Abstract
Since the time questions arose on cardiovascular safety of Rosiglitazone, FDA has suggested guidelines on conduct of studies on anti-diabetic drugs so as to prove that the cardiovascular risk is acceptable. Based on the cardiovascular risks of pre-approval clinical trials, guidelines have been made to conduct cardiovascular safety outcome trials (CVSOTs) prior to the drug approval or after the drug has been approved. Unlike the trials comparing the efficacy of antidiabetic agents, the CVSOTs examine the cardiovascular safety of a drug in comparison to standard of care. These trials are expensive aspects of drug development and are associated with various technical and operational challenges. More cost effective models of assessing cardiovascular safety like use of biomarkers, electronic medical records, pragmatic and factorial designs can be adopted. This article critically looks at the antidiabetic drug approval from a cardiovascular perspective by asking a few questions and arriving at answers.
Keywords: Anti diabetic drugs, Cardiovascular, Trials
Cardiovascular diseases (CVDs) are the leading cause of death in subjects with type 2 diabetes.1, 2 The primary aim of diabetes management is to prevent death and morbidity due to CVD and microvascular diseases. Multifactorial interventions targeting lifestyle changes, weight loss, lipids, blood pressure, hyperglycemia and use of antiplatelet agents have been shown to reduce the risk of CVD.1, 4 However, there has been a growing concern on the adverse cardiovascular outcomes in trials of certain anti-hyperglycemic agents (AHA) and drug combinations used to control hyperglycemia.4, 5, 6 It would be counterproductive if a drug used to treat diabetes itself increases the CVD risk. Following a meta-analysis of randomized controlled trials of Rosiglitazone, Nissen and Wolski5 concluded that there an increased risk of myocardial infarction and death in subjects on Rosiglitazone. This triggered a series of discussion on the need to more closely evaluate anti-diabetic therapies from a cardiovascular perspective. In 2008, FDA issued a guidance to pharmaceutical industry on the conduct of clinical studies to prove that anti-diabetic drugs confer to acceptable levels of CV safety.7 In this article, we try to answer the anti-diabetic drug approval process from a cardiovascular perspective. The authors selected few significant questions, which needed to be answered. A PubMed search was done with terms diabetes and cardiovascular outcome and cardiovascular trials. All article abstracts were screened, and articles answering our questions were selected.
1. What was the traditional FDA specifications for anti-diabetic drug approval?
Prior to the guidance, the process of drug approval required the sponsors to submit the phase 2 and phase 3 trial data on at least 2500 subjects exposed to the investigational product. At least 1300–1500 of these subjects should be exposed to the investigational product for >1 year and at least 300–500 subjects exposed to the investigational product for >18 months.8 The end point of efficacy was glycosylated hemoglobin (HbA1c).7 As per guidelines, these trials used the investigational agent as monotherapy or as an add on therapy. The cardiovascular adverse effects of these therapies were made out from the cardiovascular events that would occur during the course of the trial. These cardiovascular events were not pre-specified and not centrally independently adjudicated. Since the subjects included in these trials were younger, of low CV risk (patients with CV events usually excluded), shorter duration of disease and in shorter trial duration, the number of CV events accrued during the course of the trial would be low. The low event rates and lack of independent adjudication lead to poor estimates of CV safety of these agents.
2. What were the salient points in the FDA guidance issued in 2008?
The guidance issued by FDA in 2008 recommended that a new anti-diabetic drug should not increase cardiovascular risk to an unacceptable extent.7 The key recommendations are summarized in Table 1.
Table 1.
Salient points of the FDA guidance: Diabetes mellitus – developing drugs and therapeutic biologicals for treatment and prevention (from references 7, 35).
| 1. An upper bound of the 95% CI for the risk ratio of important CV events of 1.3 should be used as a key criterion for excluding unacceptable CV risk for new treatments of type 2 diabetes. |
| 2. Study patients must include individuals with relatively advanced disease, elderly patients, and patients with some degree of renal impairment. |
| 3. A minimum of 2 years’ CV safety data must be provided. |
| 4. All phase 2 and phase 3 studies should include a prospective independent adjudication of CV events. Adjudicated events should include CV mortality, myocardial infarction (MI), and stroke and can include hospitalization for acute coronary syndrome (ACS), urgent revascularization procedures, and possibly other end points. |
| 5. To satisfy the new statistical guidelines, the analysis of CV events may include a meta-analysis of all placebo controlled trials, add-on trials (i.e., drug vs. placebo, each added to standard therapy), and active-controlled trials, and/or an additional single, large safety trial may be conducted that alone, or added to other trials, would be able to satisfy this upper bound before a new drug application/biologics license application (NDA/BLA) is approved. |
The FDA also defined the point estimates and upper limit of 95% confidential intervals of risk ratios (1.3 and 1.8) for cardiovascular events in comparison to control group, which should prompt industry to design a post marketing or pre-marketing cardiovascular outcome trials (CVOTs).7, 8 CVOT since then have become an integral part of the drug approval process of anti-diabetic therapies. CVOT, despite its simplicity in design is often misunderstood as trials of glycemic efficacy by both practitioners and experts.9 With CVOT like Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR-TIMI 53), Trial to Evaluate Cardiovascular Outcomes after Treatment with Sitagliptin (TECOS), Examination of cardiovascular outcomes with alogliptin (EXAMINE), Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) and EMPA-REG OUTCOME already completed and with more trials to follow, it is important that all stake holders including clinicians in diabetes and cardiovascular practice know the key features of these trials.10, 11, 12, 13
