant independent predictor of aphasia outcome [WAB-R AQ right hemisphere cluster t(22) = 4.70, P = 0.0001, cerebellum cluster t(22) = 0.917, P = 0.37; Spontaneous Speech right hemisphere cluster t(22) = 4.44, P = 0.0002, cerebellum cluster t(22) = 1.47, P = 0.16]. Residual plots show relationships between grey matter volume in each of the clusters identified above and the WAB-R scores (Figs 3B–6B, 3C and 6C). Full regression results are provided in Supplementary Table 2. No significant correlation was found between the mean grey matter volumes of identified clusters and time from stroke across patients (all P > 0.10). This is expected, as all patients were at least six months post-stroke, and there were no relationships between time from stroke and aphasia scores in the group, controlling for covariates as above (all P > 0.10).
Relationship between grey matter volume and cognitive functions
The VBM analyses demonstrated that partially overlapping regions of right temporoparietal cortex relate to WAB-R AQ and the language production subscores. To further specify the possible cognitive/language functions underlying these results, we tested correlations between the grey matter volumes in these clusters and measures of executive function (Cognitive Linguistic Quick Test executive composite), verbal working memory (digit span forward and backward), spatial working memory (Corsi blocks forward and backward), speech praxis (Apraxia Battery for Adults-2, subtest 2A), and output phonology (pseudoword repetition), partialling out confounding factors (age, gender, level of education, lesion size, total volume of grey and white matter). The partial correlation analyses showed that the mean grey matter volumes of the right hemisphere clusters were associated with digit span forward (AQ right hemisphere cluster: R = 0.514, P = 0.007; Repetition cluster: R = 0.396, P = 0.045; Naming cluster R = 0.378, P = 0.057; Spontaneous Speech right hemisphere cluster: R = 0.528, P = 0.006). The Spontaneous Speech right hemisphere clusters were also associated with pseudoword repetition (R = 0.397, P = 0.045). Full partial correlation results are provided in Supplementary Table 3.
Comparison with right hemisphere grey matter volume in control subjects
Next to examine whether these relationships reflected neuroplasticity after stroke, we compared mean grey matter volumes from aphasia patients with healthy controls in the clusters identified in the VBM analyses. Age, gender, level of education, and handedness were included as confounding variables. The marginal mean grey matter volumes in patients were significantly higher than control subjects in the right hemisphere clusters related to AQ [F(1,56) = 5.58, P = 0.022] (Fig. 3D), Repetition [F(1,56) = 5.01, P = 0.029] (Fig. 4C), and Naming/Word-Finding [F(1,56) = 6.77, P = 0.012] (Fig. 5C). In contrast, marginal mean grey matter volumes within the cerebellar cluster related to AQ were significantly lower in patients than control subjects [F(1,56) = 13.77, P = 0.0005] (Fig. 3D). For Spontaneous Speech-related clusters, the marginal mean grey matter volumes in the cerebellar cluster was significantly lower in patients than controls [F(1,56) = 8.26, P = 0.006], but no significant difference was found in the right hemisphere cluster [F(1,56) = 1.74, P = 0.193] (Fig. 6D). To ensure that local grey matter volumes were not influenced by stroke-related differences in global right hemisphere tissue volume, we repeated the above analyses adding total right hemisphere grey and white matter volume as a confounding variable. The results for all right hemisphere clusters remained significant: AQ [F(1,55) = 4.86, P = 0.032], Repetition [F(1,55) = 4.31, P = .04] and Naming/Word-Finding [F(1,55) = 6.03, P = 0.017].
To further ensure that these between group effects did not reflect a general consequence of stroke regardless of aphasia, we identified 10 patients from our other research protocols with chronic left hemisphere stroke but no history of aphasia (Supplementary material). Controlling for age, gender, handedness and total volume of grey matter and white matter, the mean grey matter volumes in patients with aphasia were significantly greater than in the 10 non-aphasic patients in the right hemisphere clusters related to AQ [F(1,36) = 4.84, P = 0.034], Repetition [F(1,36) = 5.73, P = 0.022], and Naming/Word-Finding [F(1,36) = 4.95, P = 0.032]. For Spontaneous Speech, the difference was marginally significant [F(1,36) = 3.94, P = 0.055]. The marginal mean grey matter volumes within the cerebellar clusters were significantly lower in aphasic patients than the non-aphasic control patients [AQ cluster: F(1,36) = 6.41, P = 0.016; Spontaneous Speech cluster: F(1,36) = 4.47, P = 0.042].
