Abstract
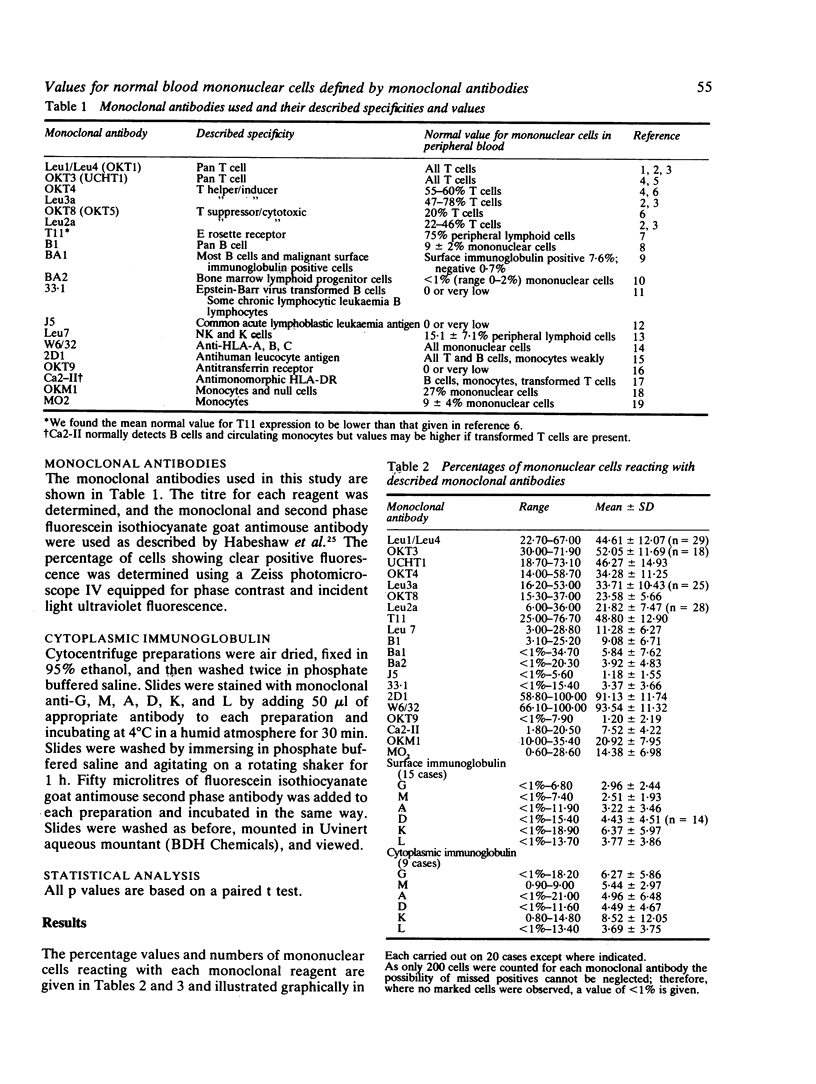
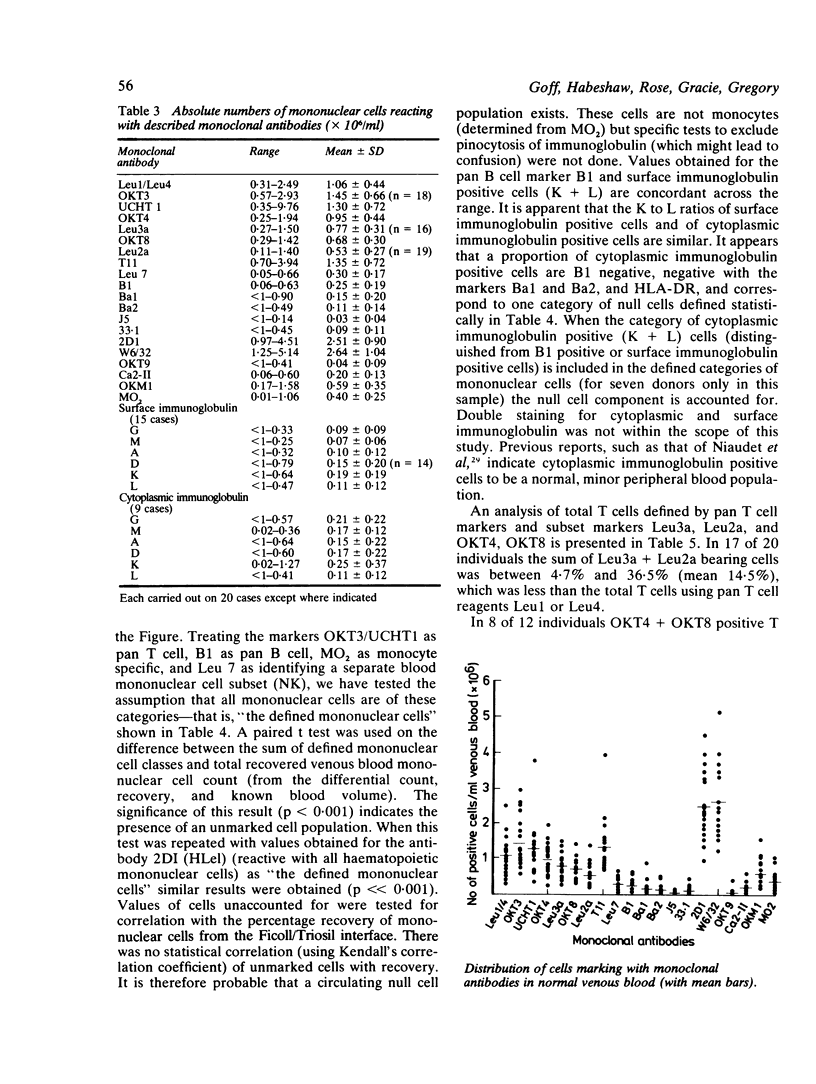
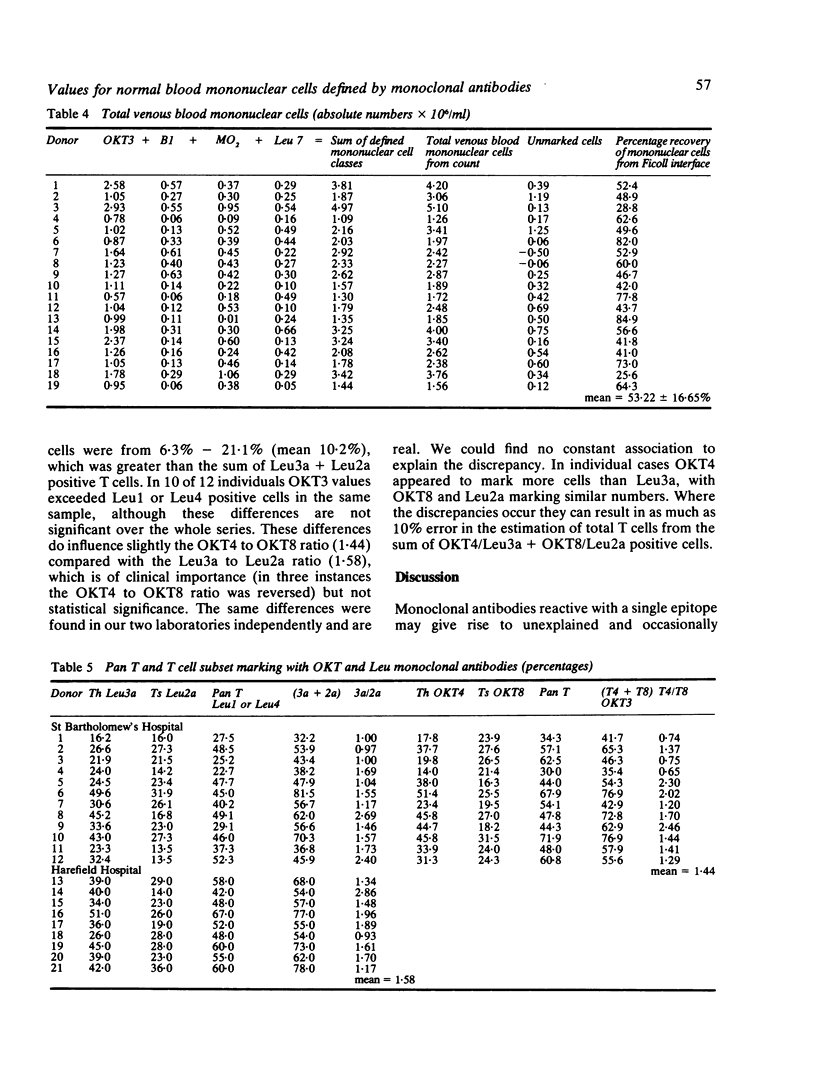
We present the data obtained from routine quantitation of normal venous blood mononuclear cells using 19 monoclonal antibodies against defined mononuclear cell surface antigens. The results indicate different values for percentage and absolute numbers of T lymphocytes and of T lymphocyte subsets depending on the monoclonal antibodies used to quantify these populations. In most instances the total number of identified cells was significantly less than the total number of recovered blood mononuclear cells, which suggests the existence in blood of a null cell population. Evidence is advanced to support previous observations that at least a proportion of this population is of B cell lineage, expressing cytoplasmic immunoglobulin but lacking other class or lineage specific markers. The value of a diverse monoclonal panel in routine quantitation of peripheral blood mononuclear cells is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Abramson C. S., Kersey J. H., LeBien T. W. A monoclonal antibody (BA-1) reactive with cells of human B lymphocyte lineage. J Immunol. 1981 Jan;126(1):83–88. [PubMed] [Google Scholar]