3. How are regulatory CVOT different from trials like UKPDS, PROactive, ACCORD, ADVANCE and VADT?
Any trial reporting a single or composite of cardiovascular end points is labeled as a CVOT. Holman et al. analyzed trials with >1000 subjects and >1 year duration for his analysis of CVOT.14 These can be of various types
-
(A)
Trials reporting cardiovascular outcomes according to treatment goals (e.g. Action to Control Cardiovascular Risk in Diabetes (ACCORD), Veterans Affairs Diabetes Trial (VADT), Hyperglycemia and its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus (HEART2D)).15, 16
-
(B)
Trials reporting CV outcomes as a part of other total outcomes (e.g. UKPDS, DCCT). They may test 2 treatment goals with different regimes.17, 18
-
(C)
Trials looking at HbA1c goals and specific drugs and/or strategies e.g. Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE), Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial.19
-
(D)
Trials comparing CV outcomes of 2 different agents e.g. Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes (CAROLINA), A Trial Comparing Cardiovascular Safety of Insulin Degludec Versus Insulin Glargine in Subjects With Type 2 Diabetes at High Risk of Cardiovascular Events (DEVOTE) and Thiazolidinediones or Sulfonylureas and Cardiovascular Accidents Intervention Trial (TOSCA.IT).20, 21
-
(E)
Trials looking at cardiovascular safety/benefits of specific drugs (e.g. SAVOR-TIMI 53, TECOS, EXAMINE, ELIXA, Liraglutide Effect and Action in Diabetes: (LEADER), EMPA REG OUTCOME, Canagliflozin cardiovascular assessment Study (CANVAS), Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN), Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE TIMI 58), Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA), Ertugliflozin CVOT).10, 11, 22, 23
Although broadly divided into 5 groups, there is some overlap in this classification. The last 2 groups (D and E) of CVOT trial design is used for satisfying the current FDA requirement of acceptable safety of AHA. In fact they could be appropriately called “CV safety outcome trials (CVSOT)” to differentiate it from other trials reporting CV outcomes.24 DEVOTE trial stands out as the only regulatory trial of insulin, where Degludec is compared to Glargine.14
Often the UKPDS is quoted to highlight the favorable cardiovascular benefits of Metformin in obese subjects with type 2 diabetes. The UKPDS study recruited subjects with type 2 diabetes at diagnosis with low cardiovascular risk. In UKPDS glucose control study, the prevalence of CV risk factors were low: 0.3% of subjects were on lipid lowering therapy, 1.6% on antiplatelet therapy, 12% on antihypertensive medication, around 2% had proteinuria and 34% were current smokers.17 The standard of care for CV risk factors in 1977, when the study started were different from the ones we use now. Subjects with history of myocardial infarction within 1 year or with heart failure were excluded.17 This is evident with the low event rates in the trial despite lack of CV protective therapies.17 Of the 4209 randomized subjects in UKPDS, only 342 subjects were in Metformin arm. This is miniscule by current CVSOT standards.25 Even though a 10-year follow-up of the trial showed a favorable CV outcome, the lack of event adjudication, changing standard of care over the course of the trial, small number of patients on follow-up, non-trial format follow-up and follow-up losses would rate the outcomes inferior to current format of CVSOT.26 The Glucose Lowering In Non-diabetic hyperglycemia Trial (GLINT) will examine the effects of Metformin on cardiovascular outcomes in subjects with high CV risk and non-diabetes dysglycemia.27
The ACCORD, VADT, and ADVANCE were trials testing different targets of glycemic control rather than strategies.15, 16, 19 ACCORD reported cardiovascular outcomes in groups of subjects randomized to achieve HbA1c <6% vs. a target of 7.0–7.9% in subjects with high CV risk.15 In ADVANCE, the therapeutic strategy involved Diamicron MR (sustained release gliclazide preparation) to target an HbA1c <6.5% vs. standard care in subjects at high CV risk.19 In ADVANCE, subjects, who were the control arm, were not treated to equal glycemic targets and drugs with potential similar mechanisms (i.e. Sulphonylureas) were used in the control arm.19 In VADT, subjects with high CV risk were randomized to receive treatment with AHA to achieve stricter HbA1c targets in the intensive treatment arm (1.5% less than standard group) and outcomes were compared at the end of the trial.16
The PROactive and Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes (RECORD) trials did look at CV outcomes with Pioglitazone and Rosiglitazone respectively.28 PROactive included subjects with prior CV events and attempted to achieve glycemic equipoise in both the arms. Outcome events were adjudicated independently.28 The RECORD trial did include some subjects with CVD and followed a policy to titrate glycemic therapy to achieve a target HbA1c. Cardiovascular events were adjudicated. However the low event rates and high drop out in the trial limited the conclusions.29
