Abstract
Percutaneous coronary intervention (PCI) is effective in opening the infarct related artery and restoring thrombolysis in myocardial infarction flow 3 (TIMI-flow 3) in large majority of ST-elevation myocardial infarction (STEMI). However there remain a small but significant proportion of patients, who continue to manifest diminished myocardial reperfusion despite successful opening of the obstructed epicardial artery. This phenomenon is called no-reflow. Clinically it manifests with recurrence of chest pain and dyspnea and may progress to cardiogenic shock, cardiac arrest, serious arrhythmias and acute heart failure. No reflow is regarded as independent predictor of death or recurrent myocardial infarction. No reflow is a multi-factorial phenomenon. However micro embolization of atherothrombotic debris during PCI remains the principal mechanism responsible for microvascular obstruction. This review summarizes the pathogenesis, diagnostic methods and the results of various recent randomized trials and studies on the prevention and management of no-reflow.
Abbreviations: CFR, coronary flow reserve; CFV, coronary flow velocity; CMRI, cardiac magnetic resonance imaging; CTFC, corrected TIMI frame count; ICCU, intensive coronary care unit; IMH, intra myocardial/mural hemorrhage; IPC, ischemic pre-conditioning; IRA, infarct related artery; IS, infarct size; IVUS, intravascular ultrasound; LV, left ventricle; MACE, major adverse cardiovascular event; MBG, myocardial blush grade; MCE, myocardial contrast echocardiography; MPV, mean platelet volume; MVO, microvascular obstruction; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; PET, positron emission tomography; PLR, platelet lymphocyte ratio; PPCI, primary percutaneous coronary intervention; ROC, receiver operating characteristics/curve; STEMI, ST-elevation myocardial infarction; STR, ST-segment resolution; TIMI, thrombolysis in myocardial infarction; TMPG, TIMI myocardial perfusion grade
Keywords: Primary percutaneous intervention, ST-elevation myocardial infarction, Thrombus aspiration
1. Introduction
Primary percutaneous coronary intervention (PPCI) is the gold standard of treatment of ST segment elevation myocardial infarction (STEMI).1 PPCI restores thrombolysis in myocardial infarction flow 3 (TIMI 3) in over 90% of patients. However there remain a small proportion of patients, who continue to exhibit overt impairment of myocardial reperfusion despite successful opening of infarct related epicardial artery (IRA). This phenomenon is called no-reflow, which is largely because of severe microvascular obstruction (MVO). Sudden loss of epicardial flow following ballooning or stenting may also occur in incomplete lesion dilation, epicardial vascular spasm, epicardial dissection or in situ thrombosis. These procedural failures are totally different clinical events and need careful exclusion. No reflow in human has a negative effect on the clinical outcome negating the potential benefit of PPCI in STEMI.2, 3, 4 Indeed no reflow is regarded as an independent predictor of death and myocardial infarction.5, 6, 7
No reflow may set in soon after completion of PCI (within 1–2 h). Recognition of no reflow is essential if it occurs in the catheterization laboratory (cath lab).8 Ideally the patient should not leave the cath lab unless no reflow has been satisfactorily managed. Clinically no reflow may present with the recurrence of chest pain, cardiogenic shock with hypotension, malignant arrhythmias or acute dyspnea due to pulmonary edema secondary to heart failure. No reflow is a progressive phenomenon and its presentation may be delayed. Angiographic no reflow after PCI is associated with reduced myocardial salvage, larger infarct size and increased long term 5 year mortality.9 Early detection, preventive measures and treatment of no reflow may alter the final outcome of PCI.
2. Classification
Galiuto10 proposed a pathological classification of no reflow, which is defined as inability to reperfuse a region of myocardium under prolonged ischemia despite re-opening of infarct related artery (IRA). The classification is based upon pathophysiology and therapeutic options of no reflow.
2.1. Structural no reflow
Microvessels within the necrotic myocardium region under prolonged ischemia exhibit (a) damage and loss of capillary integrity with endothelial swelling and odema and (b) microvascular obstruction. Structural no reflow is largely irreversible. The extent of lesion depends upon the severity and duration of ischemia.
2.2. Functional no reflow
Patency of microvasculature is compromised due to spasm, microthrombotic embolization and reperfusion injury, with accumulation of neutrophils and platelets with activation of neurohumoral system. Functional no reflow may be reversible to a varying degree.
3. Incidence
No reflow is an under-reported complication. A low incidence of 1–3% has been recorded in large registries based on TIMI flow grade, myocardial blush grade and ST resolution.11 Modern more sensitive methods of assessing no reflow and microcirculatory dysfunction include myocardial contrast echocardiography (MCE) and cardiac magnetic resonance imaging (CMRI), which have recorded a higher incidence (10–30%). Fortunately no reflow may resolve in due course of time in as many as 50% patients.10 However, the immediate prognosis of no reflow often remains uncertain and grave especially in cath lab.
4. Pathogenesis
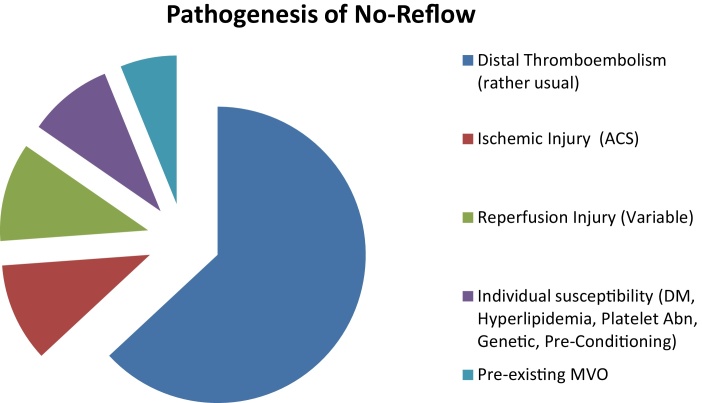
The goal of reperfusion therapy by percutaneous coronary intervention in acute myocardial infarction is to restore optimal blood flow in the infarct related artery (IRA) in order to ensure adequate blood supply to the ischemic but yet viable myocardium and to reduce infarct size and mortality. In no reflow, myocardial reperfusion is not achieved inspite of patent IRA. No reflow is a multifactorial phenomenon and five mechanisms have been recognized (Niccoli et al.)12: (i) pre-existing microvascular dysfunction, (ii) distal micro-thrombo-embolization, (iii) ischemic injury, (iv) reperfusion injury and (v) individual susceptibility. All these factors are inter-related in a complex manner (Fig. 1).
Fig. 1.
Pathogenesis of no reflow.
ACS, acute coronary syndrome; DM, diabetes mellitus; MVO, microvascular obstruction.
4.1. Pre-existing microvascular dysfunction
MVO may be either structural or functional or both. MVO impairs coronary flow reserve (CFR) and increases the vulnerability of affected myocardium to the PCI induced injury. Thus the benefits of re-opening of the obstructed epicardial artery are greatly compromised by pre-existing microvascular dysfunction. Pre-existing MVO may be related to advancing age, abnormal insulin resistance and lipid metabolism (diabetes and hyperlipidemia), chronic inflammatory diseases and individual susceptibility.12 Endothelial dysfunction is regarded as an independent predictor of adverse cardiac events.13, 14, 15
4.2. Distal micro-thrombo-embolization
Micro-embolism during PCI is a predominant cause of no-reflow in humans. Micro-thrombo-emboli refer to thrombus debris or micro-material from fissured and ruptured atheromatous plaques from the infarct related artery (IRA) going downstream during balloon dilatation or stenting. Myocardial perfusion starts falling, when embolic microspheres block >50% of coronary capillaries (Niccoli et al.).12 During PCI, 0–25 microspheres may travel downstream without causing MVO. The number and size of micro-emboli may vary in different individuals. When the number >25–200 or the size of micro-emboli is >200 μm, it can cause severe MVO. Thrombus burden and plaque erosions can be assessed by intra-vascular ultrasound (IVUS) and optical coherence tomography (OCT).
4.3. Ischemic injury
Kloner et al.16 demonstrated that temporary ligation of coronary artery in dogs for a period of >90 min resulted in ischemic anatomical changes in the capillaries of ischemic zone. These were seen by electron microscopy, which revealed significant capillary damage in the form of swollen endothelial cells with intra-luminal protrusions and platelet and fibrin thrombi. These changes were followed by occurrence of endothelial gaps and loss of integrity of capillary wall with extravascular collection of erythrocytes.
4.4. Reperfusion injury
The purpose of reperfusion is to reverse the ill-effects of ischemia. However, when ischemia >3 h, reperfusion may aggravate endothelial injury. Reperfusion leads to massive infiltration of ischemia zone with neutrophils and platelets. Activated neutrophils produce potent vasoconstrictors and inflammatory mediators, which release oxygen free radicals from the mitochondria. These result in release of adhesion molecules and bio-active factors including nitric oxide, prostacyclins and endothelins.17, 18 Combined together the ischemia and reperfusion injuries favour intra-myocardial hemorrhage (IMH) which has been demonstrated by cardiac magnetic resonance imaging (CMRI) in up to 40% patients with no-reflow.19 IMH is closely related to infarct size (IS) and is a predictor of severe MVO and adverse clinical outcome.20
4.5. Individual susceptibility
This could be genetic and acquired.
-
(a)
Genetic factors may modulate adenosine-induced vasodilatation. Genetic variation and sex-specific allelic variant genes have been recently linked with microvascular dysfunction.21
-
(b)
Individual susceptibility is also influenced by ischemic preconditioning (IPC). Pre-infarction angina may be helpful in preventive MVO after PCI.22
-
(c)
Usual well known risk factors for atherosclerosis (e.g. diabetes, hyperlipidemia, hypertension, etc.) may also have a role in microvascular dysfunction. Exact mechanisms need further elucidation.
