Abstract
Background
Lung cancer screening may provide a “teachable moment” for promoting smoking cessation. This study assessed smoking cessation and relapse rates among individuals undergoing follow-up low-dose chest computed tomography (CT) in a clinical CT lung screening program and assessed the influence of initial screening results on smoking behavior.
Methods
Self-reported smoking status for individuals enrolled in a clinical CT lung screening program undergoing a follow-up CT lung screening exam between 1st February, 2014 and 31st March, 2015 was retrospectively reviewed and compared to self-reported smoking status using a standardized questionnaire at program entry. Point prevalence smoking cessation and relapse rates were calculated across the entire population and compared with exam results. All individuals undergoing screening fulfilled the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Lung Cancer Screening v1.2012® high-risk criteria and had an order for CT lung screening.
Results
A total of 1,483 individuals underwent a follow-up CT lung screening exam during the study interval. Smoking status at time of follow-up exam was available for 1,461/1,483 (98.5%). A total of 46% (678/1,461) were active smokers at program entry. The overall point prevalence smoking cessation and relapse rates were 20.8% and 9.3%, respectively. Prior positive screening exam results were not predictive of smoking cessation (OR 1.092; 95% CI, 0.715–1.693) but were predictive of reduced relapse among former smokers who had stopped smoking for 2 years or less (OR 0.330; 95% CI, 0.143–0.710). Duration of program enrollment was predictive of smoking cessation (OR 0.647; 95% CI, 0.477–0.877).
Conclusions
Smoking cessation and relapse rates in a clinical CT lung screening program rates are more favorable than those observed in the general population. Duration of participation in the screening program correlated with increased smoking cessation rates. A positive exam result correlated with reduced relapse rates among smokers recently quit smoking.
Keywords: Smoking cessation, lung cancer, cancer screening tests, multislice computed tomography (multislice CT)
Introduction
Computed tomography (CT) lung screening may promote smoking cessation by serving as a “teachable moment” or a, “…naturally occurring life transition or health event thought to motivate individuals to spontaneously adopt risk-reducing health behaviors” (1). CT lung screening research studies have reported smoking cessation rates of 11% to 24% in the first two years of screening versus a 5% to 7% annual rate in the general population (2,3). Similarly, smoking relapse rates in screening studies range from 10–34% for smokers quit one year or less (4-7), versus a 50–90% annual relapse rate in the general population (8-10). Smoking cessation and smoking abstinence rates have been reported to continue to improve with increased duration of participation in the screening program (11-13). Some studies, including the National Lung Screening Trial (NLST), have reported increased smoking cessation rates for participants with positive exam results (4,13). A possible dose response relationship has also been reported with increasing rates of smoking cessation observed among individuals with more suspicious exam results (4,13). The purpose of our study is to report smoking cessation and smoking relapse rates in participants undergoing follow-up low-dose CT imaging evaluation in a clinical CT lung screening program and to assess the influence of screening results on smoking behavior.
Methods
Screening process
All individuals enrolled in the screening program fulfilled the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Lung Cancer Screening v1.2012 (NCCN Guidelines®) high risk criteria for lung cancer (Table 1) and had a physician order for CT lung screening. Patients were not eligible for screening if they had known metastatic disease, had been diagnosed with lung cancer within the previous five years, or had symptoms concerning for lung cancer. The CT lung screening program staff confirmed all participant eligibility at program entry including; self-reported current smoking status, duration of smoking cessation for former smokers, and smoking history in pack-years using a scripted questionnaire. Each enrolled patient was mailed a frequently asked question (FAQ) document detailing information about the screening process including smoking cessation information and available cessation resources prior to the screening exam. FAQ documents were also made available at the screening site and at many of the referring physician offices. Patients with exam findings suspicious for lung cancer were notified of their exam results directly by their ordering physician. All other patients received written notification of their exam findings within two weeks of the screening exam with a recommended next exam date. For exams with no nodules, small nodules not meeting positive size criteria, or stable nodules, a repeat CT lung screening was recommended in one year. For positive exams with “probably benign” nodules, typically not exceeding 8 mm in mean diameter, a follow-up CT lung screening exam was recommended in 3 or 6 months. Patients with “suspicious” exams, typically nodules greater than 8 mm in mean diameter or growing nodules, a pulmonary consultation was recommended with follow-up imaging performed in some cases at the discretion of the pulmonologist and multidisciplinary care team. At the time of the follow-up exam the CT technologist performing the exam asked each patient if they were actively smoking and recorded the patient response in the electronic medical record (EMR). All patients who underwent a follow-up CT lung screening exam between 1st February, 2014 and 31st March, 2015 and had their current smoking status recorded in the EMR were included in the study. The study was institution review board (IRB) approved with a waiver of individual patient consent.