4. How does a regulatory Cardiovascular Safety Outcome Trials (CVSOT) differ from trials of glycemic efficacy?
In trials of glycemic efficacy, subjects with diabetes with a certain degree of glycemic control (as defined by HbA1c), baseline drugs, age, and BMI criteria are randomized to receive an investigational drug or a comparator/placebo. The subjects are followed up for a period of time to assess the drug efficacy in reducing parameters of glycaemia (e.g. Fasting Plasma Glucose, Postprandial plasma glucose or HbA1c). The other parameters, which were commonly compared included hypoglycemia risk, weight gain, blood pressure, lipid profile and measures of beta cell function and insulin sensitivity.30
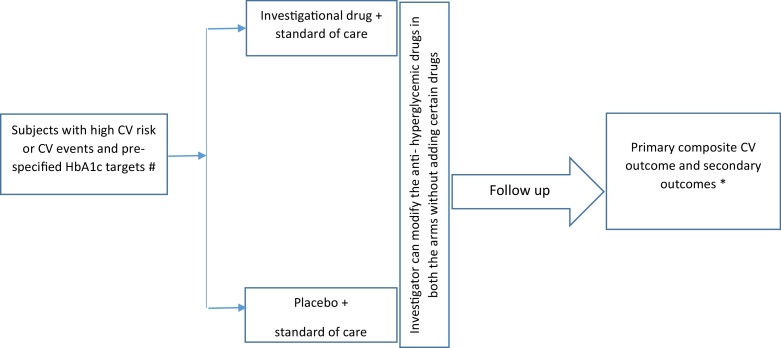
In contrast, CVSOT are trials designed to find out how a drug performs in comparison to standard care in terms of pre-defined cardiovascular end points. These studies randomize patients with diabetes and high cardiovascular event rates so as to accrue the required number of CV events in the limited time periods of the trial. In CVSOT, subjects with high cardiovascular risk or preexisting atherosclerotic vascular disease are randomized to 2 arms: one arm receives a standard of care and the other arm receives the investigational drug in addition to the standard of care. Investigators are blinded to the therapies and are allowed to titrate glycemic therapies in both the arms similarly except that the tested drug (or a drug, which acts by a similar mechanism) not be added during the course of trial. These trials are event driven i.e., the trial stops, when a certain number of events accumulate in the trial. It is expected that the glycemic control in both the arms of the trial are similar (glycemic equipoise) since it is left to the investigator to adjust the glycemic therapy in both the arms.12 (Fig. 1). CVSOT differs from trials of glycemic efficacy in a number of ways (Table 2).
Fig. 1.
Conduct of a cardiovascular outcome trial. # High CV risk features may be predefined in the trial (e.g. age >50 years, dyslipidemia, hypertension, albuminuria, smoking etc.) and CV events (previous ACS, CABG, previous PCI, carotid stenosis, peripheral vascular disease, heart failure). * The primary end point is a composite of first occurrence of non-fatal MI, non-fatal stroke or CV death. The secondary endpoints may be a hospitalization for ACS, urgent revascularization, heart failure, all-cause mortality or a combination of these.
Table 2.
Comparison of trials of glycemic efficacy and CVSOTs.
| Glycemic efficacy trial | CVSOT | |
|---|---|---|
| Objective | Efficacy of drug compared to placebo or comparator | Compare CV outcomes |
| Number of patients | 300–600 (based on sample size calculation) | In thousands |
| Duration | 26–104 weeks | Many years or event driven |
| Back ground glycemic therapies titration | Limited to rescue therapies and dose changes for hypoglycemia | More flexibility for investigator |
| Comparator | Placebo or active comparator | Usually placebo (exceptions are CAROLINA, DEVOTE and TOSCA.IT) |
| Inclusion/Exclusion | Mainly low risk patients or minimal CV risk | High CV risk factors (SAVOR TIMI 53), known atherosclerotic vascular disease (SAVOR TIMI 53, TECOS, EMPA REG OUTCOME), recent CV event (EXAMINE, ELIXA) |
| Primary outcomes | HbA1c and or FPG reduction | Composite of major adverse cardiovascular events (MACE) |
CAROLINA: Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Patients with Type 2 Diabetes. DEVOTE: A Trial Comparing Cardiovascular Safety of Insulin Degludec versus Insulin Glargine in Subjects with Type 2 Diabetes at High Risk of Cardiovascular Events. TOSCA.IT Thiazolidinediones or Sulfonylureas and Cardiovascular Accidents Intervention Trial.