- Aisenberg A. C. Current concepts in immunology: Cell-surface markers in lymphoproliferative disease. N Engl J Med. 1981 Feb 5;304(6):331–336. doi: 10.1056/NEJM198102053040606. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Linch D., Delia D. Isolation of human haematopoietic progenitor cells using monoclonal antibodies. Nature. 1980 Sep 25;287(5780):332–333. doi: 10.1038/287332a0. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Brewer E. J. The Pediatric Rheumatology Collaborative Study Group--the first nine years. J Rheumatol. 1982 Jan-Feb;9(1):1–2. [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L., Detels R., Gottlieb M. Immune-cell augmentation (with altered T-subset ratio) is common in healthy homosexual men. N Engl J Med. 1983 Apr 7;308(14):842–843. doi: 10.1056/NEJM198304073081414. [DOI] [PubMed] [Google Scholar]
- Habeshaw J. A., Bailey D., Stansfeld A. G., Greaves M. F. The cellular content of non Hodgkin lymphomas: a comprehensive analysis using monoclonal antibodies and other surface marker techniques. Br J Cancer. 1983 Mar;47(3):327–351. doi: 10.1038/bjc.1983.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeshaw J. A., Young G. A. Quantitation of subclasses of mononuclear cells in normal human blood by membrane receptor studies. Br J Haematol. 1975 Jan;29(1):43–55. doi: 10.1111/j.1365-2141.1975.