Table 1. National Comprehensive Cancer Network (NCCN) group 1 and group 2 lung cancer screening criteria.
| Variable | NCCN group 1 (n=1,105) | NCCN group 2 (n=356) |
|---|---|---|
| Age | 55–74 | 50–74* |
| Smoking history | ≥30 pack years | ≥20 pack years |
| Smoking status | Current or former | Current or former |
| Quit duration | <15 years | Any time |
| Additional risk factors | None required | At least one of the following required: (I) family history of lung cancer (parent, sibling, or child); (II) personal history of chronic lung disease; (III) occupational exposure to known lung carcinogen(s)**; (IV) personal history of smoking related cancer (excluding skin cancer and metastatic disease) |
*, >50 in NCCN Guidelines®; **, carcinogens include arsenic, asbestos, beryllium, cadmium, soot, chromium, diesel fumes, nickel, silica, coal smoke, and radon (occupational or documented residential).
Statistical analysis
Multivariable binary logistic regression models were developed for the group of active smokers at program entry, the group of all former smokers at program entry, and the group of former smokers who reported 2 years or less of smoking cessation at program entry. Self-reported smoking at the most recent scan was the dependent variable. A priori estimates of minimum sample sizes were developed using Peduzzi’s rule of thumb of one predictor variable per ten events (14). Demographic differences between current smokers and former smokers were assessed using Pearson’s Chi-Square test (two sided) for categorical variables and the independent samples T test for continuous variables. Analysis of variance (ANOVA) was used for the analysis of deviance to compare nested models. The significance level for differences was set at P≤0.05 for all statistical analysis. All data are reported as mean ± standard deviation, range or percentage as appropriate. All statistical analysis was performed using the statistical software platform R version 3.1.2 (15). The independent variables evaluated during model development were continuous variables; age, pack-years smoking history, years of smoking cessation (with zero indicating smoking at program entry), total number of screening exams, number of positive scans, and total duration of years in the program and categorical variables; gender, NCCN high-risk group, initial scan result, individual history of cancer, and referring physician. Probably benign or suspicious scan results were included in the “positive” category and all other exams were “negative”. Positive/suspicious exam thresholds were based on the NCCN Guidelines® for exams performed prior to 12th July, 2014 and ACR Lung-RADS™ version 1 thereafter. To assess the influence of exam results not suspicious for lung cancer but indicative of potential infection the logistics models were run once with these results coded as positive and again with these results coded as negative.
Results
Of 1,483 patients undergoing follow-up exams during the study interval, 1,461 (98.5%) had a current smoking status recorded in the EMR. At program entry 46.4% (678/1,461) were active smokers. A total of 23.8% (186/783) of former smokers at program entry had stopped for two years or less. Tables 2 and 3 summarize group characteristics.
Table 2. Patient characteristics of full sample.
| Variable (n=1,461) | Results |
|---|---|
| Age (years) | 62.5±5.98 |
| Pack year history | 49.0±21.9 |
| Duration in program (years) | 1.45±0.63 |
| Number of positive scans | 0.83±1.15 |
| Years quit (all) | 5.4±8.4 |
| Years quit (former smokers) | 10.1±9.2 |
| Smoking at initial scan | 678 (46.4%) |
| Smoking at most recent scan | 610 (41.8%) |
| Male | 779 (53.3%) |
| Female | 682 (46.7%) |
| Group 1 | 1,105 (75.6%) |
| Group 2 | 356 (24.4%) |
| Prior history of cancer | 249 (17.0%) |
| Outside referring PCP | 255 (17.5%) |
| 3+ scans | 725 (49.6%) |
| 1+ positive scans | 545 (37.3%) |
| Negative initial scan | 1039 (71.1%) |
| S positive | 88 (6.0%) |
Values are reported as mean ± standard deviation. Values in () percent of sample.