-
(d)
Baseline reactivity of inflammatory cells may modulate the severity of no reflow but a recent study failed to demonstrate any significant association between systemic inflammation and no reflow.23
5. Predictors of no reflow
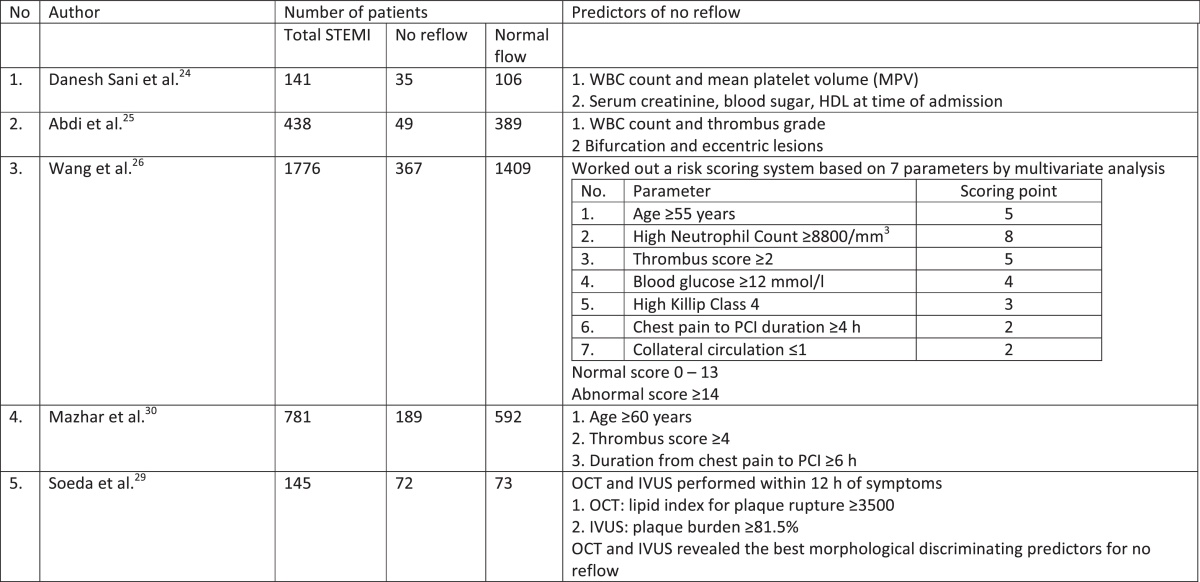
Several recent studies have investigated clinical predictors of no reflow in patients with STEMI treated with primary PCI. A few studies are summarized in Table 1. White blood cell (WBC) count, thrombus grade/score, age ≥60 years, mean platelet volume (MPV), duration between onset of chest pain and PCI (≥4 h), hyperglycaemia and raised serum creatinine were a few predictors.24, 25 Wang et al.26 in their study of 1776 STEMI patients worked out a risk scoring system based on a 7 parameters by multivariate analysis. Definite scoring number was given to each parameter e.g. high neutrophil count ≥8800/cm3 – 8 points; age ≥55 years – 5points; thrombus grade ≥2–5 points, high blood sugar ≥12 mmol/L – 4 points; prolonged chest pain before PCI ≥4 h – 2 points; high Killip Class IV – 3 points and collateral circulation ≤1–2 points respectively. All the variable points were added to build a final risk score of no reflow. Overall score for patients ranged from 0 to 29. The optimum cut off score was ≥14 (sensitivity 76% and specificity 70.8%). Receiver operating characteristics (ROC) analysis demonstrated a good risk prediction with area under curve static of 0.800 (95% confidence interval: 0.7772–0.826). The authors concluded that incidence of no reflow may be predicted with an acceptable accuracy with score higher than 14. Platelet lymphocyte ratio (PLR) on admission was a strong predictor of no reflow in a recent study of Topark et al.27
Table 1.
Predictors of no reflow.
STEMI, ST elevation myocardial infarction; WBC, white blood count; HDL, high density cholesterol; OCT, optical coherence tomography; IVUS, intravascular ultrasound.
In a recent publication on the morphological predictors for no reflow after PPCI in STEMI patients, Soeda et al.28, 29 studied 145 patients with STEMI who underwent optical coherence tomography (OCT) and intravascular ultrasound (IVUS) within 12 h of symptoms onset. The authors identified 72 patients, who had plaque rupture as the cause for STEMI. These 72 patients comprised of 28 patients with no reflow (38%) and 44 patients (62%) without no reflow. Receiver operating curve (ROC) analysis revealed OCT derived lipid index <3500 (area under curve 0.77, p < 0.001) and IVUS derived plaque burden >81.5% (area under curve 0.70, p = 0.002) were the best morphological discriminators for no reflow. Mazhar et al.30 studied PCI data base of the Canberra Hospital, Australia and identified 781 STEMI patients, who underwent PPCI and observed 189 patients with no reflow. By multivariate analysis to determine the predicting factors of no reflow, the authors found that age ≥60 years, thrombus score ≥4 and duration between chest pain and PCI > 360 min were the principal predictors of no reflow. No reflow was also associated with a higher mortality at 12 months follow-up.
6. Diagnostic methods
6.1. Coronary angiography
This is the simplest method to diagnose no reflow in the cath lab. After successful PCI, the dye flows instantaneously into the infarct related artery (IRA). Many simple and complex angiographic algorithms have been described including thrombolysis in myocardial infarction (TIMI) flow, corrected TIMI frame count (CTFC), TIMI myocardial perfusion grade (TMPG) and myocardial blush grade (MBG).31, 32, 33 However, in practice, TIMI flow grade and MBG are most commonly used. TIMI flow refers to the intensity and extent of visualization of IRA and the speed of flow of dye. TIMI flow is graded 0–3. MBG refers to the intensity of radio-opacity of the myocardial tissue and the speed, with which the enhancement clears. The filling appears as a myocardial blush, a ground glass appearance of the myocardium on the coronary angiogram. MBG is also graded as 0–3. TMPG is used to characterize the filling and clearance of the myocardial perfusion. The filling appears as myocardial blush (or ground glass appearance of the myocardium). TMPG defines the intensity of the blush and then focuses on the clearance of the contrast opacity from the myocardium. TMPG is graded 0–3.31
Thus TIMI flow grading and CTFC evaluate the epicardial flow, while MBG and TMPG evaluate the microvascular flow. TIMI flow grading and MBG are used together to define angiographic no reflow as TIMI flow <3 (with any MBG grade) or TIMI flow 3 with MBG 0–1.22 Successful reperfusion is TIMI flow 3 with MBG 2/3.
6.2. Electrocardiography (ECG) (ST segment resolution-STR)
Study of STR in serial ECG is a bedside method of assessing myocardial perfusion following PCI. A rapid decrease of ST elevation is highly suggestive of reperfusion. STR at 60 min after PCI should exceed 70%. STR <70% at 60 min is a marker of no reflow.34, 35, 36 A rapid decrease of ST segment is highly specific (91%) and fairly sensitive (77%) parameter of myocardial reperfusion.
6.3. Intracoronary guidewires
-
(i)
Intracoronary Doppler guidewire is used to measure coronary flow velocity (CFV) and coronary flow reserve (CFR). These parameters are regarded as standard methods for assessing micro vascular function. Three characteristic components are seen in no reflow37: (a) systolic flow reversal, (b) reduced systolic antegrade flow and (c) forward diastolic flow with rapid deceleration slope. Coronary blood flow velocity patterns of no reflow are caused by severe capillary damage associated with increased capillary resistance.38, 39 Doppler guidewire can detect showers of micro-emboli and help in calculation of micro-circulatory index.
-
(ii)
Intracoronary pressure measurement: a double lumen catheter with a side hole is employed to measure intracoronary pressure gradient in IRA. Absence of pressure gradient indicates absence of obstruction in IRA. By using pressure/thermistor guidewire placed distally in IRA, index of micro-circulatory resistance (IMR) can be calculated which is related to acute microvascular damage in no reflow.
6.4. Myocardial contrast echocardiography (MCE)
MCE has been regarded as one of the best methods to predict no reflow.40, 41, 42, 43 MCE is performed by injecting intravenously an ultrasound contrast agent containing small microbubbles and intramyocardial contrast opacification is visualized and recorded. Absence of opacification detects no reflow/dysfunctional microvascular circulation. MCE is best recorded after 24–48 h after PPCI since MCE performed immediately after PPCI may under estimate the size and extent of no reflow.
6.5. Coronary magnetic resonance imaging (CMRI)
CMRI is regarded as the most sensitive and specific method to assess the extent of no reflow. The ideal time to get the highest predictive value of MRI is 1 week after myocardial infarction although it can be done at 48–72 h after PCI. This a non-invasive Gadolinium based technique performed in two steps (i) Early CMRI is performed soon after injecting gadolinium and recorded during the first pass of the contrast agent and (ii) late or delayed contrast enhanced MRI performed 10–20 min after the injection of contrast. The early phase represent no reflow, while the delayed phase depicts the extent of myocardial necrosis. CMRI is utilized to assess (a) infarct size (IS) and (b) intramural hemorrhage (IMH).44
7. Prognostic implications of no reflow
No reflow portends a poor short term and long-term prognosis in humans. The immediate clinical course in the hospital following no reflow may be complicated with malignant arrhythmias, re-infarction, cardiac rupture (in form of ventricular septal defect or rupture of valve structure causing severe mitral regurgitation or even left ventricle wall rupture) and pump failure.5 There is an increased risk of death at 30 days with a relative risk (RR) of 2.1 (p < 0.038). The effects of no reflow on left ventricle remodelling are equally adverse. The end diastolic volume of left ventricle starts increasing after no reflow and eventually results in LV dilation and reduced left ventricle (LV) ejection fraction (EF) and heart failure. LV remodelling is almost negligible in the absence of no reflow. Five-year hospitalization and mortality is more common in no reflow patients.6, 9
Ndreppa et al.9 studied the infarct size (IS) and mortality in 1406 STEMI patients, who underwent PPCI, which was followed by no reflow in 410 and normal reflow in 996 patients. The infarct size was measured by single photon emission computed tomography (PET). The patients were followed with the aim of finding primary outcome of 5-year mortality. The infarct size was 15% (6–29%) of LV in no reflow group vs 8.0% (2–21%) of LV in normal flow group (p < 0.001). There were 59 deaths (14.3%) in no reflow group vs 73 deaths (7.3%) in normal reflow group. The Kaplan–Meier estimates of 5-year mortality were 18.2% in no reflow group vs 9.5% in normal reflow group (p ≤ 0.001). The authors concluded that no reflow was a strong predictor of 5-year mortality.