Discussion
In the first study to examine relationships between right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke, we found that grey matter volumes in the right temporoparietal cortex were independently and positively associated with language production outcomes after controlling for key individual differences. In addition, grey matter volumes in the right temporoparietal cortex were greater in left hemisphere stroke survivors with aphasia than either healthy controls or left hemisphere stroke survivors with no history of aphasia. These findings suggest right hemisphere compensation for language deficits after stroke that is at least partly related to beneficial right hemisphere structural plasticity in chronic post-stroke aphasia.
Contrast with recent ideas about the right hemisphere role in aphasia
Our results conflict with some common notions about the role of the right hemisphere in aphasia recovery. Some have suggested that right hemisphere recruitment depends on the left hemisphere lesion size (Heiss et al., 2003; Heiss and Thiel, 2006), specifically that in chronic aphasia individuals with large lesions must rely on the right hemisphere more than those with small lesions, and that these individuals recover poorly because the right hemisphere is ineffective in compensating for language deficits (Anglade et al., 2014). However, we covaried for stroke size and found compensatory relationships in the right hemisphere across the group, indicating that right hemisphere compensation was independent of lesion size. Further, grey matter volumes of right temporoparietal cortex contributed significant independent variance to speech production outcomes, demonstrating that right hemisphere recruitment, at least in this context, did contribute positively to aphasia recovery.
One key factor in coming to this conclusion is that we did not examine direct relationships between outcomes and right hemisphere recruitment, which ignores the primary driver of aphasia severity, the stroke itself. Instead, we first estimated the likely language outcomes based on stroke size, PCAD and demographic factors and then asked whether right hemisphere recruitment relates to deviations from those predicted outcomes. By addressing the problem in this way, we have demonstrated that right hemisphere language homologues, at least in posterior language areas, do contribute positively to aphasia outcomes in the chronic phase of recovery.
This finding also conflicts with prior suggestions that right hemisphere compensation occurs early in recovery, but not in the chronic phase. One prominent longitudinal study of post-stroke aphasia reported increased right frontal activity correlated with language recovery in the subacute period, followed later by decreased activity and a corresponding shift back to the normal left dominance of language in the chronic period (Saur et al., 2006). Other studies have suggested that right hemisphere activation in the chronic stage could be associated with poor language recovery following stroke (Szaflarski et al., 2013). Here, we found structural evidence of right hemisphere compensation in the chronic period.
Differences with some previous results may relate to the difference in the metrics of right hemisphere compensation used. Whereas prior studies have primarily used task-related functional brain activity to examine language networks in aphasia (Belin et al., 1996; Heiss et al., 2003; Winhuisen et al., 2005; Richter et al., 2008; Allendorfer et al., 2012), we instead examined the relationship between grey matter structure and language. Despite its many merits, task-related functional brain activity is related to task difficulty (Just et al., 1996), which depends on aphasia severity (Fridriksson and Morrow, 2005). Further, functional imaging studies are only sensitive to the specific areas of the brain activated by the particular task used. Thus, examining brain structure rather than functional activity may reveal different effects. One recent study examined white matter integrity in chronic post-stroke aphasia using diffusion tensor imaging, and similarly found relationships between right hemisphere networks and language outcomes (Forkel et al., 2014). Here, we have provided parallel evidence for relationships between right hemisphere grey matter structure and language outcomes. The demonstration of larger grey matter volumes in stroke survivors with aphasia than both controls and stroke survivors with no history of aphasia extends these findings to suggest that right hemisphere compensation in aphasia occurs, at least in part, through structural plasticity. Because we used a cross-sectional design rather than a longitudinal study with one specific intervention, our results suggest that compensation by right hemisphere grey matter structures may serve as a general mechanism of aphasia recovery.
