The presence of β-amyloid in the brain may represent a preclinical stage of Alzheimer’s disease, but this is controversial. Jagust examines the association between β-amyloid and biomarkers of Alzheimer’s disease in cognitively normal older people, concluding that β-amyloid is harmful to the brain in a manner consistent with Alzheimer’s disease.
Keywords: ageing, Alzheimer’s disease, amyloid, neurodegeneration, biomarkers
The presence of β-amyloid in the brain may represent a preclinical stage of Alzheimer’s disease, but this is controversial. Jagust examines the association between β-amyloid and biomarkers of Alzheimer’s disease in cognitively normal older people, concluding that β-amyloid is harmful to the brain in a manner consistent with Alzheimer’s disease.
Abstract
Although the amyloid-β protein associated with the Alzheimer’s disease plaque has been detectable in living people for over a decade, its importance in the pathogenesis of Alzheimer’s disease is still debated. The frequent presence of amyloid-β in the brains of cognitively healthy older people has been interpreted as evidence against a causative role. If amyloid-β is crucial to the development of Alzheimer’s disease, it should be associated with other Alzheimer’s disease-like neurological changes. This review examines whether amyloid-β is associated with other biomarkers indicative of early Alzheimer’s disease in normal older people. The preponderance of evidence links amyloid-β to functional change, progressive brain atrophy, and cognitive decline. Individuals at greatest risk of decline seem to be those with evidence of both amyloid-β and findings suggestive of neurodegeneration. The crucial question is thus how amyloid-β is related to brain degeneration and how these two processes interact to cause cognitive decline and dementia.
Introduction
The amyloid hypothesis of Alzheimer’s disease has been the dominant theory of disease causation for decades ( Selkoe, 1991 ; Hardy and Selkoe, 2002 ). This theory essentially holds that accumulation of the amyloid-β protein, the key constituent of the Alzheimer’s disease plaque, is sufficient to cause a series of downstream events resulting in synaptic dysfunction, inflammation, neuronal death and eventually dementia. More recently, increasing attention has been drawn to the possibility that amyloid-β exerts deleterious effects on the brain through its interactions with the tau protein ( Roberson et al. , 2007 ; Stancu et al. , 2014 ), the constituent of the neurofibrillary tangle that is more closely related to cognitive outcomes than is the amyloid-β plaque ( Nelson et al. , 2012 ). Considerable evidence for the amyloid hypothesis arises from Mendelian inherited gene mutations that have enabled both animal models and studies of presymptomatic humans. Clinical research has benefitted considerably from the availability of biomarkers for amyloid-β in humans. These data show strong evidence of brain amyloid-β deposition long before neurological changes and cognitive decline occur in people who carry autosomal dominant Alzheimer’s disease-causing mutations ( Bateman et al. , 2012 ; Fleisher et al. , 2012 ).
A number of technical factors are important in interpreting biomarker data. Amyloid PET biomarkers have been shown to correlate well with amyloid load at autopsy ( Clark et al. , 2012 a ; Curtis et al. , 2015 ; Murray et al. , 2015 ; Sabri et al. , 2015 ) and correlate inversely with CSF measures of amyloid-β 1–42 ( Landau et al. , 2013 ) although the information provided with both techniques may not be identical ( Mattsson et al. , 2015 b ). For example, PET imaging detects aggregated, fibrillar forms of the amyloid-β protein, while abundant evidence from animal models suggests that soluble forms are most likely to be deleterious ( Walsh et al. , 2002 ). While PET and CSF measures of amyloid-β occur on a continuum, it is often useful to classify individuals as ‘amyloid positive’ or ‘amyloid negative’ based on a threshold. Selection of a threshold represents a choice between sensitivity (use of a lower value to define positivity) and specificity (use of a higher value to define positivity) and thus has important effects upon how individuals are classified or misclassified ( Villeneuve et al. , 2015 ). Biomarkers are also available to define downstream effects of neurodegeneration such as brain atrophy (measured with MRI), altered neural function (measured with PET and glucose metabolism or functional MRI) and impaired cognition. These neurodegeneration biomarkers are not specific for underlying pathological processes; nevertheless human data using such biomarkers have been integrated into a model in which amyloid-β is an initiating event, followed by neurodegeneration, and lastly cognitive decline ( Jack et al. , 2010 , 2013 ).