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Kersey J. H., LeBien T. W., Abramson C. S., Newman R., Sutherland R., Greaves M. P-24: a human leukemia-associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981 Mar 1;153(3):726–731. doi: 10.1084/jem.153.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N. R., Bishop S., Jefferis Use of antibody-coated red cells for the sensitive detection of antigen and in rosette tests for cells bearing surface immunoglobulins. J Immunol Methods. 1977;15(3):279–289. doi: 10.1016/0022-1759(77)90065-5. [DOI] [PubMed] [Google Scholar]
- Niaudet P., Greaves M., Horwitz D. Phenotypes of 'null' lymphoid cells in human blood. Scand J Immunol. 1979;9(4):387–393. doi: 10.1111/j.1365-3083.1979.tb03178.x. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Ritchie A. W., Oswald I., Micklem H. S., Boyd J. E., Elton R. A., Jazwinska E., James K. Circadian variation of lymphocyte subpopulations: a study with monoclonal antibodies. Br Med J (Clin Res Ed) 1983 Jun 4;286(6380):1773–1775. doi: 10.1136/bmj.286.6380.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Pesando J. M., Notis-McConarty J., Lazarus H., Schlossman S. F. A monoclonal antibody to human acute lymphoblastic leukaemia antigen. Nature. 1980 Feb 7;283(5747):583–585. doi: 10.1038/283583a0. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3848–3852. doi: 10.1073/pnas.78.6.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]