These CVSOT define their outcomes as a combination of Major Adverse Cardiovascular Events (MACE). These are nonfatal myocardial infarction, nonfatal stroke and cardiovascular death. Other major cardiovascular events of interest are hospitalization for angina, hospitalization for heart failure, urgent revascularization for unstable angina and death from any cause. Most CVOT use a composite of MACE with or without other CV endpoints of interest as their primary outcome. E.g. SAVOR-TIMI 53, EMPA-REG OUTCOME and EXAMINE used a primary endpoint of composite MACE, TECOS used a composite of MACE and hospitalization for unstable angina as the primary outcome. Secondary outcomes may be a combination or individual components of these cardiovascular end points.10, 11, 12, 13
5. Do CVSOT reflect the true CV benefits of drugs?
The follow-up of UKPDS has shown the benefits of early intensive glycemic control on CV events in newly diagnosed subjects with type 2 diabetes.26 The Steno 2 study has shown the benefits of multifactorial intervention controlling glycaemia, lipids and blood pressure to strict targets in reducing both microvascular and macrovascular disease.3 This gives the evidence for better control of risk factors on incidence of CV disease in type 2 diabetes. The beneficial effect of AHA on the CV system is a function of improved glycemic control, effects on known CV risk factors (e.g. lipids, weight, and blood pressure) and other currently “unknown factors”. Taking out the effects of improved glycemic control by attaining glycemic equipoise in both the arms of the CVOT and achieving a high percentage of statin use, antiplatelet use and Renin–Angiotensin–Aldosterone system inhibitors in both the arms leaves only the “unknown “factors to play out in deciding the CV effects of the AHA. The subjects in these trials are older, having diabetes of longer duration and established CV disease or risk factors. This makes retarding progress of atherosclerotic process a rare possibility within the period of study. However these trials may demonstrate potential for harm (inferiority), lack of adverse CV outcome (non-inferiority) or cardiovascular benefits (superiority) by mechanisms other than interference with the atherosclerotic process.
The EMPA-REG OUTCOME, the CVOT of empagliflozin showed significant improvements in composite primary outcome (MACE), death from cardiovascular causes, hospitalization from heart failure and all-cause mortality. The possible mechanisms underlying these improvements are likely to be “multidimensional” and “speculative” as per the authors.13 The time course of primary outcome event reduction favors an effect of Empagliflozin on the volume status of the subjects.31
6. What important information can be got from CVSOT?
Most CVSOT are designed to prove non-inferiority and later superiority if the non-inferiority criteria are met.10, 13, 23 These trials establish the cardiovascular safety of investigational agents based on hard CV end points in subjects at high risk of CV events. Since these events are pre-specified and centrally adjudicated, there is uniformity of reporting and event capture across multiple sites across geographical areas. This is a major step compared to the pre-guidance era, where the CV events were pre-specified and recorded as serious adverse events but not centrally adjudicated. Central adjudication of end points has been found to improve reporting of events in some studies but not all.32, 33 This may apply even to hard end points like acute myocardial infarction.32 Pooling of data from phase 2/phase 3 trials, especially if they are not adjudicated centrally may give an entirely different picture from a CVOT as seen with Saxagliptin.10, 34
To capture the number of CV events to attain non-inferiority, large number of trial subjects are required. Even larger number of subjects are needed if the drug is trying to prove superiority.35, 36 The sheer number of subjects studied in these trials gives an opportunity to study rare adverse effects of these drugs. Beyond the CV outcomes, trials like TECOS, SAVOR-TIMI 53 and EXAMINE gave valuable insights into risk of pancreatitis, pancreatic cancer, renal outcomes, fracture risk and risk of various malignancies in the study population.11, 12 These outcomes of interest are pre specified and centrally adjudicated. Further study of exploratory end points in certain trials e.g. calcitonin levels (LEADER), NT-pro BNP (SAVOR-TIMI 53) and fractures (CANVAS) could give some mechanistic insight into complications that are of interest.10, 22, 23 From CVSOT we have come to realize that individual molecules may have off target actions, which may be different from actions specific to the class of molecules. E.g. increased risk of heart failure due to hospitalization was shown SAVOR-TIMI 53, but not in EXAMINE or TECOS.10, 12
Certain adverse effects may emerge only after significant patient years of exposure. This is possible with large patient numbers in CVSOT. The CVOT of dual PPAR αγ agonist, Aleglitazaar (AleCardio) involved high risk subjects with recent acute coronary event as the study population. The trial was stopped prematurely by the data monitoring committee due to safety issues associated with heart failure, gastrointestinal bleeds and weight gain, which was felt to outweigh the expected CV benefits the drug.37 Many of these serious adverse effects of Aleglitazaar were not seen in pooled trials of shorter duration.38 Casting a wider net of inclusion criteria to include elderly individuals, individuals with various stages of CKD and hepatic dysfunction, older women and multiethnic subjects would help identify more potential adverse outcomes.