Biomarker studies have also generated major critiques of the amyloid hypothesis. For example, therapeutic trials of amyloid-β-directed immunotherapies have used PET scanning to demonstrate reductions in brain amyloid-β without clinical improvement in Alzheimer’s disease patients ( Salloway et al. , 2014 ). Another salient critique is that both neuropathological and PET data show evidence of extensive amyloid-β pathology in cognitively normal older people ( Bennett et al. , 2006 ; Morris et al. , 2010 ). This evidence of amyloid-β deposition without cognitive dysfunction, and amyloid-β reduction without cognitive improvement raises substantial questions about the validity of amyloid-β as a causative agent ( Herrup, 2015 ). In part to accommodate this evidence, a framework for the staging of preclinical Alzheimer’s disease has been developed in which the deposition of amyloid-β alone is prima facie evidence of preclinical Alzheimer’s disease in its earliest, or first stage ( Sperling et al. , 2011 ) ( Fig. 1 A). Subsequent neurodegeneration (changes in brain structure and function) marks the second stage in preclinical Alzheimer’s disease, leading to subtle, asymptomatic cognitive decline, which reflects a third stage. Therapy directed at amyloid-β, it is argued, must therefore be initiated at the earliest possible stage in order to be effective. Amyloid-β immunotherapeutic clinical trials in older people with brain evidence of amyloid-β but no cognitive symptoms are underway based on this reasoning ( Sperling et al. , 2014 ).
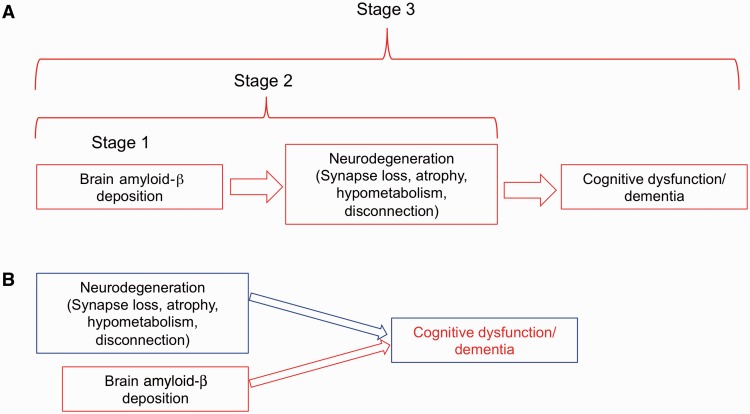
Figure 1.
Two different conceptualizations of how amyloid-β affects neurological outcomes. A summarizes a preclinical staging scheme for Alzheimer’s disease in which amyloid-β deposition is the initiating event. In this scheme, brain amyloid-β deposition alone constitutes Stage 1 of preclinical Alzheimer’s disease, followed by Stage 2 in which amyloid-β leads to neurodegeneration. In Stage 3, subtle cognitive dysfunction insufficient to establish dementia or mild cognitive impairment, occurs. This scheme posits neurodegeneration as the invariable mediator between amyloid-β and cognition. B suggests an alternative view in which neurodegeneration and amyloid-β are independent processes. Neurodegeneration without amyloid-β has been referred to as suspected non-Alzheimer pathology (SNAP). In this scheme, either neurodegeneration or amyloid-β alone may lead to cognitive dysfunction, although the two together may produce synergistic harmful effects.
Similarly, another test of the amyloid hypothesis is whether there is convincing evidence that amyloid-β affects brain structure, function, and cognition in normal older people well before the onset of clinically significant cognitive impairment or dementia. While there are extensive data concerning relationships between amyloid-β and such downstream effects in patients with Alzheimer’s disease and mild cognitive impairment (MCI) and in those with autosomal dominantly inherited forms of Alzheimer’s disease, this review is limited to studies of cognitively normal older people, with the expectation that this might represent the earliest stage of Alzheimer’s disease and the appropriate window in which to detect the earliest harmful effects of amyloid-β. It seems reasonable to expect that if amyloid-β is the initiating event in the Alzheimer pathological cascade, and if normal older people with brain amyloid-β have preclinical Alzheimer’s disease, then harmful effects should be detectable as Alzheimer’s disease-like changes in these individuals.
Is amyloid-β associated with brain atrophy in normal older people?