Table 3. Patient characteristics of major subgroups.
| Variable | Smoking at initial scan (n=678) | Quit at initial scan (n=783) | P |
|---|---|---|---|
| Smoking at most recent scan | 537 | 73 | |
| Quit at most recent scan | 141 | 710 | |
| Age (years) | 61.3±5.91 | 63.6±5.82 | <0.001 |
| Pack year history | 47.8±20.1 | 50.0±23.2 | 0.056 |
| Duration in program (years) | 1.43±0.63 | 1.46±0.64 | 0.35 |
| Number of positive scans | 0.80±1.09 | 0.85±1.20 | 0.42 |
| Male | 386 (56.9%) | 393 (50.2%) | 0.01 |
| Female | 292 (43.1%) | 390 (49.8%) | |
| Group 1 | 560 (82.6%) | 545 (69.6%) | <0.001 |
| Group 2 | 118 (17.4%) | 238 (30.4%) | |
| Prior history of cancer | 122 (18.0%) | 127 (16.2%) | 0.37 |
| Outside referring PCP | 113 (16.7%) | 142 (18.1%) | 0.46 |
| 3+ scans | 335 (49.4%) | 401 (51.2%) | 0.49 |
| 1+ positive scans | 257 (37.9%) | 288 (36.8%) | 0.66 |
| Negative initial scan | 479 (70.6%) | 560 (71.5%) | 0.71 |
| S positive | 40 (5.9%) | 48 (6.1%) | 0.85 |
Values are reported as mean ± standard deviation. Values in () percent of sample.
Smoking cessation and relapse rates
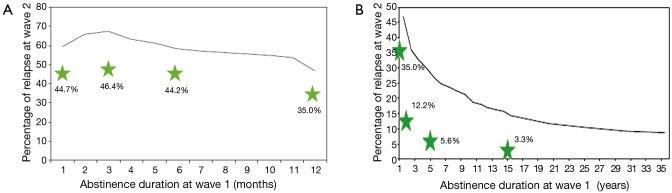
Of current smokers at program entry, 20.8% (141/678) reported smoking cessation at the most recent follow-up exam. With an average time of enrollment of 1.43 years, this results in an annualized rate of 14.5%. Of former smokers at program entry 9.3% (73/783) reported smoking relapse at the most recent follow-up exam. In former smokers with greater than 2 years of cessation the relapse rate was 3.2%. Participants with shorter periods of smoking cessation at program entry had higher relapse rates: 44.2% for smoking cessation of six months or less and 35% for 12 months or less (Figure 1A,B).
Figure 1.
Comparison of smoking relapse rates with time abstinent at entry into the screening program for the study as compared to population relapse rates from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (8). Data are shown for patients who report being quit at time of entering the screening program. Relapse is determined by patient self-reporting as smoking at their most recent scan. Abstinent times at program entry are patient self-reported years quit. (A) shows relapse rates for those quit less than 1 year and (B) relapse rates for those quit 1 year or longer. Reproduced with permission.
Model results (Table 4)
Table 4. Binary logistic regression model results.
| Independent variable | Smoking at initial scan (n=678) | Quit at initial scan (n=783) | Quit ≤2 years at initial scan (n=186) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |||
| Age | 0.994 | 0.963–1.027 | 0.731 | 0.988 | 0.944–1.034 | 0.603 | 0.960 | 0.903–1.019 | 0.185 | ||
| Duration in program | 0.647 | 0.477–0.877 | 0.005 | — | — | — | — | — | — | ||
| Gender | 1.826 | 1.254–2.670 | 0.002 | 1.527 | 0.907–2.601 | 0.114 | 1.198 | 0.599–2.423 | 0.611 | ||
| Initial scan result | 1.092 | 0.715–1.693 | 0.688 | 0.538 | 0.232–1.185 | 0.134 | 0.330 | 0.143–0.710 | 0.006 | ||
| Years quit (− initial scan) | — | — | — | 0.764 | 0.692–0.831 | <0.001 | — | — | — | ||
| Years quit (+ initial scan) | — | — | — | 0.743 | 0.516–0.982 | — | — | — | — | ||
| Years quit × initial scan result* | — | — | — | 0.972 | 0.746–1.182 | 0.804 | — | — | — | ||
| Years quit | — | — | — | — | — | — | 0.294 | 0.158–0.510 | <0.001 | ||
*, interaction term.
For each year enrolled in the screening program current smokers had a 0.647 OR of continued smoking (95% CI, 0.477–0.877, P=0.005). Male current smokers at program entry had 1.83 OR of continued smoking (95% CI, 1.25–2.67, P=0.002). Neither the number of positive scans nor the initial scan results were significantly correlated with smoking cessation for this group.