Mechanisms of right hemisphere compensation in aphasia
The microstructural basis of the right hemisphere grey matter hypertrophy in these results remains unclear. Potential mechanisms underlying grey matter plasticity include axonal sprouting, dendritic branching and synaptogenesis, neurogenesis as well as angiogenesis (Zhao et al., 2006). Indeed, all of these changes have been observed previously in animal models of stroke recovery (Kerr et al., 2011) and thus may be involved here.
Our findings of compensatory hypertrophy are also in line with structural plasticity studies showing focal changes in grey matter during learning in healthy adults (Maguire et al., 2000; Golestani et al., 2002; Gaser and Schlaug, 2003; Draganski et al., 2004, 2006; Taubert et al., 2010, 2012). Additionally, evidence from studies on second language learning in healthy adults shows that second language competence positively correlates with activations or grey matter volume in right hemisphere networks including the superior temporal gyrus and supramarginal gyrus (Jeong et al., 2010; Raboyeau et al., 2010; Van Ettinger-Veenstra et al., 2012; Hosoda et al., 2013). Studies in stroke have also shown longitudinal changes in grey matter volume (Dang et al., 2013; Fan et al., 2013), sometimes associated with therapy for motor deficits (Gauthier et al., 2008) or even listening to music (Sarkamo et al., 2014). In aphasia, constraint-induced language therapy and melodic intonation therapy have been shown to enhance integrity of the right arcuate fasciculus (Schlaug et al., 2009; Breier et al., 2010). Here we have demonstrated that similar changes may occur in right hemisphere grey matter morphology. Further, the changes observed here reflect a general mechanism of language recovery in chronic aphasia, and are not restricted to direct, potentially transient, treatment effects.
We observed a relationship between right temporoparietal grey matter volumes and speech production measures across patients with various lesion sizes and locations. One might expect that post-stroke plasticity would vary based on these individual differences, as well as differences in speech therapy and other behavioural experiences. Indeed, there may be considerable individual differences in patterns of recovery (Torres et al., 2013). For this reason, we performed our whole brain analyses to identify areas in which grey matter volumes related to aphasia outcomes. Thus, the focality of the results is related to the brain–behaviour relationship, not the spatial distribution of hypertrophy across patients. Pertinent to these results, we have previously demonstrated that right hemisphere language activation is consistently localized across functional imaging studies of aphasia using varied populations and methods. Moreover, right hemisphere functional activity is not limited to simple one-for-one compensation by individual nodes homotopic to the area of damage (Turkeltaub et al., 2011). As such, one would expect right hemisphere brain–behaviour relationships to be consistently localized across individuals despite individual differences, as we found here.
The driver for the hypertrophy in this area is likely recruitment of alternate right hemisphere processors capable of compensating for a specific language or cognitive process involved in speech production across various specific tasks. This compensation may be a consequence of experience, whether formal speech therapy, self-therapy with apps or at-home exercises, or simply the experience of struggling to communicate with aphasia. These effects are not likely driven by a specific speech therapy experience because the nature and quantity of speech therapy varies between individuals substantially. Rather, various experiences likely drive recruitment and hypertrophy of right hemisphere processors capable of supporting key cognitive/language functions involved in speech production after damage in the left hemisphere language network.
Cognitive and language compensation by the right hemisphere
We found that the grey matter volumes in right temporoparietal areas are related to speech production, but not comprehension. Furthermore, we identified a relationship between the grey matter volumes of identified right hemisphere regions and forward digit span, a measure of verbal working memory capacity, as well as pseudoword repetition, which relies on both verbal working memory and other phonological output processes. Importantly, we allowed patients to point to a number line when responding on the digit span to ensure that scores did not simply reflect motor speech deficits. Moreover, no relationships were observed between grey matter volumes and other cognitive faculties, including backwards digit span, which relies on executive control of working memory. These results suggest that the right hemisphere areas identified here contribute to aphasia outcomes not through broad domain general effects on cognition, but through effects on particular aspects of language production, including verbal working memory capacity and phonological output processing.