Cross-sectional studies have defined a regional predilection of brain atrophy in Alzheimer’s disease that, while not entirely specific, tends to follow a typical pattern. This includes volume loss of the hippocampus ( Seab et al. , 1988 ; Jack et al. , 1992 ) and a pattern of atrophy involving medial and lateral parietal cortex and temporal neocortex that has been characterized as an ‘Alzheimer’s disease signature’ ( Dickerson et al. , 2009 ). Brain atrophy suggestive of Alzheimer’s disease has been reported in a number of cross-sectional studies of normal older people with brain amyloid-β ( Storandt et al. , 2009 ; Bourgeat et al. , 2010 ; Becker et al. , 2011 ; Dore et al. , 2013 ). CSF measures of amyloid-β 1–42 have also been associated with whole brain atrophy ( Fagan et al. , 2009 ). However, not all studies have found such relationships in cross-sectional data. In the Alzheimer’s Disease Neuroimaging Initiative (ADNI), no relationship was detected between brain amyloid-β and regional brain atrophy in 280 normal older people, 30% of whom had evidence of brain amyloid deposition ( Mattsson et al. , 2015 a ) although evidence for such associations were seen in those with MCI. Other studies have also failed to confirm cross-sectional relationships ( Wirth et al. , 2013 a ), including some studies that reported greater atrophy progression in people with brain amyloid-β ( Schott et al. , 2010 ; Andrews et al. , 2013 ). Thus, reports of associations between amyloid-β and cross-sectional measures of brain atrophy are not consistent and may also not necessarily reflect a typical Alzheimer’s disease pattern ( Whitwell et al. , 2013 ).
Longitudinal data have considerable advantages over cross-sectional data in progressive disorders. Many studies have shown accelerated brain atrophy rates in normal older people with evidence of brain amyloid-β by PET or CSF measurement ( Schott et al. , 2010 ; Chetelat et al. , 2012 ; Ewers et al. , 2012 ; Andrews et al. , 2013 ). In one study, investigators found a relationship between the rate of accumulation of brain amyloid-β over time and the rate of hippocampal and cortical atrophy ( Villemagne et al. , 2013 ). However, longitudinal results are also neither straightforward nor consistent and different methods for data analysis may produce disparate results even in subjects from the same cohort ( Driscoll et al. , 2011 ; Clark et al. , 2012 b ). More recent studies have begun to probe subtle differences in older subjects with brain amyloid-β by classifying them according to the suggested preclinical stages of Alzheimer’s disease ( Sperling et al. , 2011 ) ( Fig. 1 A). When normal older people are categorized using this approach, an unforeseen but substantial proportion (∼25%) of normal individuals show evidence of neurodegeneration without amyloid-β. These cases are often referred to as SNAP as they reflect suspected non-Alzheimer pathology ( Jack et al. , 2012 ). SNAP is a complex and controversial entity as it is entirely biomarker-driven and without a clear pathological substrate; nevertheless its existence implies that amyloid-β and neurodegeneration can be independent processes ( Fig. 1 B). While the data are limited, some reports indicate that individuals with amyloid-β alone do not show progressive atrophy, while those with amyloid-β and evidence of neurodegeneration do ( Desikan et al. , 2011 ; Knopman et al. , 2013 ). These same studies indicate that neurodegeneration without amyloid-β (SNAP) may not be progressive. These data in the aggregate suggest a relationship between brain amyloid-β and atrophy that is subtle and complex. Detection of this relationship is somewhat dependent on methods, but appears to be most robust in longitudinal data. In addition, the evidence that amyloid-β is associated with progressive atrophy may be strongest when amyloid-β is associated with other biomarkers of brain injury. This means, of course, that the best predictor of brain injury progression is not necessarily amyloid-β alone, but amyloid-β and brain injury.
Is amyloid-β associated with altered neural function in normal older people?
Glucose metabolism
Patients with Alzheimer’s disease have long been known to demonstrate a characteristic pattern of hypometabolism seen on FDG-PET imaging that involves areas in temporal and parietal cortex including the posterior cingulate cortex and precuneus ( Minoshima et al. , 1997 ). While this hypometabolic pattern is not entirely specific for Alzheimer’s disease, it is strongly associated and has been used in much the same way as the Alzheimer’s disease signature pattern of atrophy on MRI with which it shares considerable topography. Appearance of this typical pattern in older people with brain amyloid-β deposition would therefore constitute reasonable evidence of a harmful, Alzheimer’s disease-like effect on brain function.