7. What are the possible limitations in information gathered in CVSOT?
As alluded earlier, the true CV benefits of the drug (which is the function of glycemic lowering and effect on known risk factors) a may not be derived from CVSOT. The possible beneficial results of CVSOT cannot be applied to a population, where it has not been studied. E.g. the CV mortality reduction with subjects on empagliflozin in the EMPA-REG OUTCOME trial cannot possibly applied to a younger group of type 2 diabetes subjects with low CV risk.13 However, this could be the population, where the drug is probably going to be used more. Since CVSOT have strict entry criteria, they may not have a population similar to the spectrum of subjects in the community that would use the drug. Hence some adverse effects may surface only during the post marketing surveillance e.g. euglycemic diabetic ketoacidosis related to SGLT-2 inhibitors were identified in the post marketing program rather than in large CVSOT.39 CVSOT includes comparison of an investigational agent to standard care. However, standard of care may consist of therapies and combination of therapies with unproven CV track records.35 This may include agents like SU and Pioglitazone, which may have their own baggage of potential CV adverse effects. The potential of slight excess of these therapies in the arms may confound the results of these trials, which may not be measurable.40
A composite outcome is made up of 2 or more component outcomes. Any patient, who experiences any one of the outcomes is considered to have experienced the composite outcome. Using composite outcomes increases statistical efficiency because of higher event rates, reduces sample size, costs and time.41 Chosen carefully, composite outcomes will reflect a sum total of meaningful endpoints of interest, which may have a common pathophysiology or clinical implication. In CVSOT, the recommended primary outcome is a composite outcome of CV death, non-fatal MI and non-fatal ischemic stroke.13, 42 However, the treatment effect sizes may vary between the components of the composite outcome e.g. in EMPA-REG OUTCOME, the risk of cardiovascular death 0.62 (95% CI: 0.49–0.77) influenced the primary composite outcome (HR: 0.86 (95% CI: 0.74–0.99)) much more than the other 2 components: risk of non-fatal MI 0.87 (95% CI: 0.70–1.09) and non-fatal stroke 1.24 (95% CI: 0.92–1.67).13 Unless specifically explained, it is likely that readers will consider that the drug had similar effects on each of the individual outcomes.
Further, for certain findings of a trial, a potential mechanistic explanation may not be available and it may even conflict the current knowledge on the subject. E.g. the increased risk of heart failure in the Saxagliptin arm of SAVOR-TIMI despite a positive effect of GLP-1 on heart failure in animal models.43 Rapid reduction of CV mortality starting within the first year in subjects using empagliflozin in the EMPAREG OUTCOME trial, which conflicts with the known potential glycemic benefits and the known potential CV risk benefits of the drug.13 However, these would be an impetus to undertake smaller mechanistic studies to explore the findings of the trial.
8. What are the operational and technical challenges with CVSOT?
The cost of successfully bringing a drug to the market is tagged at 1.5 billion USD.44 The true cost of drug development including late stage failures are 4–5 billion USD and may go up to 12 billion USD.44 CVSOT are expensive aspects of drug development program and are likely to escalate the costs, which are passed to the end-consumer. These trials involve large number of subjects, investigators and sites across different countries. This would involve challenges in lab shipments, IVRS based randomization, different cost structures, temperature controlled transport of drugs, customs clearance across borders, translation of documents and variable ethics committee practices.45 This may lead to sponsors selectively choosing “trial friendly” countries and those countries, where regulatory requirements necessitate enrolling local population before approval of drug. There would be challenges in adhering to local regulatory practices and local practices of “standard of care”. Since these trials are of long duration, there will be challenges in patient retention, changes in investigator and staffing of trial sites and interpretation of missing data.14, 45 Ethnic variations may be present in efficacy and adverse effects to a drug in addition to challenges in weight based dosing (e.g. Asian patients may have lower body weight but may use the same dose as Caucasian population). There are challenges further in doing CV safety trials with insulin.46 Unlike other new AHA getting approved for diabetes, insulin is associated with hypoglycemia. Undertaking CVSOT with insulin will run challenges of increased risk of hypoglycemia, need to have treat-to-target models to ensure glycemic equipoise and challenges of blinding when using 2 different types of insulin. Further, there is an increased risk of CV events in subjects with severe hypoglycemia, which should be factored into the trial analysis.15
Event rate for primary endpoints is one of the factors, which determine sample size of a trial. Low observed event rate during the course of the trial may lead to increased recruitment of high-risk patients and these may adversely reflect on outcome of trials.42 One of the challenges would be to design trials to satisfy needs for different regulatory agencies. EMA requires sponsor to have phase 2/3 studies, which may last up to 18–24 months and include sufficient subjects with high CV risk, renal dysfunction and elderly. These data would be expected to be entered into a meta-analysis. The need for CVSOT will be decided if there is an adverse CV signal or unacceptable lack of precision.47 FDA has specified more definite criteria for conduct of CVSOT.7 The Japanese Pharmaceuticals and Medicines Development Agency does not require CVSOT for drug approval of AHA.45
Further, these financial resources could otherwise be used for more potential health research priorities. CVSOT in the current format would only help to identify a segment of subjects, where the drug has potential to be used safely. This would automatically be extrapolated to be safety in a population with low risk although the converse may not be true with regards to potential benefit. There is a concern that CVSOT, when conducted in a community will tend to recruit a large number of subjects. This would potentially limit the number of subjects could enter other drug developmental programs.