In a recent meta-analysis of 10 studies out of 134 publications between January 2004 and April 2012, van Kranenbrug et al.45 concluded that microvascular obstruction is responsible for no reflow, which is an independent predictor of MACE and cardiac death at 2 years.
8. Management of no reflow
Optimal therapeutic strategies for the management of no-reflow depend on the extent of structural and functional damage in any individual patient.10 In structural no reflow, the integrity of microvessels is irreversibly damaged, while in functional no reflow, the microvascular obstruction is characterized by a dynamic time course and is potentially reversible. The therapeutic approach should depend upon the prevailing mechanism of functional no reflow. However, therapeutic drug may fail to reach the target lesion due to prevailing obstruction. Hence prevention of no reflow is far better than treatment. In humans, distal athero-thrombo-embolization has been recognized as the predominant mechanism for no reflow. The various strategies to tackle thromboembolism are (i) manual thrombus aspiration, (ii) mechanical thrombectomy, (iii) direct stenting without prior ballooning and often combined with prior thrombus aspiration, (iv) embolic protection devices and (v) intracoronary abciximab. Ischemic (and reperfusion) injuries play a devastating role in some patients. To avoid ischemic injuries, the patient is to be prepared for PPCI at the earliest (preferably within 40–90 min). Prior to PPCI, full dose of aspirin (325 mg), atorvastatin (80 mg) and antiplatelet therapy (prasugrel 60 mg or ticagrelor 180 mg or clopidogrel 600 mg) must be given. Newer P2Y12 receptor blockers (prasugrel and ticagrelor) are more powerful antiplatelet agents and are preferred over clopidogrel in absence of any contrainidication. Attempts should be made to stabilize and control the prevailing risk factors (e.g. hyperglycemia, arrhythmia, severe hypotension or acute heart failure).
9. Thrombus aspiration
Manual aspiration and mechanical thrombectomy have been used successfully prior to stenting during PPCI in STEMI patients. Various trials are summarized in Table 2.
Table 2.
Randomized thrombectomy trials for the prevention of reperfusion no reflow.
| (A) Manual thrombus aspiration | |||
|---|---|---|---|
| Trial | Author | Number of patients | Results |
| 1. REMEDIA | Burzotta et al.46 | 99 | Improved MBG and ST resolution |
| 2. TAPAS | Svilass et al.47 Vlaar et al.48 |
1071 | Improved MBG and reduction in 30 days mortality Reduced mortality at 1 year |
| 3. PIHRATE | Dudek et al.49 | 196 | Aspiration thrombectomy and direct stenting is safe and effective and results in better angiographic results with no difference in 6 months mortality |
| 4. TASTE | Frobert et al.50 | 11,709 | No reduction in 30 days mortality, ST or recurrent MI |
| 5. TOTAL | Jolly et al.51 | 10,732 | No reduction in mortality at 6 months, increased stroke at 30 days |
| (B) Mechanical thrombectomy | |||
|---|---|---|---|
| Trial | Author | Number of patients | Results |
| 1. JET stent study | Migliorini et al.52 | – | Improved STR Reduced MACE at 1 year |
| 2. X-sizer (Ev 3) | Lefevre et al.53 | 201 | Improved ST resolution |
MBG, myocardial blush grade. MI, myocardial infarction; STR, ST resolution; MACE, major cardiovascular event.
9.1. Manual aspiration trials
-
1.
REMEDIA (Randomized Evaluation of the Effect of Mechanical reduction of distal Embolization by Thrombus Aspiration) Study was conducted on 90 patients of STEMI presenting within 12 h of chest pain. Thrombus aspiration by Pronto (vascular solutions) improved MBG and STR.46
-
2.
TAPAS Trial (Thrombus aspiration during primary coronary Interventions)47: 535 STEMI patients underwent thrombus aspiration by Export (Medtronic vascular) prior to PCI while 536 underwent conventional PCI without thrombus aspiration. Assessment of MBG grading showed that thrombus aspiration resulted in better reperfusion vs PCI alone (p < 0.001). One year follow-up of TAPAS trial showed reduced mortality with better ejection fraction in thrombus aspiration group.48
-
3.
PIHRATE Trial (Polish Italian Hungarian Randomized Thrombectomy Trial): 196 STEMI <6 h underwent either thrombus aspiration followed by direct stenting (n = 100) or balloon dilatation and stenting (n = 96). Angiographic parameters of microvascular reperfusion assessed by MBG grading were superior in the thrombus aspiration group vs standard ballooning followed by stenting (p < 0.001).49 Thrombus aspiration followed by direct stenting was advised in this study.
-
4.
TASTE Trial (Thrombus Aspiration during ST-segment Elevation myocardial infarction): It was an open label prospective trial at 29 PCI centres in Sweden (11,709 patients) and one center each in Iceland and Denmark (247 patients). Out of these 7244 patients were randomized for either thrombus aspiration before PCI (n-3621) or conventional PCI (n-3623). Trial failed to demonstrate any difference in mortality, recurrent myocardial infarction and stent thrombosis at 30 days in the two groups.50
-
5.
TOTAL Trial51: 10,732 STEMI patients undergoing PCI were randomly assigned to either upfront manual thrombus aspiration (n-5033) or PCI alone (n-5030). Trial showed no statistical difference in the primary outcome of recurrent MI, heart failure, stent thrombosis or mortality between the two groups. However, there was an increase in the incidence of stroke at 48 h and 30 days in the thrombus aspiration group with high mortality (30.8%) within stroke patients within 180 days.
9.2. Mechanical thrombectomy
-
1.
ANGIOJET rheolytic thrombectomy
It involved delivery of pressured heparin saline (in the coronaries), which travels backwards creating a low pressure zone with powerful vacuum effect, drawing the thrombus into the intra-coronary catheter. The recent randomized Jet Stent Trial52 showed improvement in STR in the treated group, followed by reduced MACE on 1-year follow-up. Angiojet procedure is, however, complex and routine rhelotytic thrombectomy prior to PCI is not beneficial.
-
2.
X-SIZER (EV-3 Plymouth): X-AMINE TRIAL
Use of X-sizer device in X-AMINE Trial reduced the distal embolization and improved ST resolution.53 However MBG and 6 months MACE and mortality did not show any benefit.
9.3. Infuse AMI trial (intracoronary bolus abciximab)54
In a randomized trial, 452 patients due to occlusion of left anterior descending artery (LAD) with large anterior wall myocardial infarction were studied at 37 sites in 6 countries, presenting within 4 h of STEMI. The patients were divided into 2 groups: (i) thrombectomy group (n-174) vs no thrombectomy group (n-179) and (ii) abciximab group (n-181) vs no abciximab group (n-172). Abciximab was administered by an intracoronary catheter (Clearway) at the site of lesion/infarct. The end point was the measurement of infarct size (IS) assessed by cardiac MRI at 30 days. There was a significant reduction of IS in abciximab group and not in thrombectomy group. Infuse AMI Trial was further analyzed comparing IS with successful MBG (2/3) vs those with unsuccessful MBG (0–1). Abciximab reduced infarct size (IS) by 30% in patients with successful MBG (2/3) and reduced mortality with improvement in left ventricular ejection fraction (EF) at 30 days (Brenner et al.).55
10. Meta-analysis studies
Various randomized trials on thrombus aspiration, mechanical thrombectomy and embolic protection trials have undergone meta-analysis by various authors. The results of these trials are at variance. These trials are summarized in Table 3. Following conclusions were drawn from these meta-analyses.
-
1.
Manual thrombus aspiration is simpler and superior to mechanical thrombectomy and is associated with reduced MACE at 6–12 months.56, 57, 62
-
2.
Manual thrombus aspiration may be beneficial in improving MBG and TIMI flow 3 but was not associated with reduced MACE and mortality at 6–12 months.58, 59, 60, 61
-
3.
Two recent meta-analysis by Jolly et al.63 and Elgendy et al.64 have raised doubt about the beneficial effect of thrombectomy on mortality. There is an additional risk of stroke.
Table 3.
Meta-analysis of randomized studies on thrombectomy vs PCI alone.
| Author | Number of trials analyzed | Number of patients | Follow-up period | Results |
|---|---|---|---|---|
| 1. Bavry et al.56 | 30 | 6415 | 6 months | Aspiration thrombectomy reduced mortality while mechanical thrombectomy and distal embolic protection did not reduce mortality |
| 2. Burzotta et al.57 | 11 | 2686 | 1 year | Manual thrombectomy improved 1 year survival. Additional benefit when patients treated with IIb/IIIa antagonist |
| 3. Mongeon et al.58 | 21 | 4299 | 30 days | Mortality up to 30 days not reduced although ST resolution was better No reduction in recurrent MI/stroke |
| 4. Tamhane et al.59 | 8 – manual device (Export, Pronto, Diver) 5 – mechanical device (Angiojet, X-Sizer) 4 – vacuum (Rescue TVAC) |
3909 | 30 days | 30 days mortality not reduced although MBG and TIMI flow 3 improved Stroke incidence increased in thrombectomy (14/1403) vs PCI alone (3/1413) |
| 5. De Luca et al.60 | 21 | 4514 | 30 days | Manual thrombectomy improved ST resolution but did not reduce mortality Higher risk of stroke in thrombectomy patients within 30 days |
| 6. Jolly et al.63 | 20 10 |
21,173 19,585 |
180 days 180 days |
Mortality 3.8% in thrombectomy group vs 4.3% in PCI only group Stroke in thrombectomy group was 0.8% vs 0.5% in PCI only group |
| 7. Elgendy et al.64 | 17 | 20,960 | – | (1) No significant reduction in death, re-infarction with routine aspiration thrombectomy (2) Additional risk of stroke |
| 8. Kumbhani et al.62 | 18 – manual aspiration 7 – mechanical thrombectomy |
3936 1598 Total 5534 |
12 months | Manual aspiration but not mechanical thrombectomy beneficial in reducing MACE and mortality at 6–12 months |
| 9. Deng et al.61 | 26 | 11,780 | 24 months | No evidence of definite benefit by manual aspiration although MACE was less frequent |
MI, myocardial infarction; MBG, myocardial blush grade; TIMI, thrombolysis in myocardial infarction; PCI, percutaneous coronary intervention.