These right temporoparietal areas thus likely compensate for homologous left hemisphere areas, as the left temporoparietal cortex is commonly implicated in verbal working memory capacity and phonological output processes. Lesion–symptom mapping studies have implicated the left posterior superior temporal gyrus and the supramarginal gyrus in verbal working memory capacity (Leff et al., 2009), phonological retrieval (Pillay et al., 2014), and phonemic paraphasias (Schwartz et al., 2012), possibly through a role in phonological short term storage (Baldo and Dronkers, 2006). Similarly, neuroimaging studies have implicated the left posterior superior temporal gyrus in phonological access (Graves et al., 2007) and verbal working memory capacity (Richardson et al., 2011). The left supramarginal gyrus is commonly activated during phonological decision-making in healthy subjects (Devlin et al., 2003; Buchsbaum and D'Esposito, 2009). Recent transcranial magnetic stimulation studies have found that disruption of the right supramarginal gyrus interferes with performance of phonological but not semantic decision tasks (Hartwigsen et al., 2010), and impairs single word production in healthy adults (Sollmann et al., 2014). These findings suggest that right temporoparietal areas may be good candidates to compensate for damage in the left hemisphere language network after brain injury. These areas may undergo hypertrophy after stroke due to increased reliance on either learned compensatory strategies or due to the extra verbal working memory load associated with communicating with aphasia. Additional studies, ideally using inhibitory transcranial magnetic stimulation in people with aphasia, will help to confirm that the right temporoparietal cortex compensates for speech production deficits by contributing to verbal working memory capacity and phonological output processes.
Alternate interpretations
It should be noted that our evidence for plasticity in the right hemisphere is based on cross-sectional intergroup comparisons. Controlling confounding factors as much as possible, we still found robust effects suggesting structural hypertrophy. Even stronger evidence of structural hypertrophy in these areas will come from future longitudinal studies. If not related to plasticity, the relationship between right temporoparietal cortex and language outcomes likely reflects premorbid interindividual differences in these areas that protect from language deficits after left hemisphere stroke. Indeed, the degree of left lateralization of language predicts the severity of language deficits induced by transient lesions using transcranial magnetic stimulation (Knecht et al., 2002). Here, relative bilaterality of language might be reflected in greater grey matter volumes in the right hemisphere, which makes an individual’s language system more resilient in the face of left hemisphere stroke. Alternatively, greater grey matter volumes in these areas may reflect relatively greater premorbid ability in paralinguistic or attentional processes involving the right temporoparietal cortex (Geranmayeh et al., 2014). Perhaps individuals with greater premorbid attention obtain more benefits from speech–language therapy, which could facilitate a better recovery and concomitant expansion of verbal working memory capacity through other neuroplastic mechanisms not measured here. Although additional longitudinal data will strengthen the evidence for post-stroke plasticity presented here, the current results still demonstrate for the first time a positive association between focal right hemisphere grey matter structure and language outcomes after left hemisphere stroke.
Relationships with grey matter in other undamaged areas of the brain
The VBM analyses also demonstrated a relationship between grey matter volumes in the cerebellum and language outcomes, specifically Spontaneous Speech and as a consequence, overall aphasia severity. The location of the cerebellar clusters is close to areas previously implicated in speech output processes in neuroimaging studies (Callan et al., 2007). If the atrophied areas of the cerebellum played a role in language prior to the stroke, this could cause additional deficits beyond those caused by the direct damage of the stroke. Additional studies using diffusion tensor imaging tractography would help to confirm the cause of atrophy in these cerebellar areas.
Notably, we observed no relationships between grey matter volumes and language outcomes in the left hemisphere. This could be attributed to variability in stroke distributions, which could result in different patterns of peri-lesional compensation between stroke survivors. However, previous studies of aphasia have shown group-level functional activity in the left hemisphere (Fridriksson, 2010; Turkeltaub et al., 2011), suggesting that specific alternate brain areas in the left hemisphere are engaged after stroke, and that ‘peri-lesional’ left hemisphere recruitment needn’t involve the tissue immediately adjacent to the lesion. Perhaps distortions in spatial normalization near the lesion or atrophy in some cortical layers due to severed axonal connections with the lesion (Rowan et al., 2007) interfered with the ability to identify compensatory alterations of other neuronal circuits in these areas.