9. Can there be any improvements in conduct of CVSOT?
With some limitations, CVSOT can be considered a gold standard to establish the CV safety of AHA. There has been an outcry from both professionals and patient groups on escalating costs of antidiabetic drug development, where CVSOT are involved potentially draining resources, delaying drug development and approval.14, 24, 44, 48, 49 With a thought toward minimizing the cost of drug development without compromising patient safety, alternatives methods may need to be considered.
Using a larger bouquet of CV outcomes in addition to 3 point MACE (CV mortality, MI, and stroke) as primary composite end point would be one of these. This can include new onset angina, evidence of reversible ischemia, hospitalization for acute coronary syndrome, urgent revascularization procedures, heart failure hospitalizations, ECHO proven Regional Wall Motion Abnormalities, Left Ventricular End Diastolic Volume, asymptomatic carotid artery stenosis and peripheral vascular disease. However, increasing components of composite outcome may increase event rates leading to shorter trial duration, but reduce precision of study and mask treatment effects.50 This would also run a risk of underestimating the possible benefits of drug in reducing certain related outcomes.
Phase 2 and Phase 3 trials of antidiabetic drugs should include the use of CV biomarkers and in addition to hard end points. This can include but not limited to more established biomarkers such as QT intervals, heart rate, hsCRP, carotid-intima media thickness, lipids, and body weight. Newer biomarkers in the horizon such as fibrinogen, amyloid A, Pentraxin 3, homocysteine, and soluble CD40 ligand can also be considered.51 These trials should also look out for signs with potential association with CV risk like ECG abnormalities, edema, heart rate changes, weight gain, unfavorable lipid and blood pressure changes. Although individual parameters by themselves may not be decisive, combinations of biomarkers and clinical parameters may show trend toward benefit or harm. A conceptual developmental framework for CV safety assessment at various stages of drug development has been proposed.24 However, previous trials have shown that, where the disease process is multifactorial, favorable surrogate markers may not always translate into positive hard outcomes.37, 52, 53
High-risk subjects are chosen in CVSOT since their CV event rates are higher. But in a background of cardio protective therapies (statins, antiplatelet and antihypertensive), the event rates are still low necessitating larger patient population. If we were to identify patient characteristics (clinically, genomics or by metabolomics), more targeted therapy could be studied (precision medicine) in larger number of subjects in early phase studies.44 This could potentially reduce the need for CVSOT and late stage drug failures.44
Current format of CVSOT would achieve the aim of excluding cardiovascular toxicity during the course of study that would last few years. However, longer follow-up of these subjects would help understand off-target effects of the drug and its adverse effects including carcinogenicity. This may be resource intensive and may require a regulatory–sponsor financial and technical collaboration.
Since clinical trials are conducted outside the routine clinical care system, they involve extra staff, investigators and documentation, which contributes to massive cost. In an era of electronic health records, it would be possible to integrate clinical trial participation with routine clinical care. Electronic records would help subject identification for participation in trials, capture baseline data without duplication, identify concomitant medications and help follow events of interest.14 Various alternatives for CVSOT based on large observational data, electronic health records and Big Data” has been proposed by the Cardiac Safety Research Consortium.24 Various alternative trial designs like “factorial design “and “pragmatic design” has been proposed for CVSOT.8 Establishment of a strong pharmacovigilance program could help identify potential risk with AHA. Diabetes being a very prevalent disease, the number of subjects exposed to a newly introduced AHA within a short time would be potentially large. An integrated pharmacovigilance system based on electronic records and spread across different geographic areas, where the drug will help generate useful safety and efficacy data.
10. Conclusion
CVSOT can be considered as a gold standard for establishing the cardiovascular safety of anti-diabetic agents. These trials are not done to evaluate the glycemic efficacy of a drug but to understand if the drug increases the risk for cardiovascular outcomes. These trials are multicentric and involve significant logistic and operational challenges. However they serve to give valuable information beyond CV safety. A more cost effective approach to cardiovascular safety assessment of these agents balancing both costs and benefits would be the ideal way forward.