Former smokers had a relapse OR of 0.764 (95% CI, 0.692–0.831, P<0.001) for each year quit at program entry after a positive exam and 0.743 (95% CI, 0.516–0.982, P<0.001) for each year quit at program entry after a negative initial exam. Former smokers with cessation duration two years or less at program entry had a relapse OR of 0.330 following a positive initial scan (95% CI, 0.143–0.710; P=0.006). Each additional year of cessation at program entry resulted in a 0.294 relapse OR (95% CI, 0.158–0.510; P<0.001). There were no appreciable differences in model results with inclusion of scans indicative of infection in the positive scan category. The final models coded these as negative scans.
Discussion
To our knowledge this is the first study assessing rates of smoking cessation and relapse in a clinical CT lung cancer screening program.
Our results support the idea that lung cancer screening is a teachable moment for smoking cessation with an observed annualized smoking cessation rate (14.5%) at least double annual cessation rates in the general population (5–7%) and consistent with the 11–24% smoking cessation rates in the first or second year of lung cancer screening clinical trials and academic studies (2-5). These results are especially encouraging since only about 20% of people entering a lung cancer screening program say they are ready to quit (13,16).
Relapse rates in our study show the same trends as those in the general population but are approximately 10 to 15 percentage points lower (Figure 1A,B). The 9.3% overall relapse rate in the program is consistent with the relapse rates of 10% seen in lung cancer screening in clinical trials and academic studies (4,7). The steep initial decline in relapse rates after the first year reinforces the need (and opportunity for those enrolled in a screening program) to provide help with remaining tobacco free to those recently quit.
A positive initial scan result reduced the odds of relapse among former smokers who had stopped smoking for two years or less at program entry; however a positive initial scan result was not significantly correlated with quitting smoking for the group of active smokers. Results from the NLST (13) and Mayo Clinic (4) studies however showed increased smoking cessation rates for participants with positive (abnormal) scan results. These seemingly contradictory results are likely attributed to differences in study design and sample size in the Mayo and NLST studies compared to the present study. In the Mayo report, the criteria used for a positive scan resulted in 57% of participants receiving an initial positive scan result as compared to 29.4% for our study. The NLST study group of smokers at the start of a screening program had a much larger sample size than ours; 14,661 in total, as compared to our 678 smokers. Furthermore, both the Mayo and NLST studies were longitudinal studies with smoking status recorded at each scan as compared to our study comparing smoking status after only the most recent scan.
The rate of patients with positive CT lung screening exams in this study (29.4%) substantially exceeds our observed CT lung screening baseline positive exam rate (11.8%) due to the study inclusion criteria requiring at least one follow-up exam. Patients with a negative exam are recommended to return in one year; however patients with a positive exam typically return for a repeat exam within six months. Therefore, for exams performed after April 1, 2014 only those with positive results were included in the study leading to the inflated rate of patients with positive exams.
Duration of program enrollment did correlate with increased rates of smoking cessation for active smokers. The reasons for this are unclear. One contributing factor may be the repeated opportunities to discuss smoking cessation with a healthcare professional that occur throughout the screening process; we estimate that over a two year screening period there are 11 different opportunities for an enrolled patient to express quit readiness to program staff.
Our results are consistent with others studies, including the NLST, in refuting the “permission to smoke” theory showing no association between a negative screening result and decreased cessation. Our finding that men are less likely to engage in smoking cessation contrasts with prior studies reporting smoking cessation as more challenging for women (17,18). Population studies generally show similar cessation rates for men and women (3).
The findings in this study are limited to individuals undergoing follow-up CT lung screening and therefore excluded non-compliant patients who did not return for the recommended repeat annual screening or follow-up exam. In addition most individuals with suspicious findings who may have undergone further evaluation with PET-CT or biopsy including virtually all patients eventually diagnosed with lung cancer were not assessed. Given the reported positive correlation between smoking cessation rates and more suspicious exam results, the inclusion of individuals with the most suspicious findings including those diagnosed with lung cancer would likely increase the observed smoking cessation rate (4,13).
Self-reporting of smoking status may be prone to error. Since the participants gave their smoking status as part of a lung cancer screening protocol and not as part of a smoking cessation study, it is less likely that social bias would influence the answer. An assessment of self-reported smoking status among participants in a lung cancer screening trial showed excellent correlation of self-reported status to urinary cotinine verification (19).
There are many different factors influencing smoking cessation and relapse that are potential confounders including; level of education, marital status, nicotine addiction level, anxiety about lung cancer or other smoking related illnesses, perceived benefits of quitting, and “outside” smoking cessation interventions. As this was an observational study of lung cancer screening in a clinical practice, these confounders should be consistent with those in a similar populations of participants in clinical lung cancer screening.