Conclusion
The current findings provide the first evidence that grey matter structure in right hemisphere language area homologues is associated with language recovery in chronic post-stroke aphasia, and suggests that post-stroke hypertrophy in these areas accounts, at least in part, for these effects. This implies that structural plasticity in the right temporoparietal cortex may serve as a general compensatory mechanism for speech production regardless of sources of interindividual variability in post-stroke aphasia. These findings may provide novel targets for enhancement using non-invasive brain stimulation techniques in chronic post-stroke aphasia.
Supplementary Material
Acknowledgements
We thank Katherine Spiegel, Mackenzie Fama, Rachael Harrington, Alexa Desko, Lauren Taylor, Laura Hussey, Jessica Friedman, and Molly Stamp for contributing to data collection, and our participants for their involvement in the study.
Glossary
Abbreviations
- AQ
aphasia quotient
- PCAD
proportion of critical area damaged
- SVR-LSM
support vector regression-based lesion–symptom mapping
- VBM
voxel-based morphometry
- WAB-R
Western Aphasia Battery-Revised
Funding
This study was supported by the National Center for Advancing Translational Sciences via the Georgetown-Howard Universities Center for Clinical and Translational Science (KL2TR000102), Doris Duke Charitable Foundation (2012062), Vernon Family Trust, National Basic Research Program of China (2011CB707804); National Natural Science Foundation of China (81000500, 81371277), Joint Funds of the Natural Science Foundation of China (U1032005), Project of Science and Technology New Star of Pearl River (2012J2200089) , Chinese Government Scholarship Program, and Alzheimer’s Drug Discovery Foundation (20130805).
Supplementary material
Supplementary material is available at Brain online.
References
- Allendorfer JB, Kissela BM, Holland SK, Szaflarski JP. Different patterns of language activation in post-stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Med Sci Monit 2012; 18: CR135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade C, Thiel A, Ansaldo AI. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Inj 2014; 28: 138–45. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage 2000; 11 (6 Pt 1): 805–21. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26: 839–51. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology 2006; 20: 529–38. [DOI] [PubMed] [Google Scholar]
- Barlow T. On a case of double hemiplegia, with cerebral symmetrical lesions. BMJ 1877; 2: 103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, OS JD, et al. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol 2011; 18: 935–43. [DOI] [PubMed] [Google Scholar]
- Basso A, Gardelli M, Grassi MP, Mariotti M. The role of the right hemisphere in recovery from aphasia. Two case studies. Cortex 1989; 25: 555–66. [DOI] [PubMed] [Google Scholar]
- Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S, et al. Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology 1996; 47: 1504–11. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Pulvermuller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol 2011; 7: 86–97. [DOI] [PubMed] [Google Scholar]
- Breier JI, Juranek J, Maher LM, Schmadeke S, Men D, Papanicolaou AC. Behavioral and neurophysiologic response to therapy for chronic aphasia. Arch Phys Med Rehab 2009; 90: 2026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Randle S, Maher LM, Papanicolaou AC. Changes in maps of language activity activation following melodic intonation therapy using magnetoencephalography: two case studies. J Clin Exp Neuropsyc 2010; 32: 309–14. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. Repetition suppression and reactivation in auditory-verbal short-term recognition memory. Cereb Cortex 2009; 19: 1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan DE, Kawato M, Parsons L, Turner R. Speech and song: the role of the cerebellum. Cerebellum 2007; 6: 321–7. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KM. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke 1999; 30: 2331–40. [DOI] [PubMed] [Google Scholar]
- Corsi PM. Human memory and the medial temporal region of the brain. Diss Abstr Int 1972; 34: 819B. [Google Scholar]
- Dabul B. Apraxia battery for adults-2. Austin: Pro-Ed; 2000. [Google Scholar]
- Dang C, Liu G, Xing S, Xie C, Peng K, Li C, et al. Longitudinal cortical volume changes correlate with motor recovery in patients after acute local subcortical infarction. Stroke 2013; 44: 2795–801. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci 2003; 15: 71–84. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature 2004; 427: 311–2. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci 2006; 26: 6314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Zhu C, Chen H, Qin W, Ji X, Wang L, et al. Dynamic brain structural changes after left hemisphere subcortical stroke. Hum Brain Mapp 2013; 34: 1872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez B, Cardebat D, Demonet JF, Joseph PA, Mazaux JM, Barat M, et al. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke 2004; 35: 2171–6. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Dell'Acqua F, Kalra L, Murphy DG, Williams SC, et al. Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain 2014; 137 (Pt 7): 2027–39. [DOI] [PubMed] [Google Scholar]
- Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci 2010; 30: 11558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Morrow L. Cortical activation and language task difficulty in aphasia. Aphasiology 2005; 19: 239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage 2012; 60: 854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci 2003; 23: 9240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke 2008; 39: 1520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranmayeh F, Brownsett SL, Wise RJ. Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain 2014; 137 (Pt 10): 2632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialanella B, Bertolinelli M, Lissi M, Prometti P. Predicting outcome after stroke: the role of aphasia. Disabil Rehabil 2011; 33: 122–9. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci 2008; 28: 990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron 2002; 35: 997–1010. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez M, Christian AB, Davis C, Hillis AE. Role of aphasia in discharge location after stroke. Arch Phys Med Rehabil 2013; 94: 851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol 2010; 9: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gordon JK. A neural signature of phonological access: distinguishing the effects of word frequency from familiarity and length in overt picture naming. J Cogn Neurosci 2007; 19: 617–31. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000; 406: 147–50. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang 2011; 118: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR. Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci USA 2010; 107: 16494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 1999; 45: 430–8. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang 2006; 98: 118–23. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Kessler J, Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage 2003; 20 (Suppl 1): S42–9. [DOI] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Duffau H. Rethinking voxel-wise lesion-deficit analysis: a new challenge for computational neuropsychology. Cortex 2015; 64: 413–6. [DOI] [PubMed] [Google Scholar]
- Hosoda C, Tanaka K, Nariai T, Honda M, Hanakawa T. Dynamic neural network reorganization associated with second language vocabulary acquisition: a multimodal imaging study. J Neurosci 2013; 33: 13663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Sugiura M, Sassa Y, Wakusawa K, Horie K, Sato S, et al. Learning second language vocabulary: neural dissociation of situation-based learning and text-based learning. Neuroimage 2010; 50: 802–9. [DOI] [PubMed] [Google Scholar]
- Johnson JP, Sommers RK, Weidner WE. Dichotic ear preference in aphasia. J Speech Hear Res 1977; 20: 116–29. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science 1996; 274: 114–6. [DOI] [PubMed] [Google Scholar]
- Karbe H, Kessler J, Herholz K, Fink GR, Heiss WD. Long-term prognosis of poststroke aphasia studied with positron emission tomography. Arch Neurol 1995; 52: 186–90. [DOI] [PubMed] [Google Scholar]
- Kerr AL, Cheng SY, Jones TA. Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord 2011; 44: 538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Sheppard A, MacKenzie R. Localization in transcortical sensory aphasia. Arch Neurol 1982; 39: 475–8. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The minor cerebral hemisphere as a source of aphasic speech. Arch Neurol 1971; 25: 302–6. [DOI] [PubMed] [Google Scholar]
- Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci 2002; 5: 695–9. [DOI] [PubMed] [Google Scholar]
- Kurland J, Cortes CR, Wilke M, Sperling AJ, Lott SN, Tagamets MA, et al. Neural mechanisms underlying learning following semantic mediation treatment in a case of phonologic alexia. Brain Imaging Behav 2008; 2: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 2009; 132: 3401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 2000; 97: 4398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain 2014; 137 (Pt 9): 2522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Treglia E, Kaplan E, Baker EH, et al. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep 2009; 9: 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Breitenstein C. Functional imaging studies of treatment-induced recovery in chronic aphasia. Aphasiology 2008; 22: 1251–68. [Google Scholar]
- Moore BD, III, Papanicolaou AC. Dichotic-listening evidence of right-hemisphere involvement in recovery from aphasia following stroke. J Clin Exp Neuropsychol 1988; 10: 380–6. [DOI] [PubMed] [Google Scholar]