Conflicts of interest
The authors have none to declare.
Authors contribution
Mathew John (MJ), Ambika Gopalakrishnan Unnikrishnan (AGU), Sanjay Kalra (SK), Tiny Nair (TN).
Concept: MJ, AGU, SK.
Design: MJ, SK, TN.
Literature search: MJ, AGU, SK, TN.
Analysis of literature: MJ, AGU, SK, TN.
Initial preparation of manuscript: MJ, SK.
Editing: TN, SK.
Review of manuscript: MJ, AGU, SK, TN.
Final approval of manuscript: MJ, AGU, SK, TN.
All the authors, whose names are mentioned have contributed significantly to merit authorship of the manuscript. All the authors have read and approved the final copy of the manuscript.
Contributor Information
Mathew John, Email: drmathewjohn@yahoo.com.
Ambika Gopalakrishnan Unnikrishnan, Email: unnikrishnanag@gmail.com.
Sanjay Kalra, Email: brideknl@gmail.com.
Tiny Nair, Email: tinynair@gmail.com.
References
- 1.Fox C.S., Golden S.H., Anderson C. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777–1803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go A.S., Mozaffarian D., Roger V.L. Executive summary: heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Gaede P., Lund-Andersen H., Parving H.-H. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 4.Rao A.D., Kuhadiya N., Reynolds K. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: a meta-analysis of observational studies. Diabetes Care. 2008;31:1672–1678. doi: 10.2337/dc08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Salsburg D.S. The UGDP study. JAMA. 1971;218:1704–1705. [PubMed] [Google Scholar]
- 7.US Food and Drug Administration Guidance for industry: Diabetes mellitus — Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes [Internet]. [cited 2015 Nov 22]; Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf.
- 8.Bethel M.A., Sourij H. Impact of FDA guidance for developing diabetes drugs on trial design: from policy to practice. Curr Cardiol Rep. 2012;14:59–69. doi: 10.1007/s11886-011-0229-7. [DOI] [PubMed] [Google Scholar]
- 9.Hiatt W.R., Kaul S., Smith R.J. The cardiovascular safety of diabetes drugs – insights from the rosiglitazone experience. N Engl J Med. 2013;369:1285–1287. doi: 10.1056/NEJMp1309610. [DOI] [PubMed] [Google Scholar]
- 10.Scirica B.M., Bhatt D.L., Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 11.White W.B., Cannon C.P., Heller S.R. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 12.Green J.B., Bethel M.A., Armstrong P.W. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 13.Zinman B., Wanner C., Lachin J.M. Empagliflozin cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 14.Holman R.R., Sourij H., Califf R.M. Cardiovascular outcome trials of glucose-lowering drugs or strategies in type 2 diabetes. Lancet Lond Engl. 2014;383:2008–2017. doi: 10.1016/S0140-6736(14)60794-7. [DOI] [PubMed] [Google Scholar]
- 15.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein H.C., Miller M.E. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duckworth W., Abraira C., Moritz T. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet Lond Engl. 1998;352:837–853. [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 19.ADVANCE Collaborative Group, Patel A., MacMahon S. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 20.Marx N., Rosenstock J., Kahn S.E. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA®) Diabetes Vasc Dis Res. 2015;12:164–174. doi: 10.1177/1479164115570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccaro O., Masulli M., Bonora E. The TOSCA.IT trial: a study designed to evaluate the effect of pioglitazone versus sulfonylureas on cardiovascular disease in type 2 diabetes. Diabetes Care. 2012;35:e82. doi: 10.2337/dc12-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marso S.P., Poulter N.R., Nissen S.E. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823–830. doi: 10.1016/j.ahj.2013.07.012. e5. [DOI] [PubMed] [Google Scholar]
- 23.Neal B., Perkovic V., de Zeeuw D. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) – a randomized placebo-controlled trial. Am Heart J. 2013;166:217–223. doi: 10.1016/j.ahj.2013.05.007. e11. [DOI] [PubMed] [Google Scholar]
- 24.Sager P.T., Seltzer J., Turner J.R. Cardiovascular Safety Outcome Trials: a meeting report from the Cardiac Safety Research Consortium. Am Heart J. 2015;169:486–495. doi: 10.1016/j.ahj.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet Lond Engl. 1998;352:854–865. [PubMed] [Google Scholar]
- 26.Holman R.R., Paul S.K., Bethel M.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 27.The Glucose Lowering In Non-diabetic hyperglycaemia Trial (GLINT) – Glucose lowering in those at risk of diabetes [Internet]. ISRCTN Registry. [cited 2015 Nov 22]; Available from: http://www.isrctn.com/ISRCTN34875079