The data indicating such an effect are limited. Some studies have suggested, in fact, that increases in brain glucose metabolism occur in older amyloid-positive cognitively normal people ( Johnson et al. , 2014 ; Oh et al. , 2014 ), which has been taken as possible evidence of brain compensation. The problem of detecting effects of amyloid-β on glucose metabolism is compounded by the fact that the apolipoprotein E4 allele, the major risk genetic risk for Alzheimer’s disease, is associated with both glucose hypometabolism and amyloid-β accumulation ( Reiman et al. , 2004 ; Morris et al. , 2010 ). While it is unclear whether glucose hypometabolism occurs as a consequence of APOE4 or amyloid-β ( Jagust et al. , 2012 ; Knopman et al. , 2014 ), one large study showed that amyloid deposition in normal ageing was associated with hypometabolism even after accounting for APOE genotype ( Lowe et al. , 2014 ). This study enrolled over 600 normal people, and found a correlation of −0.2 between glucose metabolism in Alzheimer’s disease-typical regions and retention of the PET amyloid-β tracer. These results do suggest that amyloid-β has a small deleterious effect on glucose metabolism in ageing.
Resting state functional connectivity
Another method of studying brain function uses blood oxygen-dependent imaging with functional MRI in a task-free or resting state. These studies make use of the fact that spontaneous synchrony in the functional MRI signal occurs between topographically dispersed brain regions, indicating that these regions co-activate and function together in large scale networks ( Biswal et al. , 1995 ). One such network is the default mode network (DMN), so named because it is deactivated during most externally-directed cognitive tasks ( Raichle et al. , 2001 ) appearing to reflect an internally directed default mode of brain function. This network is of considerable interest because it becomes disconnected in patients with Alzheimer’s disease ( Greicius et al. , 2004 ) and shares considerable topographic overlap with the distribution of brain amyloid-β ( Buckner et al. , 2005 ). As Alzheimer’s disease progresses, other brain networks also show functional disruption ( Brier et al. , 2012 ).
Amyloid-β deposition has consistently been linked to alterations in DMN function in cross-sectional data. These studies have shown evidence of both decreases and increases in brain network connectivity in different parts of the DMN ( Hedden et al. , 2009 ; Sheline et al. , 2010 b ; Mormino et al. , 2011 ) in relation to amyloid-β. It is also increasingly obvious that connectivity in multiple resting state networks, in addition to the DMN, is modified in the presence of amyloid-β deposition in normal older people ( Brier et al. , 2014 ; Lim et al. , 2014 a ). In fact, the pattern of connectivity changes transcends specific networks and appears to align with cortical hubs, or brain regions that interconnect different neural regions and systems ( Buckner et al. , 2009 ). Both the pattern of cortical hubs and the pattern of regional network connectivity that is affected by amyloid-β reflect brain regions that are hypometabolic in patients with Alzheimer’s disease ( Elman et al. , 2014 a ). This finding is congruent with other evidence that brain metabolism is related to measures of connectivity ( Tomasi et al. , 2013 ; Riedl et al. , 2014 ).
While results appear to be generally consistent, a few caveats must be kept in mind. First, the approach to resting state functional MRI data analysis is far from standardized, and repeated and longitudinal measurements remain problematic. Second, the APOE4 genotype appears to affect functional brain connectivity ( Machulda et al. , 2011 ) even in the absence of detectable amyloid-β ( Sheline et al. , 2010 a ), so that these effects probably need to be examined separately. Nevertheless, considerable existing data suggest a relationship between brain amyloid and alterations in network connectivity in normal older people.
Is amyloid-β associated with cognitive dysfunction in normal older people?
While measurement of biomarkers that reflect neurodegeneration provide important clues about the effects of amyloid-β on the brain, cognitive function is the crucial variable linking misfolded proteins to dementia. Initial cross-sectional studies investigating associations between cognition and amyloid-β in cognitively normal older people were conflicting. Because such studies define the sample a priori as cognitively normal, any evidence of cognitive dysfunction must be subtle and difficult to detect. Perhaps unsurprisingly while a number of studies have found evidence of cognitive deficits in association with amyloid-β ( Chetelat et al. , 2011 ; Rentz et al. , 2011 ; Rodrigue et al. , 2012 ) some have failed to find this association ( Aizenstein et al. , 2008 ). A meta-analysis examining data from over 3000 subjects supports a relationship, particularly between amyloid-β and episodic memory, although the strength of this relationship was very small, an unsurprising finding that likely accounts for disagreement in the literature ( Hedden et al. , 2013 ).