11. Guidelines on STEMI and myocardial reperfusion
The guidelines on management of STEMI and myocardial reperfusion have been issued by European Society of Cardiology (ESC) (2012)65 and European Association of cardio-thoracic surgery (EACTS) (2014).66 The guidelines have also been issued by American College of Cardiology (ACC), American Heart Association (AHA) and Society of Cardiovascular Interventions (SCAI) (2011, 2013).1, 67 However, keeping in mind the newer information and observations of TASTE, TOTAL and INFUSE AMI trials and recent meta-analysis on thrombectomy by Jolly et al. and Elgendy et al., ACC/AHA/SCAI have recently modified their earlier recommendation as given below (Levine et al.).68
| 2011/2013 recommendation | 2015 focused update Recommendations |
Comments |
|---|---|---|
|
Class IIa Manual aspiration thrombectomy is reasonable for patients undergoing primary PCI (Level of Evidence: B) |
Class IIb The usefulness of selective and bailout aspiration thrombectomy in patients undergoing primary PCI is not well established (Level of Evidence: C-LD) |
Modified recommendation (Class changed from “IIa” to “IIb” for selective and bailout aspiration thrombectomy before PCI) |
|
Class III: No Benefit Routine aspiration thrombectomy before primary PCI is not useful (Level of Evidence: A) |
New recommendation (“Class III: No Benefit” added for routine aspiration thrombectomy before PCI) |
PCI, percutaneous coronary intervention; LD, limited data.
12. Direct stenting
The dilatation of coronary artery prior to stenting may result in dislodgement of atheromatous debris from thrombus and cause distal embolization and no reflow. Direct stenting without prior balloon dilatation has been advised to reduce the incidence of no reflow. However, direct stenting without prior ballooning may be difficult. More over the results of direct stenting have been controversial.
Dudek et al.49 have advocated direct stenting following thrombus aspiration (PIHRATE Trial) resulting in better microvascular perfusion assessed by MBG.
13. Deferred stenting
Deferred stenting in selected high risk patients may reduce the incidence of no reflow.69 During the deferred period of 6–12 h, the patient is treated in intensive coronary care unit (ICCU) and receives supportive therapy. In a recent randomized study of 411 STEMI patients, the results of deferred stenting were compared with immediate stenting.70 The authors concluded that in high risk STEMI patients deferred stenting reduced incidence of no reflow as judged by TIMI flow grade and cardiac MRI.
14. M-guard stent
M-guard stent is a new technology. The M-guard stent design is built to prevent distal embolization and no reflow following PCI. It consists of a bare metal stent with cobalt chromium strut with polyethylene theraphthalate mesh (micronet) covering anchored to the external surface of the strut. M-guard has undergone three non-randomized and two randomized trials. The results are summarized in Table 4. Piscione Trial71 was a multicentric Italian prospective registry of 100 patients. Full revascularization was achieved in 90% patients with a MACE of 7.9% and mortality of 7%. MAGICAL trial72 enrolled 60 patients with almost similar results. REWARD MI trial73 is a recent retrospective study of 150 patients with good results. The randomized MASTER I Trial involved 433 patients.74, 75 M-guard stenting was particularly beneficial in STEMI patients with large thrombus burden, restoring myocardial reperfusion and reducing no-reflow. However, M-guard stent has exhibited three drawbacks: (i) frequent instent restenosis due to bare metal strut, (ii) inability to reach or cross the lesion (4.1%) and (iii) rarely dislodgement of stent during attempted withdrawal due to failure to cross (0.9%). There is not data for side branch occlusion, though there is a theoretical risk due to stent design. In view of above the manufacturers decided to cancel the MASTER II Trial and recall the M-guard stents. The manufacturers are now trying to develop a new drug eluting version of M-guard stent.76
Table 4.
Trials on M-guard stent.
| Name of trial/author | Number of patient | FU | STR > 70% | MBG 3 | Side effects |
|---|---|---|---|---|---|
| Non-randomized trial | |||||
| 1. Piscione et al.71 | 100 | 24 months | 90% | 90% | MACE – 7.9% |
| 2. MAGICAL (Dudek et al.)72 | 60 | 36 months | 61% | 90% | Mortality – 7% |
| 3. REWARD MI (Fernandez Gisnal)73 | 150 | 10 months | 86% | 74% | – |
| Randomized trials | |||||
| 1. MASTER I (Stone et al.)74 (Costa et al.)75 |
433 | 12 months | 57.8% | 74% | Stent dislodgement (0.9%) Instent restenosis |
| 2. MASTER II (Gracida et al.)76 | Designed for 1114 patients but trial stopped in middle | 30 days | 56.9% | – | M-guard stent withdrawn in 2015 |
15. Pharmacological treatment of no reflow
Pharmacological drug management has been the sheet anchor of modern therapy utilized by interventional cardiologist in the cath lab to manage no reflow in acute setting. Drugs are administered by intracoronary route and delivered directly into the IRA by guiding catheter or through infusion catheters, since the drugs given intravenously may not reach the target site due to microvascular obstruction in no reflow. Distal infusion catheter (Clear way Medical Corporation, USA) and Multifunction Probe catheter (Boston Scientific, MA) are the two most suitable catheters for drug administration at the target site.77 Pharmacological strategies are summarized in Table 5.
Table 5.
Pharmacologic strategies for no reflow.
| Drug | Route | Dose | Side effects | |
|---|---|---|---|---|
| 1. | Adenosine78, 79, 80, 81, 82, 83, 84, 85, 86 | IC, IV | IV: 70 μg/kg/min × 3 h IC: 48–200 μg bolus |
Transient heart block, hypotension, bronchospasm |
| 2. | Nicorandil93, 94, 95, 96 | IC, IV | IV: 8 mg/h infusion IC: 2 mg bolus |
– |
| 3. | Sodium nitroprusside97, 98, 99, 100 | IC | 50–200 μg bolus | Hypotension |
| 4. | Calcium channel drugs87, 88, 89, 90, 91, 92 | |||
| (a) Verapamil | IC | 100–250 μg bolus or 100 μg/min up to 1000 μg | Hypotension, heart block | |
| (b) Diltiazem | IC | 400 μg | –do– | |
| (c) Nicardipine | IC | 50–200 μg bolus (upto 500 μg) | –do– | |
| 5. | Abciximab101, 104, 105 Eptifibatide103 Tirofiban102 |
IC IV IV IV |
IC: 0.25 mg/kg bolus IV: 0.25 mg/kg bolus followed 0.125 mg/kg/h 2 μg/kg/min IV: 10 μg/kg |
Bleeding |
| 6. | Epinephrine122 | IC | 50–200 μg | Arrhythmias |
16. Adenosine
Adenosine is a potent vasodilator. It inhibits neutrophil adhesion and migration and reduces formation of oxygen free radicals. Adenosine was administered intravenously in AMISTAD I (Acute Myocardial Infarct STudy of ADenosine) as infusion of 70 μg/kg/min for 3 h in 236 STEMI patients in a randomized fashion.78 Adenosine reduced the infarct size (IS) measured by SPECT in 33%. In AMISTAD II study, adenosine was administered as IV infusion (50 μg/kg/min or 70 μg/kg/min) in a randomized study of 2118 patients.79 The infusion was started 15 min before PCI and continued for 3 h. The study confirmed the reduction of infarct size (IS) but follow-up results on MACE and mortality were inconclusive.
Adenosine has also been administered by intracoronary (IC) route (dose 24–48 μg as bolus) in no reflow patients and found effective.80 REOPEN TRIAL evaluated intracoronary adenosine or nitroprusside.After thrombus aspiration in 240 STEMI patients during PCI with upstream infusion of GpIIb/IIIa.81 The patients were randomly divided into 3 groups: (i) adenosine group (n = 80) given IC 120 μg bolus followed by 2 mg in 33 ml saline slow infusion over 2 min, (ii) nitroprusside group (n = 80) given 60 μg bolus followed by 100 μg in 33 ml 5% glucose slow infusion over 2 min and (iii) placebo group (n = 80) in which 33 ml saline was given over 2 min. STR >70% at 90 min was found in 71% adenosine vs 54% in nitroprusside vs 51% in saline group. 30 days MACE (heart failure and recurrent MI) was 10% in adenosine group vs 14% in nitroprusside group and 20% in saline group. The authors concluded that adenosine was more beneficial than nitroprusside.
More recently 46 STEMI patients, who had developed no reflow following PCI received IV Tirofiban and then randomized into three groups: adenosine (n = 16), verapamil (n = 15) and placebo (n = 15) depending upon IC drug administered.82 In this trial adenosine was not found beneficial, while verapamil was effective. REFLOW STEMI is an ongoing trial to compare the effects of adenosine, nitroprusside and placebo. Primary end point is the measurement of infarct size at the end of 48–72 h by CMRI. The results are awaited.