- Moore WH, Jr, Weidner WE. Bilateral tachistoscopic word perception in aphasic and normal subjects. Percept Mot Skills 1974; 39: 1003–11. [DOI] [PubMed] [Google Scholar]
- Moore WH, Jr, Weidner WE. Dichotic word-perception of aphasic and normal subjects. Percept Mot Skills 1975; 40: 379–86. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang 2005; 93: 95–105. [DOI] [PubMed] [Google Scholar]
- Pillay SB, Stengel BC, Humphries C, Book DS, Binder JR. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann Neurol 2014; 76: 738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, et al. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. J Cogn Neurosci 2010; 22: 1299–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboyeau G, Marcotte K, Adrover-Roig D, Ansaldo AI. Brain activation and lexical learning: the impact of learning phase and word type. Neuroimage 2010; 49: 2850–61. [DOI] [PubMed] [Google Scholar]
- Richardson FM, Ramsden S, Ellis C, Burnett S, Megnin O, Catmur C, et al. Auditory short-term memory capacity correlates with gray matter density in the left posterior STS in cognitively normal and dyslexic adults. J Cogn Neurosci 2011; 23: 3746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain 2008; 131 (Pt 5): 1391–401. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000; 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Rowan A, Vargha-Khadem F, Calamante F, Tournier JD, Kirkham FJ, Chong WK, et al. Cortical abnormalities and language function in young patients with basal ganglia stroke. Neuroimage 2007; 36: 431–40. [DOI] [PubMed] [Google Scholar]
- Sarkamo T, Ripolles P, Vepsalainen H, Autti T, Silvennoinen HM, Salli E, et al. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: a voxel-based morphometry study. Front Hum Neurosci 2014; 8: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain 2006; 129: 1371–84. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca's aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci 2009; 1169: 385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Marchina S, Zipse L, Wan CY. From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurol 2010; 5: 657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, et al. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage 2010; 50: 1392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain 2012; 135 (Pt 12): 3799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollmann N, Tanigawa N, Ringel F, Zimmer C, Meyer B, Krieg SM. Language and its right-hemispheric distribution in healthy brains: an investigation by repetitive transcranial magnetic stimulation. Neuroimage 2014; 102 (Pt 2): 776–88. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Allendorfer JB, Banks C, Vannest J, Holland SK. Recovered vs. not-recovered from post-stroke aphasia: the contributions from the dominant and non-dominant hemispheres. Restor Neurol Neurosci 2013; 31: 347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 2010; 30: 11670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Villringer A, Ragert P. Learning-related gray and white matter changes in humans: an update. Neuroscientist 2012; 18: 320–5. [DOI] [PubMed] [Google Scholar]
- Torres J, Drebing D, Hamilton R. TMS and tDCS in post-stroke aphasia: integrating novel treatment approaches with mechanisms of plasticity. Restor Neurol Neurosci 2013; 31: 501–15. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, et al. The right hemisphere is not unitary in its role in aphasia recovery. Cortex 2012; 48: 1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, Hamilton RH. Are networks for residual language function and recovery consistent across aphasic patients? Neurology 2011; 76: 1726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ettinger-Veenstra H, Ragnehed M, McAllister A, Lundberg P, Engstrom M. Right-hemispheric cortical contributions to language ability in healthy adults. Brain Lang 2012; 120: 395–400. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the wechsler memory scale-revised. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 2005; 36: 1759–63. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. The right inferior frontal gyrus and poststroke aphasia - A follow-up investigation. Stroke 2007; 38: 1286–92. [DOI] [PubMed] [Google Scholar]
- Yarnell P, Monroe P, Sobel L. Aphasia outcome in stroke: a clinical neuroradiological correlation. Stroke 1976; 7: 516–22. [DOI] [PubMed] [Google Scholar]
- Zaghi S, Acar M, Hultgren B, Boggio PS, Fregni F. Noninvasive brain stimulation with low-intensity electrical currents: putative mechanisms of action for direct and alternating current stimulation. Neuroscientist 2010; 16: 285–307. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, Wang Z. Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp 2014; 35: 5861–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 2006; 26: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.