Longitudinal studies, however, have repeatedly shown cognitive decline in people with brain amyloid-β. Initial studies examining how the presence or absence of brain amyloid-β predicted subsequent decline showed that older people with amyloid-β experienced more decline than those without, regardless of whether amyloid-β was indicated by increased retention of PET tracers or reduced CSF measurements ( Fagan et al. , 2007 ; Resnick et al. , 2010 ; Landau et al. , 2012 ; Lim et al. , 2014 b ). In one study, rates of amyloid-β deposition correlated with rates of episodic memory decline ( Villemagne et al. , 2013 ). With the publication of criteria for preclinical staging of Alzheimer’s disease, studies have increasingly examined how the presence of amyloid-β and neurodegeneration, together and separately, affect cognitive decline. Investigators studying several large cohorts from ADNI ( Toledo et al. , 2014 ), Amsterdam ( van Harten et al. , 2013 ), Harvard ( Mormino et al. , 2014 ), the Mayo Clinic ( Knopman et al. , 2012 ), and Washington University ( Vos et al. , 2013 ) have used this approach to contrast progression by preclinical stage of Alzheimer’s disease. In general, advancing stage was associated with a greater risk of progressive cognitive decline so that those with evidence of amyloid-β with neurodegeneration (i.e. preclinical stages 2/3) had the highest risk. The situation for those in Stage 1, with only evidence of amyloid-β is not as straightforward; two studies found that such individuals with CSF evidence of low amyloid-β alone declined ( van Harten et al. , 2013 ; Vos et al. , 2013 ) while in the three other cohorts using PET measures of amyloid-β, decline in those with Stage 1 was less evident. These studies differ in many ways besides the method of amyloid-β measurement; they used different outcomes, sample selection and length of follow-up. However as a group they indicate that the risk from amyloid-β alone, while likely important, is lower than the risk from amyloid-β in the presence of additional abnormal biomarkers. This has, in fact, been seen as an interaction between amyloid-β and neurodegenerative biomarkers in other studies, suggesting that the joint effect of abnormal amyloid-β and neurodegeneration is greater than the additive contribution of either marker alone ( Wirth et al. , 2013 b ; Mormino et al. , 2014 ).
Summary
Is amyloid-β harmful to the brain? The literature is replete with evidence that individuals who harbour amyloid-β show a number of neurological effects that are similar to those seen in Alzheimer’s disease. While studies are sometimes contradictory, there is substantial evidence that amyloid-β is associated with cross-sectional and progressive brain atrophy, cross-sectional network dysfunction and longitudinal cognitive decline. A closer examination of the data indicates that simply classifying individuals based on the presence or absence of amyloid-β does not give the full picture. Studies that have categorized normal older people according to preclinical stages of Alzheimer’s disease strongly indicate that those with evidence of both amyloid-β and biomarkers suggesting neurodegeneration show the greatest evidence of decline. Thus examining individuals simply in terms of the presence or absence of amyloid-β is not as informative as defining where they stand along a putative pathway of preclinical Alzheimer’s disease progression.
Many of these studies, especially those that are cross-sectional, demonstrate associations but do not prove causality. Scientific inquiry, especially in human research, benefits from studies of association especially when results converge across different methods and laboratories. However, traditional views of the scientific method hold that disproving a hypothesis is more valuable than accumulating positive evidence. In this regard, the only true human experiments manipulating amyloid-β levels—clinical trials of amyloid-lowering therapies—refute the importance of amyloid-β in Alzheimer’s disease. This has not, however, disproven the amyloid hypothesis primarily because it is not a true hypothesis but rather a complex model of disease causation. Over time, this model has been refined and generated numerous hypotheses, one of which is that Alzheimer’s disease has a prolonged incubation period during which amyloid-β promotes neurological damage. Failure of clinical trials in late stage disease is fully compatible with this model and begs for studies to be done in early and presymptomatic individuals. The limitations of cross-sectional association studies are in part ameliorated through longitudinal studies that permit observation of the evolution of disease from asymptomatic stages. While incomplete, the studies reviewed here show that amyloid-β deposition is a very early event that appears to play a harmful role in brain ageing especially when it is associated with neurodegeneration.
In this setting, the appropriate question, therefore, may not be whether amyloid-β is harmful to the brain, but how amyloid-β is harmful to the brain. The mechanisms that link amyloid-β to neurodegeneration are poorly understood and perplexing. For example, in Alzheimer dementias with focal features such as posterior cortical atrophy or progressive aphasia, the pattern of amyloid-β deposition does not reflect the pattern of brain degeneration ( Rabinovici et al. , 2008 ; Rosenbloom et al. , 2011 ). Individuals may respond differently to amyloid-β deposition based on the ability to compensate ( Elman et al. , 2014 b ) or the presence of genetic factors that influence immunity and inflammation ( Tanzi, 2015 ). Multiple biomarkers must be measured to characterize neurodegeneration, which could be important for ‘staging’ individuals by defining how progressed they are—an important factor for selection of individuals in clinical trials. The addition of tau imaging offers the potential of more specific biomarkers for degenerative brain processes ( Villemagne et al. , 2015 ) that may shed light on these issues.