Su et al.83 made an updated search of 11 RCTs involving 1027 STEMI patients. 10 RCTs evaluated adenosine only. There was no evidence that adenosine reduces short term or long term all cause mortality or recurrent myocardial infarction. Adverse effects of adenosine were bradycardia, hypotension and atrioventricular block. However adenosine as treatment did reduce angiographic no reflow. Gao et al.84 performed a PRISMA COMPLAINT meta-analysis and extracted the data from 15 RCTs with 1736 patients. Data revealed that there was better STR and improved TIMI flow grade after adenosine but no definite improvement in LVEF or mortality.
Adjedj et al.85 have recently studied intracoronary adenosine dose-dependent relationship with hyperaemia in 30 patients by measurement of Doppler derived coronary flow velocity (CFV). The optimal IC bolus dose of adenosine was 100 μg in right coronary artery (RCA) and 200 μg in left coronary artery (LCA) to induce maximum hyperemia and with minimal side effects. No reflow and infarct size depend upon the dose of adenosine (Yetgin et al.).86
17. Calcium channel blockers
Verapamil, diltiazem and nicardepine have been studied for their beneficial effects in no reflow. These drugs act by blocking l-type channels in the cell membrane of myocardium and cause endothelial dependent relaxation of micro-vessels. These also reduce oxygen demand by the myocardium and minimize the damage caused by oxygen free radicals. Verapamil was administered by intracoronary route through coronary infusion catheter distal to the stent in dose of 100–250 μg as bolus followed by 100 μg/min infusion till a maximum of 1000 μg (1 mg) in 23 STEMI patients with no reflow.87 TIMI flow improved in 87% patients along with reduction of corrected TIMI frame count (CTFC) (from 56 ± 9 to 24 ± 4, p < 0.0001). In a randomized study of 150 STEMI patients, verapamil and adenosine were equally effective for prevention of no reflow and improving TIMI frame count.88 In RECOVER AMI trial, 102 patients of no reflow were randomly divided in 3 groups: (i) verapamil (n = 34), (ii) diltiazem (n = 34) and (iii) nitroglycerine (n = 34). Diltiazem given via intracoronary route (dose 400 μg diluted in normal saline) followed by 90 mg orally bd in the ward. The end results were measured by CTFC 3 h after PCI. There was significant reduction in CTFC in diltiazem and verapamil groups.89 Verapamil was administered in dose of 100–200 μg by intra-coronary route immediately after the diagnosis of no reflow in 25 patients (with TIMI flow <2).90 21 patients (84%) responded with TIMI flow 3. Two patients developed transient hypotension, which reversed in 3–5 min. Three patients developed severe bradycardia, which also reversed by intravenous atropine (0.5–1 mg). In a recent meta-analysis, Wang et al.91 analyzed 8 RCTs with 494 patients (162 on drug and 163 as control) treated with verapamil or diltiazem, which equally decreased no reflow significantly (RR 0.3, 95% CI: 0.16–0.57, p = 0.0002) and reduced CTFC (weighted mean difference = −9.24, 95% CI: 13.91–4.57, p = 0.0001). In addition, verapamil and diltiazem improved wall motion abnormality and reduced 6 months MACE. However both drugs did not provide additional improvement in left ventricular ejection fraction (EF) at 6 months.
Nicardepine by intracoronary route (dose 360–460 μg) was administered to 72 patients of no reflow.92 71 patients responded to the treatment with nicardipine. TIMI flow grade improved from a mean of 1.65 ± 0.53 to 2.97 ± 0.24 (p < 0.001) and CTFC reduced from 57 ± 40 to 15 ± 12 (p < 0.0001). The drug was well tolerated.
Thus all three calcium channel blockers have produced good results in the treatment of no reflow.
18. Nicorandil
Nicorandil is an ATP-sensitive potassium channel opener and a nitric oxide (NO) donor. It modulates neutrophil activation and suppresses formation of oxygen free radicals. It is a potent vasodilator. It reduces reperfusion injury and promotes blood flow velocity in the coronaries. It improves LV function and reduces endothlin-1 (ET-1) in no reflow patients (Chen et al.).93 Nicorandil has been found beneficial in the prevention and treatment of no reflow94 and reducing major cardiac adverse events (MACE) at 5 years.95 Nicorandil has been used as IV infusion 8 mg/h or as intracoronary bolus (2 mg).96
19. Sodium nitroprusside
It is a direct donor of NO which is a potent vasodilator. The dose has to be carefully balanced against induced hypotension. It was administered by intra-coronary route (100 μg bolus) in 11 STEMI patients with no reflow and was found effective in 82% cases.97 Better angiographic reperfusion (assessed by TIMI flow) was found in 70 no reflow patients, when IC nitroprusside (50 μg) was combined with adenosine (12 μg) as bolus.98 However Amit et al.99 found negative results on the prevention of no reflow in a randomized study of 98 STEMI, when sodium nitroprusside was given intra-coronary route in bolus dose of 60 μg and results assessed by CTFC, MBG and STR. A recent retrospective randomized study evaluated the effects of nitroprusside (n = 43) or thrombectomy (n = 124) vs control (n = 97 patients). Thrombectomy provided improved perfusion grade assessed by TIMI flow compared with early intracoronary administration of nitroprusside. Neither thrombectomy nor nitroprusside had any significant impact on 30 days, 1 year and 3 year MACE or survival (Lee et al.).100
20. Glycoprotein IIB/IIIA inhibitors
These are modern potent antiplatelet drugs. Abciximab, tirofiban and eptifibatide have been used for the prevention and treatment of no reflow. In a study of 90 STEMI patients, no reflow occurred less frequently in abciximab group (7%) compared with control group (17%).101 Tirofiban given as upstream intravenous infusion achieved better STR and MBG following PCI in ON TIME TRIAL.102 Eptifibatide administered in PROTECT TIMI study improved reperfusion by TIMI flow grading in PROTECT TIMI trial.103
A recent randomized trial of IC vs IV abciximab demonstrated a significantly smaller infarct size (15.1% vs 23.4%, p = 0.001) as assessed by CMRI.104 In a much larger study (AIDA-STEMI), an open label multicentre trial,105 2065 STEMI patients presenting within 12 h with no contraindication to abciximab were randomly assigned intracoronary abciximab (n-1032)(bolus dose 0.25 μg/kg) or intravenous abciximab (infusion dose 0.125 μg/kg/min) (n = 1033). The intracoronary or intravenous abciximab did not result in any difference in the combined end point of death, re-infarction or congestive heart failure. Since IC abciximab bolus is safe, it may be preferred if abciximab is indicated. ESC/EACT guidelines (2014) recommends use of GpIIb/IIIa as a bail out procedure in no reflow patients.66
COCTAIL TRIAL (2015)106: the safety and efficacy of IC cocktail injection combined with thrombus aspiration in STEMI patients treated with primary PCI are under study. The cocktail includes bivalirudin, tirofiban and tenectaplase. The trial is sponsored and conducted at Xijing Hospital, China. The trial is currently recruiting participants and the results are still awaited.
COCTAIL II study has compared standard vs CLEARWAY infused abciximab in myocardial infarction.107
21. Ranolazine (RWISE trials)
Ranolazine is an inhibitor of late sodium current. RWISE was a randomized trial of ranolazine in patients with coronary microvascular dysfunction (Bairey Merz et al.).108 In this trial ranolazine did not improve myocardial perfusion index or the quality of life.
22. Pre-conditioning and post-conditioning
When subjected to non-lethal periods of ischemia, heart may adapt to become more resistant to a subsequent more severe acute ischemia or infarction. The cardiac adaptation is termed as ischemic pre-conditioning.109 Patients with pre-infarction angina may exhibit better clinical outcome and smaller infarct size. Ischemia post-conditioning is another similar concept of cardiac adaptation.110 Ischemic pre- and post-conditioning have been further elaborated.111, 112 Ischemic conditioning probably exerts their protective effect via their action on the mitochondrial permeability pores. The concept needs further research.
23. Newer drugs and prospectives under investigation
-
1.
Immunosuppressive ex. cycloserine inhibits opening of mitochondrial permeability transition pores and improves LVEF. It may reduce infarct size.113
-
2.
Bendavia: a mitochondria cytoprotective peptide (EMBRACE STUDY): may reduce ischemic reperfusion injury.114
-
3.
Atrial natriuretic peptide (ANP) acts via cardioprotective pathways and reduced IS with improved LVEF in J-Wind trial.115
-
4.
Exenatide given 15 min before PCI and 6 h post-PCI improved salvage index.116
-
5.
FX 06 (FIRE STUDY): a peptide from human fibrin failed to reduce IS and MVO, although FX06 reduced necrotic core zone.117
-
6.
Pexelizumab, a Humanized mono-component antibody that binds the C5 component was administered as 2.0 mg/kg bolus, or 2.0 mg/kg bolus and 0.05 mg/kg infusion. It reduced 90 days mortality but had no measurable effect on infarct size in COMMA TRIAL.118 Pexelizumab was investigated as an adjunct to PCI in improving 30 and 90 days mortality, cardiogenic shock or heart failure in APEX AMI trial but failed to show any benefit.119, 120
-
7.
Eniporide is a Na+/H+ exchange inhibitor, which has been administered as an adjunct to early reperfusion therapy for acute myocardial infarction in ESCAMI trial.121 It was given in a dose of 50–200 mg as an infusion over 10 min prior to PCI. Eniporide did not make any significant difference in the clinical outcome.
-
8.
Epinephrine has been administered for the treatment of noreflow, when all other drugs have failed and the patient remains critically unstable. However the RESTORE-SIRIO trial with epinephrine in no-reflow was prematurely withdrawn (2016).122
-
9.
Miscellaneous drugs – antioxidants, statins, endothelin receptor antagonist and Thromboxane A2 receptor antagonist may have a role to protect against no reflow.