Conclusions
CT lung screening in a clinical setting may provide a teachable moment to help people quit smoking and avoid smoking relapse.
Acknowledgements
None.
Ethical Statement: The study was institution review board (IRB) approved with a waiver of individual patient consent.
Footnotes
Conflicts of Interest: Andrea B. McKee, MD; received consultancy and speaking fees from Covidien (Medtronic). Shawn M. Regis, PhD; received consultancy fees from Covidien (Medtronic). Christoph Wald, MD, PhD, MBA, FACR; Member of the Radiology Medical Advice Network for Philips Healthcare; received consultancy fees from Phillips Healthcare. Brady J. McKee, MD; Spouse received consultancy and speaking fees from Covidien (Medtronic). The other authors have no conflicts of interest to declare.
References
- 1.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res 2003;18:156-70. 10.1093/her/18.2.156 [DOI] [PubMed] [Google Scholar]
- 2.Slatore CG, Baumann C, Pappas M, et al. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc 2014;11:619-27. 10.1513/AnnalsATS.201312-460OC [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) . Quitting smoking among adults--United States, 2001-2010. MMWR Morb Mortal Wkly Rep 2011;60:1513-9. [PubMed] [Google Scholar]
- 4.Townsend CO, Clark MM, Jett JR, et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer 2005;103:2154-62. 10.1002/cncr.21045 [DOI] [PubMed] [Google Scholar]
- 5.Ashraf H, Tønnesen P, Holst Pedersen J, et al. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64:388-92. 10.1136/thx.2008.102475 [DOI] [PubMed] [Google Scholar]
- 6.van der Aalst CM, van den Bergh KA, Willemsen MC, et al. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600-5. 10.1136/thx.2009.133751 [DOI] [PubMed] [Google Scholar]
- 7.Cox LS, Clark MM, Jett JR, et al. Change in smoking status after spiral chest computed tomography scan screening. Cancer 2003;98:2495-501. 10.1002/cncr.11813 [DOI] [PubMed] [Google Scholar]
- 8.García-Rodríguez O, Secades-Villa R, Flórez-Salamanca L, et al. Probability and predictors of relapse to smoking: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2013;132:479-85. 10.1016/j.drugalcdep.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) . Efforts to quit smoking among persons with a history of alcohol problems--Iowa, Kansas, and Nebraska, 1995-1996. MMWR Morb Mortal Wkly Rep 1997;46:1144-8. [PubMed] [Google Scholar]
- 10.Zhu S, Melcer T, Sun J, et al. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305-11. 10.1016/S0749-3797(00)00124-0 [DOI] [PubMed] [Google Scholar]
- 11.Anderson CM, Yip R, Henschke CI, et al. Smoking cessation and relapse during a lung cancer screening program. Cancer Epidemiol Biomarkers Prev 2009;18:3476-83. 10.1158/1055-9965.EPI-09-0176 [DOI] [PubMed] [Google Scholar]
- 12.Ashraf H, Saghir Z, Dirksen A, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax 2014;69:574-9. 10.1136/thoraxjnl-2013-203849 [DOI] [PubMed] [Google Scholar]
- 13.Tammemägi MC, Berg CD, Riley TL, et al. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst 2014;106:dju084. 10.1093/jnci/dju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373-9. 10.1016/S0895-4356(96)00236-3 [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available online: http://www.R-project.org/
- 16.Barry SA, Tammemagi MC, Penek S, et al. Predictors of adverse smoking outcomes in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst 2012;104:1647-59. 10.1093/jnci/djs398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetter DW, Kenford SL, Smith SS, et al. Gender differences in smoking cessation. J Consult Clin Psychol 1999;67:555-62. 10.1037/0022-006X.67.4.555 [DOI] [PubMed] [Google Scholar]
- 18.Reynoso J, Susabada A, Cepeda-Benito A. Gender differences in smoking cessation. J Psychopathol Behav Assess 2005;27:227-34. 10.1007/s10862-005-0638-2 [DOI] [Google Scholar]
- 19.Studts JL, Ghate SR, Gill JL, et al. Validity of self-reported smoking status among participants in a lung cancer screening trial. Cancer Epidemiol Biomarkers Prev 2006;15:1825-8. 10.1158/1055-9965.EPI-06-0393 [DOI] [PubMed] [Google Scholar]