Cross-sectional association studies also have the disadvantage that they reflect brain biomarkers of structure and function that do not necessarily reflect neurodegeneration but could indicate a variety of non-progressive processes. The time between the appearance of brain amyloid-β and the development of dementia is potentially decades ( Villemagne et al. , 2013 ) during which a multitude of pathological mechanisms may be operating. We need longitudinal studies to define relationships between amyloid-β, neurodegeneration, and cognition, and to better infer causation from temporal associations. How often is neurodegeneration preceded by amyloid-β, and how often might it arise independently and interact with amyloid-β to result in decline? Is Fig. 1 A or Fig. 1 B closer to the evolution of Alzheimer’s disease? In Fig. 1 A, dementia occurs via amyloid-β-induced neurodegeneration, while in Fig. 1 B dementia could evolve in relation to either amyloid-β or neurodegeneration independently. This is one of the key controversies in Alzheimer’s disease research today, but it is not simply an academic exercise. Our ability to target the right interventions to the correct processes at the appropriate time point depends on a more precise understanding of the complex chain of events that occurs over many years to produce dementia.
Acknowledgements
I am indebted to Beth Mormino, Susan Landau, and Gil Rabinovici who provided critical comments on the manuscript.
Funding
This work was supported in part by NIH grant AG034570.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. . Frequent amyloid deposition without significant cognitive impairment among the elderly . Arch Neurol 2008. ; 65 : 1509 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews KA, Modat M, Macdonald KE, Yeatman T, Cardoso MJ, Leung KK, et al. . Atrophy rates in asymptomatic amyloidosis: implications for Alzheimer prevention trials . PLoS One 2013. ; 8 : e58816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. . Clinical and biomarker changes in dominantly inherited Alzheimer’s disease . N Engl J Med 2012. ; 367 : 795 – 804 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, et al. . Amyloid-beta associated cortical thinning in clinically normal elderly . Ann Neurol 2011. ; 69 : 1032 – 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. . Neuropathology of older persons without cognitive impairment from two community-based studies . Neurol 2006. ; 66 : 1837 – 44 . [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS . Functional connectivity in the motor cortex of resting human brain using echo-planar MRI . Magn Reson Med 1995. ; 34 : 537 – 41 . [DOI] [PubMed] [Google Scholar]
- Bourgeat P, Chetelat G, Villemagne VL, Fripp J, Raniga P, Pike K, et al. . Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia . Neurology 2010. ; 74 : 121 – 7 . [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. . Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression . J Neurosci 2012. ; 32 : 8890 – 99 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Wang L, Fagan AM, Benzinger T, et al. . Unrecognized preclinical Alzheimer disease confounds rs-fcMRI studies of normal aging . Neurology 2014. ; 83 : 1613 – 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. . Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease . J Neurosci 2009. ; 29 : 1860 – 73 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. . Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory . J Neurosci 2005. ; 25 : 7709 – 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Pike KE, Ellis KA, Bourgeat P, Jones G, et al. . Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease . Brain 2011. ; 134 : 798 – 807 . [DOI] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Villain N, Jones G, Ellis KA, Ames D, et al. . Accelerated cortical atrophy in cognitively normal elderly with high beta-amyloid deposition . Neurology 2012. ; 78 : 477 – 84 . [DOI] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. . Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study . Lancet Neurol 2012a. ; 11 : 669 – 78 . [DOI] [PubMed] [Google Scholar]
- Clark VH, Resnick SM, Doshi J, Beason-Held LL, Zhou Y, Ferrucci L, et al. . Longitudinal imaging pattern analysis (SPARE-CD index) detects early structural and functional changes before cognitive decline in healthy older adults . Neurobiol Aging 2012b. ; 33 : 2733 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. . Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density . JAMA Neurol 2015. ; 72 : 287 – 94 . [DOI] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, et al. . Amyloid-beta associated volume loss occurs only in the presence of phospho-tau . Ann Neurol 2011. ; 70 : 657 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. . The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals . Cereb Cortex 2009. ; 19 : 497 – 510 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore V, Villemagne VL, Bourgeat P, Fripp J, Acosta O, Chetelat G, et al. . Cross-sectional and longitudinal analysis of the relationship between Abeta deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease . JAMA Neurol 2013. ; 70 : 903 – 11 . [DOI] [PubMed] [Google Scholar]