24. Conclusions
Diminished and impaired myocardial reperfusion despite successful opening and patency of infarct related artery (IRA) in STEMI following PCI is known as no reflow phenomenon. It may set in and manifest soon after ballooning or stenting during PCI in the cath lab (or ICCU). No reflow is a multifactorial phenomenon. Micro-embolization, ischemia and reperfusion injuries are the principal mechanisms responsible for no reflow. In cath lab, it is diagnosed by finding TIMI flow <3 or TIMI flow 3 with MBG 0–1 or by direct measurement of coronary flow reserve (CFR) using intracoronary guidewire. Failure of STR <70% (60 min after PCI) can be an indicator of no-reflow in the ICCU. The extent of cardiac damage is judged by cardiac MRI. Currently there is no consensus on how best to manage no reflow. A few predictive factors have been recognized and prevention is always better than treatment. Measures to reduce time interval from onset of chest pain to PPCI are probably the best measure to improve myocardial salvage and reduce risk of no reflow. Flow chart (Fig. 2) may help in deciding the steps to be undertaken at various stages of treatment. No-reflow may present as an emergency in cath lab with adverse prognosis. Such patients warrant urgent supportive measures to save life. Intracoronary adenosine, calcium channel blockers, nicorandil, nitroprusside or GpIIb/IIIa are administered depending upon the underlying mechanism/s and the choice of interventional cardiologist. Manual aspiration thrombectomy may have limited effectiveness in patients with large thrombus burden. Unfortunately no reflow may prove resistant to pharmacological therapy in 5–10% cases, with adverse short term and long term outcomes. A personalized attention to tide over the crisis in cath lab is required for every patient of no reflow.
Fig. 2.
Flow chart: management of no reflow.
Conflicts of interest
The authors have none to declare.
References
- 1.O’Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guidelines for the management of ST elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:485–510. doi: 10.1016/j.jacc.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe R., Dick A., Strauss B.H. Prevention and treatment of microvascular-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. J Am Coll Cardiol Interv. 2010;3:695–704. doi: 10.1016/j.jcin.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Eeckhout E., Kern M.J. Clinical perspective: the coronary no-reflow phenomena: a review of mechanisms and therapies. Eur Heart J. 2001;22:729–739. doi: 10.1053/euhj.2000.2172. [DOI] [PubMed] [Google Scholar]
- 4.Brosh D., Assali A.R., Mager A. Effect of no reflow during primary percutaneous coronary intervention for acute myocardial infarction on six month mortality. Am J Cardiol. 2007;99:442–445. doi: 10.1016/j.amjcard.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 5.Morishima I., Sone T., Okumura K. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 6.Bolognese L., Carrabba N., Parodi G. Impact of microvascular dysfunction on left ventricular remodelling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 7.Ndreppa G., Tiroch K., Keta D. Predictive factors and impact of no reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Circ Cardiovasc Interv. 2010;3:27–33. doi: 10.1161/CIRCINTERVENTIONS.109.896225. [DOI] [PubMed] [Google Scholar]
- 8.Berg R., Buhari C. Treating and preventing no reflow in the cardiac catheterization laboratory. Curr Cardiol Rev. 2012;8:209–214. doi: 10.2174/157340312803217148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndreppa G., Tiroch K., Fusaro M. 5-Year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383–2389. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 10.Galiuto L. Optimal therapeutic strategies in the setting of post-infarct no reflow: the need for a pathological classification. Heart. 2004;90:123–125. doi: 10.1136/hrt.2003.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison R.W., Aggarwal A., Ou F.S. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2013;111(2):178–184. doi: 10.1016/j.amjcard.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Niccoli G., Scalone G., Lerman A., Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv484. [DOI] [PubMed] [Google Scholar]
- 13.Hermann J., Lerman A. The endothelium: dysfunction and beyond. J Nucl Cardiol. 2001;8:197–206. doi: 10.1067/mnc.2001.114148. [DOI] [PubMed] [Google Scholar]
- 14.Marks D.S., Gudapati S., Prisant L.M. Mortality in patients with microvascular obstruction. J Clin Hypertens (Greenwich) 2004;6:304–309. doi: 10.1111/j.1524-6175.2004.03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerman A., Homes D.R., Herrmann J., Gersh B.J. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? Eur Heart J. 2007;28:788–797. doi: 10.1093/eurheartj/ehl501. [DOI] [PubMed] [Google Scholar]
- 16.Kloner R.A., Ganote C.E., Jennings R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Investig. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekkers S.C., Yazdani S.K., Virmani R., Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55(16):1649–1660. doi: 10.1016/j.jacc.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Frohlich G.M., Meier P., White S.K., Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 19.Wu K.C., Zerhouni E.A., Judd R.M. Prognostic significance of microvascular obstruction magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 20.Basso C., Rizzo S., Thiene G. The metamorphosis of myocardial infarction following coronary revascularization. Cardiovasc Pathol. 2010;19:22–28. doi: 10.1016/j.carpath.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Yoshino S., Ciluffo R., Best P.Z. A single nucleotide polymorphism associated with abnormal coronary micro-vascular function. Coron Artery Dis. 2014;25:281–289. doi: 10.1097/MCA.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niccoli G., Burzotta F., Galiuto L., Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Niccoli G., Lanza G.A., Spaziani C. Baseline systemic inflammatory status and no-reflow phenomenon after percutaneous coronary angioplasty for acute myocardial infarction. Int J Cardiol. 2007;117(3):306–311. doi: 10.1016/j.ijcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Danesh Sani H.D., Eshraghi A., Sahri B., Vejdanparast M. No-reflow phenomenon in patients with ST-elevation acute myocardial infarction, treated with primary percutaneous coronary intervention: a study of predictive factors. J Cardiothorac Med. 2014;2(4):221–226. [Google Scholar]
- 25.Abdi S., Rafizadeh O., Peighambari M., Basiri H., Bakhshandeh H. Evaluation of clinical and procedural predictive factors of no-reflow phenomenon following primary percutaneous coronary intervention. Res Cardiovasc Med. 2005;4(2):e25414. doi: 10.5812/cardiovascmed.4(2)2015.25414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J.W., Zhoud Z.Q., Chen Y.D., Want C.H., Zhu X.L. A risk score for no reflow in patients with ST-segment elevation myocardial infarction after percutaneous coronary intervention. Clin Cardiol. 2015;38(4):208–215. doi: 10.1002/clc.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topark C., Tabakci M.M., Zimsek Z. Platelet/lymphocyte ratio was associated with impaired myocardial perfusion and both in-hospital and long-term adverse outcome in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Postep Kardiol Interwencyjnej. 2015;11(4):288–297. doi: 10.5114/pwki.2015.55599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeda T., Higuma T., Abe N. Intravascular ultrasound, but not optical coherence tomography, predicts no reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction cause by plaque rupture. J Am Coll Cardiol. 2015;67(10S) 1244-071. [Google Scholar]
- 29.Soeda T., Higuma T., Abe N. Morphological predictors for no-reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction caused by plaque rupture. Eur Heart J Cardiovasc Imaging. 2016;(January) doi: 10.1093/ehjci/jev341. pii:jev341 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Mazhar J., Maschicharan M., Farshid A. Predictors and outcome of no-reflow post primary percutaneous coronary intervention for ST elevation myocardial infarction. IJC Heart Vasc. 2016;10:8–12. doi: 10.1016/j.ijcha.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson M.C., Cannon C.P., Murphy S.A., Marble S.J., Barron H.V., Braunwald E. Relationship of TIMI myocardial perfusion grades, flow grades, frame count and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–1913. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann P., Haager P., Lepper W., Franke A., Hanrath P. Relation of coronary flow pattern to myocardial blush grade in patients with first myocardial infarction. Heart. 2003;89(10):1147–1151. doi: 10.1136/heart.89.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henriques J.P.S., Zijlistra F., van’t Hof A.W.J. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107:2115–2119. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 34.Schroder R. Prognostic impact of early ST-segment resolution in acute ST-elevation myocardial infarction. Circulation. 2004;110:e506–e5010. doi: 10.1161/01.CIR.0000147778.05979.E6. [DOI] [PubMed] [Google Scholar]
- 35.Sorajja P., Gersh B.J., Costantini C. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J. 2005;26:667–674. doi: 10.1093/eurheartj/ehi167. [DOI] [PubMed] [Google Scholar]
- 36.Giugliano R.P., Sabatine M.S., Gibson C.M. Combined assessment of thrombolysis in myocardial infarction Flow grade, myocardial perfusion grade and ST-segment resolution to evaluate epicardial and myocardial reperfusion. Am J Cardiol. 2004;93:1362–1367. doi: 10.1016/j.amjcard.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Iwakura K., Ito H., Takiuchi S. Alteration in the coronary blood flow velocity pattern in patients with no reflow and reperfused acute myocardial infarction. Circulation. 1996;94:1269–1275. doi: 10.1161/01.cir.94.6.1269. [DOI] [PubMed] [Google Scholar]
- 38.Claessen B.E.P.M., Bax M., Delewi R., Meuwissen M., Henriques J.P.S., Piek J.J. The Doppler flow wire in acute myocardial infarction. Heart. 2010;96:631–635. doi: 10.1136/hrt.2009.186932. [DOI] [PubMed] [Google Scholar]
- 39.Yamamuro A., Akasaka T., Tamita K. Coronary flow velocity pattern immediately after percutaneous coronary intervention as a predictor of complications and in-hospital survival after acute myocardial infarction. Circulation. 2002;106:3051–3056. doi: 10.1161/01.cir.0000043022.44032.77. [DOI] [PubMed] [Google Scholar]