- 28.Dormandy J.A., Charbonnel B., Eckland D.J.A. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 29.Home P.D., Pocock S.J., Beck-Nielsen H. Rosiglitazone evaluated for cardiovascular outcomes – an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 30.Aschner P., Kipnes M.S., Lunceford J.K. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo R., McMurray J. EMPA-REG – the diuretic hypothesis. J. Diabetes Complicat. 2015 doi: 10.1016/j.jdiacomp.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Mahaffey K.W., Harrington R.A., Akkerhuis M. Systematic adjudication of myocardial infarction end-points in an international clinical trial. Curr Control Trials Cardiovasc Med. 2001;2:180–186. doi: 10.1186/cvm-2-4-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granger C.B., Vogel V., Cummings S.R. Do we need to adjudicate major clinical events? Clin Trials. 2008;5:56–60. doi: 10.1177/1740774507087972. [DOI] [PubMed] [Google Scholar]
- 34.Frederich R., Alexander J.H., Fiedorek F.T. A systematic assessment of cardiovascular outcomes in the saxagliptin drug development program for type 2 diabetes. Postgrad Med. 2010;122:16–27. doi: 10.3810/pgm.2010.05.2138. [DOI] [PubMed] [Google Scholar]
- 35.Hirshberg B., Raz I. Impact of the U.S. Food and Drug Administration cardiovascular assessment requirements on the development of novel antidiabetes drugs. Diabetes Care. 2011;34:S101–S106. doi: 10.2337/dc11-s202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon V., Lincoff A.M. Cardiovascular safety evaluation in the development of new drugs for diabetes mellitus. Circulation. 2014;129:2705–2713. doi: 10.1161/CIRCULATIONAHA.113.008221. [DOI] [PubMed] [Google Scholar]
- 37.Lincoff A.M., Tardif J.C., Schwartz G.G. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311:1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 38.Henry R.R., Buse J.B., Wu H. Efficacy, safety and tolerability of aleglitazar in patients with type 2 diabetes: pooled findings from three randomized phase III trials. Diabetes Obes Metab. 2015;17:560–565. doi: 10.1111/dom.12455. [DOI] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood [Internet]. [cited 2015 Nov 21]; Available from: http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm.
- 40.Zhong J., Goud A., Rajagopalan S. Glycemia lowering and risk for heart failure: recent evidence from studies of dipeptidyl peptidase inhibition. Circ Heart Fail. 2015;8:819–825. doi: 10.1161/CIRCHEARTFAILURE.114.001967. [DOI] [PubMed] [Google Scholar]
- 41.Cordoba G., Schwartz L., Woloshin S. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920. doi: 10.1136/bmj.c3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scirica B.M., Bhatt D.L., Braunwald E. The design and rationale of the saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus-thrombolysis in myocardial infarction (SAVOR-TIMI) 53 study. Am Heart J. 2011;162:818–825. doi: 10.1016/j.ahj.2011.08.006. e6. [DOI] [PubMed] [Google Scholar]
- 43.Standl E., Schnell O. DPP-4 inhibitors and risk of heart failure EXAMINEd. Lancet. 2015;385:2022–2024. doi: 10.1016/S0140-6736(15)60037-X. [DOI] [PubMed] [Google Scholar]
- 44.Jackson N., Atar D., Borentain M. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv213. Jun 15. pii: ehv213. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Hirshberg B., Katz A. Cardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerations. Diabetes Care. 2013;36:S253–S258. doi: 10.2337/dcS13-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloomgarden Z., Grunberger G. Cardiovascular safety trials: be careful what you wish for. J Diabetes. 2014;6:1–3. doi: 10.1111/1753-0407.12092. [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment of diabetes mellitus [Internet]. [cited 2015 Nov 22]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/10/WC500115945.pdf.
- 48.Matthews D.R. Cardiovascular trials and diabetes treatments: a regulatory maze. Diabetes Vasc Dis Res. 2012;9:83–84. doi: 10.1177/1479164112441380. [DOI] [PubMed] [Google Scholar]
- 49.Close K. 2015. A Complicated Case of the Heart – FDA Meets on Heart Safety of Diabetes Drugs [Internet]. diaTribe 2015. [cited 2015 Nov 22] Available from: http://diatribe.org/complicated-case-heart-fda-meets-heart-safety-diabetes-drugs. [Google Scholar]
- 50.Bethel M.A., Holman R., Haffner S.M. Determining the most appropriate components for a composite clinical trial outcome. Am Heart J. 2008;156:633–640. doi: 10.1016/j.ahj.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Yayan J. Emerging families of biomarkers for coronary artery disease: inflammatory mediators. Vasc Health Risk Manag. 2013;9:435–456. doi: 10.2147/VHRM.S45704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barter P.J., Caulfield M., Eriksson M. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 53.Caveney E.J., Cohen O.J. Diabetes biomarkers. J Diabetes Sci Technol. 2011;5:192–197. doi: 10.1177/193229681100500127. [DOI] [PMC free article] [PubMed] [Google Scholar]