- Driscoll I, Zhou Y, An Y, Sojkova J, Davatzikos C, Kraut MA, et al. . Lack of association between 11C-PiB and longitudinal brain atrophy in non-demented older individuals . Neurobiol Aging 2011. ; 32 : 2123 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Madison CM, Baker SL, Vogel JW, Marks SM, Crowley S, et al. . Effects of beta-amyloid on resting state functional connectivity within and between networks reflect known patterns of regional vulnerability . Cereb Cortex 2014a. . Advance Access published on November 7, 2014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Oh H, Madison CM, Baker SL, Vogel JW, Marks SM, et al. . Neural compensation in older people with brain amyloid-beta deposition . Nat Neurosci 2014b. ; 17 : 1316 – 18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Insel P, Jagust WJ, Shaw L, Trojanowski JQ, Aisen P, et al. . CSF biomarker and PIB-PET-derived beta-amyloid signature predicts metabolic, gray matter, and cognitive changes in nondemented subjects . Cereb Cortex 2012. ; 22 : 1993 – 2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, et al. . Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly . Ann Neurol 2009. ; 65 : 176 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM . Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults . Arch Neurol 2007. ; 64 : 343 – 349 . [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, et al. . Florbetapir PET analysis of amyloid-beta deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study . Lancet Neurol 2012. ; 11 : 1057 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V . Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI . Proc Natl Acad Sci USA 2004. ; 101 : 4637 – 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ . The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics . Science 2002. ; 297 : 353 – 6 . [DOI] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, Patel TA . Meta-analysis of amyloid-cognition relations in cognitively normal older adults . Neurology 2013. ; 80 : 1341 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. . Disruption of functional connectivity in clinically normal older adults harboring amyloid burden . J Neurosci 2009. ; 29 : 12686 – 94 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K . The case for rejecting the amyloid cascade hypothesis . Nat Neurosci 2015. ; 18 : 794 – 9 . [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers . Lancet Neurol 2013. ; 12 : 207 – 16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. . Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade . Lancet Neurol 2010. ; 9 : 119 – 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. . An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease . Ann Neurol 2012. ; 71 : 765 – 75 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, O’Brien PC, Tangalos EG . MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease . Neurology 1992. ; 42 : 183 – 8 . [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM ; Alzheimer’s Disease Neuroimaging I . Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging . J Neurosci 2012. ; 32 : 18227 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, et al. . Amyloid burden and neural function in people at risk for Alzheimer’s Disease . Neurobiol Aging 2014. ; 35 : 576 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Wiste HJ, Lundt ES, Weigand SD, Vemuri P, et al. . 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons . Neurobiol Aging 2014. ; 35 : 2096 – 106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. . Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease . Neurology 2012. ; 78 : 1576 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, et al. . Selective worsening of brain injury biomarker abnormalities in cognitively normal elderly persons with beta-amyloidosis . JAMA Neurol 2013. ; 70 : 1030 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, et al. . Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid . Ann Neurol 2013. ; 74 : 826 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. . Amyloid deposition, hypometabolism, and longitudinal cognitive decline . Ann Neurol 2012. ; 72 : 578 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Nebes R, Snitz B, Cohen A, Mathis C, Price J, et al. . Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects . Brain 2014a. ; 137 : 3327 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. . Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease . Brain 2014b. ; 137 : 221 – 31 . [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Weigand SD, Senjem ML, Vemuri P, Jordan L, Kantarci K, et al. . Association of hypometabolism and amyloid levels in aging, normal subjects . Neurology 2014. ; 82 : 1959 – 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, et al. . Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects . Arch Neurol 2011. ; 68 : 1131 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Aisen PS, Jagust W, Mackin S, Weiner M, et al. . Brain structure and function as mediators of the effects of amyloid on memory . Neurology 2015a. ; 84 : 1136 – 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. . Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease . Brain 2015b. ; 138 : 772 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE . Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease . Ann Neurol 1997. ; 42 : 85 – 94 . [DOI] [PubMed] [Google Scholar]
- Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, et al. . Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals . JAMA Neurol 2014. ; 71 : 1379 – 85 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, et al. . Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging . Cereb Cortex 2011. ; 21 : 2399 – 407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. . APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging . Ann Neurol 2010. ; 67 : 122 – 31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. . Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum . Brain 2015. ; 138 : 1370 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. . Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature . J Neuropathol Exp Neurol 2012. ; 71 : 362 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Habeck C, Madison C, Jagust W . Covarying alterations in Abeta deposition, glucose metabolism, and gray matter volume in cognitively normal elderly . Hum Brain Mapp 2014. ; 35 : 297 – 308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. . Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia . Ann Neurol 2008. ; 64 : 388 – 401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL . A default mode of brain function . Proc Natl Acad Sci USA 2001. ; 98 : 676 – 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. . Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia . Proc Natl Acad Sci USA 2004. ; 101 : 284 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, et al. . Face-name associative memory performance is related to amyloid burden in normal elderly . Neuropsychologia 2011. ; 49 : 2776 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. . Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB . Neurology 2010. ; 74 : 807 – 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Forster S, et al. . Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study . J Neurosci 2014. ; 34 : 6260 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. . Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model . Science 2007. ; 316 : 750 – 4 . [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, et al. . beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences . Neurology 2012. ; 78 : 387 – 95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M, et al. . Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution . Neurology 2011. ; 76 : 1789 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Sabbagh MN, Seibyl J, Barthel H, Akatsu H, Ouchi Y, et al. . Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study . Alzheimers Dement 2015. ; 11 : 964 – 74 . [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. . Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease . N Engl J Med 2014. ; 370 : 322 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott JM, Bartlett JW, Fox NC, Barnes J ; Alzheimer’s Disease Neuroimaging Initiative I . Increased brain atrophy rates in cognitively normal older adults with low cerebrospinal fluid Abeta1–42 . Ann Neurol 2010. ; 68 : 825 – 34 . [DOI] [PubMed] [Google Scholar]
- Seab JP, Jagust WJ, Wong STS, Roos MS, Reed BR, Budinger TF . Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease . Magn Reson Med 1988. ; 8 : 200 – 8 . [DOI] [PubMed] [Google Scholar]
- Selkoe DJ . The molecular pathology of Alzheimer’s disease . Neuron 1991. ; 6 : 487 – 98 . [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, et al. . APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42 . J Neurosci 2010a. ; 30 : 17035 – 40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. . Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly . Biol Psychiatry 2010b. ; 67 : 584 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. . Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease . Alzheimers Dementia 2011. ; 7 : 280 – 92 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. . The A4 study: stopping AD before symptoms begin? . Sci Transl Med 2014. ; 6 : 228fs13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu IC, Vasconcelos B, Terwel D, Dewachter I . Models of beta-amyloid induced Tau-pathology: the long and ‘folded’ road to understand the mechanism . Mol Neurodegener 2014. ; 9 : 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC . Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition . Arch Neurol 2009. ; 66 : 1476 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE . TREM2 and Risk of Alzheimer’s Disease–Friend or Foe? . N Engl J Med 2015. ; 372 : 2564 – 5 . [DOI] [PubMed] [Google Scholar]
- Toledo JB, Weiner MW, Wolk DA, Da X, Chen K, Arnold SE, et al. . Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition . Acta Neuropathol Commun 2014. ; 2 : 26 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND . Energetic cost of brain functional connectivity . Proc Natl Acad Sci USA 2013. ; 110 : 13642 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harten AC, Smits LL, Teunissen CE, Visser PJ, Koene T, Blankenstein MA, et al. . Preclinical AD predicts decline in memory and executive functions in subjective complaints . Neurology 2013. ; 81 : 1409 – 16 . [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. . Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study . Lancet Neurol 2013. ; 12 : 357 – 67 . [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC . Tau imaging: early progress and future directions . Lancet Neurol 2015. ; 14 : 114 – 24 . [DOI] [PubMed] [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. . Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation . Brain 2015. ; 138 : 2020 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. . Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study . Lancet Neurol 2013. ; 12 : 957 – 65 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. . Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo . Nature 2002. ; 416 : 535 – 9 . [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Tosakulwong N, Weigand SD, Senjem ML, Lowe VJ, Gunter JL, et al. . Does amyloid deposition produce a specific atrophic signature in cognitively normal subjects? . Neuroimage Clin 2013. ; 2 : 249 – 57 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ . Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals . J Neurosci 2013a. ; 33 : 5553 – 63 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M, Oh H, Mormino EC, Markley C, Landau SM, Jagust WJ . The effect of amyloid beta on cognitive decline is modulated by neural integrity in cognitively normal elderly . Alzheimers Dement 2013b. ; 9 : 687 – 98.e1 . [DOI] [PMC free article] [PubMed] [Google Scholar]