- 40.Ito H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2006;3(9):499–506. doi: 10.1038/ncpcardio0632. [DOI] [PubMed] [Google Scholar]
- 41.Lepper W., Belcik T., Wei K., Linder J., Sklenar J., Kaul S. Myocardial contrast echocardiography. Circulation. 2004;109:3132–3135. doi: 10.1161/01.CIR.0000132613.53542.E9. [DOI] [PubMed] [Google Scholar]
- 42.Galiuto L., Garramone B., Scara A. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post infarction reperfusion in predicting left ventricular remodelling: result of the multicentre AMICI study. J Am Coll Cardiol. 2008;51:552–559. doi: 10.1016/j.jacc.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 43.Sanjeev K. The no-reflow phenomenon following acute myocardial infarction: mechanism and treatment options. J Cardiol. 2014;64:77–85. doi: 10.1016/j.jjcc.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Wu K.C. CMR of microvascular obstruction and haemorrhage in myocardial infarction. J Cardiovasc Magn Reson. 2012;14:68. doi: 10.1186/1532-429X-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Kranenbrug M., Magro M., Thiele H. Prognostic value of microvascular obstruction and infarct size as measured by CMR in STEMI patients. J Am Coll Cardiol Imaging. 2014;7:930–939. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Burzotta F., Trani C., Ramagnoli E. Manual thrombus aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46:371–376. doi: 10.1016/j.jacc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 47.Svilass T., Vlaar P.J., van der Horst I.C. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–576. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 48.Vlaar P.J., Svilass T., van der Horst W.C. Cardiac death and reinfarction after 1 year in there thrombus aspiration during percutaneous coronary intervention in acute myocardial infarction study (TAPAS): a 1-year follow-up study. Lancet. 2008;371(9628):1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 49.Dudek D., Mielecki W., Burzotta F. Thrombus aspiration followed by direct stenting: a novel strategy of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Results of the Polish–Italian–Hungarian-Randomized ThrombEctomy (PIHRATE Trial) Am Heart J. 2010;160(5):966–972. doi: 10.1016/j.ahj.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Frobert O., Lagerqvist B., Olivercrona G.K. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 51.Jolly S.S., Carins J.A., Yusuf S. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med. 2015;372(15):1389–1398. doi: 10.1056/NEJMoa1415098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Migliorini A., Stabile A., Rodriguez A.E. Comparison of AngioJet rheolytic thrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;56(16):1298–1306. doi: 10.1016/j.jacc.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Lefevre T., Garcia E., Reimers B. X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution. J Am Cardiol. 2005;46(2):246–252. doi: 10.1016/j.jacc.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Stone G.W., Maehara A., Witzenbichler B. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: the INFUSE-AMI randomized trial. JAMA. 2012;307(17):1817–1826. doi: 10.1001/jama.2012.421. [DOI] [PubMed] [Google Scholar]
- 55.Brenner S.J., Maehara A., Dizon J.M. Relationship between myocardial reperfusion, infarct size and mortality; the INFUSE-AMI (intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction) trial. J Am Coll Cardiol Cardiovasc Interv. 2013;6(7):718–724. doi: 10.1016/j.jcin.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Bavry A.A., Kumbhani D.J., Bhatt D.J. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur Heart J. 2008;29:2989–3001. doi: 10.1093/eurheartj/ehn421. [DOI] [PubMed] [Google Scholar]
- 57.Burzotta F., De Vita M., Gu Y.L. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction; an individual patient-data pooled analysis of 11 trials. Eur Heart J. 2009;30:2193–2203. doi: 10.1093/eurheartj/ehp348. [DOI] [PubMed] [Google Scholar]
- 58.Mongeon F.P., Belisle P., Joseph L., Eisenberg M.J., Rinfert S. Adjunctive thrombectomy for acute myocardial infarction: a Bayesian meta-analysis. Circ Cardiovasc Interv. 2010;3:6–16. doi: 10.1161/CIRCINTERVENTIONS.109.904037. [DOI] [PubMed] [Google Scholar]
- 59.Tamhane U.U., Chetcuti S., Hameed I., Grossman P.M., Mosucci M., Gurm H.S. Safety and efficacy of thrombectomy in patients undergoing primary percutaneous coronary intervention for acute ST elevation MI: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2010;10:10. doi: 10.1186/1471-2261-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Luca G., Navarese E.P., Suryapranta H. A meta-analytic overview of thrombectomy during primary angioplasty. Int J Cardiol. 2013;166(3):606–612. doi: 10.1016/j.ijcard.2011.11.102. [DOI] [PubMed] [Google Scholar]
- 61.Deng S.B., Wang J., Xiao J. Adjunctive manual thrombus aspiration during ST-segment elevation myocardial infarction: a meta-analysis of randomized controlled trials. PLOS ONE. 2014;9(11):e 113481. doi: 10.1371/journal.pone.0113481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kumbhani D.J., Bavry A.A., Desai M.Y., Bangalore S., Bhatt D.L. Role of aspiration and mechanical thrombectomy in patients with acute myocardial infarction undergoing primary angioplasty: an updated meta-analysis of randomized trials. J Am Coll Cardiol. 2013;62(16):1409–1418. doi: 10.1016/j.jacc.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 63.Jolly S.S., Cairns J.A., Yusuf S. Stroke in the TOTAL trial: a randomized trial of routine thrombectomy vs. percutaneous coronary intervention alone in ST elevation myocardial infarction. Eur Heart J. 2015;36:2364–2372. doi: 10.1093/eurheartj/ehv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elgendy I.Y., Huo T., Bhatt D.L. Is aspiration thrombectomy beneficial in patients undergoing primary percutaneous coronary intervention? Meta-analysis of randomized trials. Circ Cardiovasc Interv. 2015;8:e002258. doi: 10.1161/CIRCINTERVENTIONS.114.002258. [DOI] [PubMed] [Google Scholar]
- 65.Steg P.G., James S.K., Atar D. ESC guideline for the management of acute myocardial infarction in patients presenting with ST segment elevation. Eur Heart J. 2012;33(20):2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 66.Authors/Task Force Members, Windecker S., Kolh P. ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic c Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 67.Levine G.N., Bates E.R., Blankenship J.C. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines and the Society of Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 68.Levine N.G., Bates E.R., Blankenship J.C. ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of 2011 ACCF/AHA/SCAI guidelines for percutaneous coronary intervention and 2013 ACCF/AHA guidelines for the management of ST-elevation myocardial infarction. Circulation. 2015 http://circ.ahajournals.org/content/early/2015/10/20/CIR.0000000000000336.full.pdf [Google Scholar]
- 69.Freixa X., Belle L., Joseph L. Immediate vs delayed stenting in acute myocardial infarction: a systemic review and meta-analysis. EuroIntervention. 2013;8:1207–1216. doi: 10.4244/EIJV8I10A185. [DOI] [PubMed] [Google Scholar]
- 70.Carrick D., Oldroyd K.G., McEntegart M. A randomized trial of deferred stenting versus immediate stenting to prevent no or slow-flow in acute ST-segment elevation myocardial infarction (DEFER-STEMI) J Am Coll Cardiol. 2014;63:2088–2098. doi: 10.1016/j.jacc.2014.02.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Piscione F., Danzi G.B., Cassese S. Multicentre experience with MGuard net protective stent in ST-elevation myocardial infarction: safety, feasibility and impact on myocardial infarction. Catheter Cardiovasc Interv. 2010;75(5):715–721. doi: 10.1002/ccd.22292. [DOI] [PubMed] [Google Scholar]
- 72.Dudek D., Dziewierz A., Rzeszutko L. Mesh covered stent in ST-segment elevation myocardial infarction. EuroIntervention. 2010;6(5):582–589. doi: 10.4244/EIJV6I5A98. [DOI] [PubMed] [Google Scholar]
- 73.Fernandez-Cisnal A., Cid-Alvarez B., Alvarez-Alvarez B. Real world comparison of the MGuard stent versus the bare metal stent for ST elevation myocardial infarction (the REWARD-MI study) Catheter Cardiovasc Interv. 2015;85(1):E1–E9. doi: 10.1002/ccd.25563. [DOI] [PubMed] [Google Scholar]
- 74.Stone G.W., Abizaid A., Silber S. Prospective, randomized, multicentre evaluation of a polyethylene terephthalat micronet mesh-covered stent (MGuard) in ST-segment myocardial infarction (The MASTER trial) J Am Coll Cardiol. 2012;60(19):1975–1984. doi: 10.1016/j.jacc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Costa R.A., Abizaid A., Lotan C. Impact of thrombus burden on outcomes after standard versus mesh-covered stents in acute myocardial infarction (from the MGuard for acute ST elevation reperfusion trial) Am J Cardiol. 2015;115(2):161–166. doi: 10.1016/j.amjcard.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 76.Gracida M., Ramaguera R., Jacobi F., Gomez-Hospital J.A., Cequier A. The MGuard coronary stent: safety, efficacy and clinical utility. Vasc Health Risk Manag. 2015;11:533–539. doi: 10.2147/VHRM.S68007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cetin M., Kiziltunc E., Cetin Z.G., Kundi H., Gulkan B., Cicekcioglu H. Case report: a practical method for no-reflow treatment. Hindawai. 2016 doi: 10.1155/2016/9596123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahaffey K.W., Puma J.A., Barbagelata N.A. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction Study of Adenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 79.Ross A.M., Gibbons R.J., Stone G.W. A randomized, double-blinded, placebo-controlled multicentre trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 80.Assali A.R., Sdringola S., Ghani M. Intracoronary adenosine administered during percutaneous intervention in acute myocardial infarction and reduction in the incidence of no reflow phenomenon. Catheter Cardiovasc Interv. 2000;51:27–31. doi: 10.1002/1522-726x(200009)51:1<27::aid-ccd7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 81.Niccoli G., Rigattieri S., De Vita M.R. Open-label, randomized, placebo-controlled evaluation of intracoronary adenosine or nitroprusside after thrombus aspiration during primary percutaneous coronary intervention for the prevention of microvascular obstruction in acute myocardial infarction: the REOPEN-AMI study (Intracoronary Nitroprusside Versus Adenosine in Acute Myocardial Infarction) J Am Coll Cardiol Cardiovasc Interv. 2013;6:580–589. doi: 10.1016/j.jcin.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Faruk Akturk I., Arif Yalcin A., Biyik I. Effects of verapamil and adenosine in an adjunct to tirofiban on resolution and prognosis of no reflow phenomenon in patients with acute myocardial infarction. Minerva Cardioangiol. 2014;62(5):389–397. [PubMed] [Google Scholar]
- 83.Su Q., Nyi S., Li L. Adenosine and verapamil for no reflow during primary percutaneous coronary intervention in people with acute myocardial infarction. Cochrane Database Syst Rev. 2015;18(May (5)):CD009503. doi: 10.1002/14651858.CD009503.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao Q., yang B., Guo Y., Zheng F. Efficacy of adenosine in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Medicine (Baltimore) 2015;94(August (32)):e1279. doi: 10.1097/MD.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adjedj J., Toh G.G., Johnson N.P. Intracoronary adenosine: dose–response relationship with hyperaemia. J Am Coll Cardiol Interv. 2015;8:1422–1430. doi: 10.1016/j.jcin.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 86.Yetgin T., Uitterdijk A., Hekkert M.L. Limitation of infarct size and no-reflow by intracoronary adenosine depends critically on dose and duration. J Am Coll Cardiol Interv. 2015;8:1990–1999. doi: 10.1016/j.jcin.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 87.Werner G.S., Lang K., Kuehnert H. Intracoronary verapamil for reversal of no-reflow during coronary angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv. 2002;57:444–451. doi: 10.1002/ccd.10375. [DOI] [PubMed] [Google Scholar]
- 88.Vijaylakshmi K., Whittaker V.J., Kunadian B. Prospective, randomized controlled trial to study the effect of intracoronary injection of verapamil and adenosine on coronary blood flow during percutaneous coronary intervention in patients with acute coronary syndromes. Heart. 2006;92(9):1278–1284. doi: 10.1136/hrt.2005.075077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang D., Qian I., Gee L. Restoration of coronary flow in patients with no reflow after primary coronary intervention of acute myocardial infarction (RECOVER AMI) Am Heart J. 2012;164:394–401. doi: 10.1016/j.ahj.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 90.Fu Q., Lu W., Huang Y.J. Verapamil reverses myocardial no-reflow after primary percutaneous coronary intervention in patients with acute myocardial infarction. Cell Biochem Biophys. 2013;67(3):911–914. doi: 10.1007/s12013-013-9581-0. [DOI] [PubMed] [Google Scholar]
- 91.Wang L., Cheng Z., Gu Y., Peng D. Review article: short-term effects of verapamil and diltiazem in the treatment of no reflow phenomenon: a meta-analysis of randomized controlled trials. Hindawi. 2015 doi: 10.1155/2015/382086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang R.I., Patel P., Walinsky P. Efficacy of intracoronary nicardipine in the treatment of no-reflow during percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006;68:671–676. doi: 10.1002/ccd.20885. [DOI] [PubMed] [Google Scholar]
- 93.Chen Z., Chen X., Li S., Huo X., Fu X., Dong X. Nicorandil improves myocardial function by regulating plasma nitric oxide and enothelin-1 in coronary slow flow. Coron Artery Dis. 2015;26(March (2)):114–120. doi: 10.1097/MCA.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ezhilan J., Juneja M.S., George T., Ramkumar S., Viswanathan V., Badrinath A. Prevention of no flow/slow flow phenomenon in primary PCI by nicorandil. Indian Heart J. 2007;59(3):246–249. [PubMed] [Google Scholar]
- 95.Ishii H., Ichimiya S., Kanashior M. Impact of single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation. 2005;112:1284–1288. doi: 10.1161/CIRCULATIONAHA.104.530329. [DOI] [PubMed] [Google Scholar]
- 96.Ono H., Osanai T., Ishizaka H. Nicorandil improves cardiac function and clinical outcome in patients with acute myocardial infarction undergoing percutaneous coronary intervention: role of inhibitory effect on reactive oxygen species formation. Am Heart J. 2004;148:E15. doi: 10.1016/j.ahj.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 97.Wang H.J., Lo P.H., Lin J.J. Treatment of slow/no-reflow phenomenon with intracoronary nitroprusside injection in primary coronary intervention for acute myocardial infarction. Catheter Cardiovasc Interv. 2004;63(2):171–176. doi: 10.1002/ccd.20149. [DOI] [PubMed] [Google Scholar]
- 98.Parikh K.H., Chag M.C., Shah K.J. Intracoronary boluses of adenosine and sodium nitroprusside in combination reverses slow/no flow during angioplasty: a clinical scenario of ischemic preconditioning. Can J Physiol Pharmacol. 2007;85(3–4):476–482. doi: 10.1139/y07-013. [DOI] [PubMed] [Google Scholar]
- 99.Amit G., Cafri C., Yaroslavtsev S. Intracoronary nitroprusside for the prevention of the no-reflow phenomenon after primary percutaneous coronary intervention in acute myocardial infarction. A randomized, double-blind, placebo-controlled clinical trial. Am Heart J. 2006;152 doi: 10.1016/j.ahj.2006.05.010. 887.e9–887.e14. [DOI] [PubMed] [Google Scholar]
- 100.Lee W.C., Chen S.M., Liu C.F. Early administration of intracoronary nitroprusside compared with thrombus aspiration in myocardial perfusion for acute myocardial infarction: a 3-year clinical follow-up study. Acta Cardiol Sin. 2015;31:373–380. doi: 10.6515/ACS20150515A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petronio A.S., de Carlo M., Ciabatti N. Left ventricular remodelling after primary coronary angioplasty in patients treated with abciximab or intracoronary adenosine. Am Heart J. 2005;150:1015. doi: 10.1016/j.ahj.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 102.Van’t Hoff A.W., ten Berg J., Heestermans T. Prehospital initiation of tirofiban in patients with ST-elevation myocardial infarction undergoing primary angioplasty (On-TIMI 2): a multicentre, double-blind, randomized controlled trial. Lancet. 2008;373:537–546. doi: 10.1016/S0140-6736(08)61235-0. [DOI] [PubMed] [Google Scholar]
- 103.Gibson C.M., Morrow D.A., Murphy S.A. A randomized trial to evaluate the relative protection against post-percutaneous coronary intervention microvascular dysfunction, ischemia, and inflammation among antiplatelet and antithrombotic agents: the PROTECT-TIMI-30 trial. J Am Coll Cardiol. 2006;47:2364–2373. doi: 10.1016/j.jacc.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 104.Thiele H., Scindler K., Friedenberger J. Intracoronary compared with intravenous bolus abciximab application in patient with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-segment myocardial infarction. Circulation. 2008;118:49–57. doi: 10.1161/CIRCULATIONAHA.107.747642. [DOI] [PubMed] [Google Scholar]
- 105.Thiele H., Wohrle J., Hambrecht R. Intracoronary versus intravenous bolus abciximab during primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction: a randomized trial. Lancet. 2012;379(9819):923–931. doi: 10.1016/S0140-6736(11)61872-2. [DOI] [PubMed] [Google Scholar]
- 106.ClinicalTrials.gov. Intracoronary cocktail injection combined with thrombus aspiration in STEMI patients treated with primary angioplasty. http://clinicaltrials.gov/ct2/show/NCT02592694.
- 107.Prati F., Di Vito L., Ramazzotti V. Randomized trial of standard versus clearway-infused abciximab and thrombectomy in myocardial infarction: rationale and design of the COCKTAIL II study. J Cardiovasc Med (Hagerstown) 2013;14(May (5)):364–371. doi: 10.2459/JCM.0b013e3283586fee. [DOI] [PubMed] [Google Scholar]
- 108.Bairey Merz N., Handberg E.M., Shufelt C.L. A randomized, placebo controlled trial of late current inhibition (Ranolazine) in coronary micro-vascular dysfunction: impact on Angina and myocardial perfusion reserve. Eur Heart J. 2015;(November) doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murry C.E., Jennings R.B., Reimer K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Z.Q., Covera J.S., Halkos M.E. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 111.Kharbanda R.K., Nielsen T.T., Redington A.N. Tanslation of remote ischemic preconditioning into clinical practice. Lancet. 2009;374(9700):1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 112.Botker H.E., Kharbanda R., Schmidt M.R. Remote ischemic conditioning before hospital admission, as a complement to angioplasty and effect on myocardial salvage in patients with acute myocardial infarction: a randomized trial. Lancet. 2010;375(9716):727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 113.Piot C., Croisille P., Staat P. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 114.Chakrabarti A.K., Feeney K., Abueg G. Rational and design of the EMBRACE STEMI Study: a phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous Bendavia on reperfusion injury in patients treated with standard therapy including primary percutaneous coronary intervention and stenting for ST-segment elevation myocardial infarction. Am Heart J. 2013;165:509–514. doi: 10.1016/j.ahj.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Kitakaze M., Asakura M., Kim J. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomized trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 116.Lonborg J., Vejlstrup N., Kelbaek H. Exenatide rescues reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2013;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 117.Atar D., Petzelbauer P., Schwitter J. Effects of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial. J Am Coll Cardiol. 2009;53(8):720–729. doi: 10.1016/j.jacc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 118.Granger C.B., Mahaffey K.W., Weaver W.D. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA TRIAL) Circulation. 2003;108(10):1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 119.Armstrong P.W., Granger C.B., Adams P.X. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297(1):43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 120.Patel M.R., Worthely S.G., Stebbins A. Pexelizumab and infarct size in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: a delayed enhancement cardiac magnetic resonance sub-study from the APEX-AMI trial. J Am Coll Cardiol Cardiovasc Imaging. 2010;3(1):52–60. doi: 10.1016/j.jcmg.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 121.Zeymer U., Suryapranata H., Monassier J.P. The Na+/H+ exchange inhibitor Eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardio-protective effects of eniporide in acute myocardial infarction (ESCAMI) TRIAL. J Am Coll Cardiol. 2001;38(6):1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- 122.2016, February. The RESTORE-SIRIO TRIAL – Clinical Trials.gov, Report. [Google Scholar]