Abstract
AIM
To test antibiotic-loaded coating for efficacy in reducing bacterial biofilm and development of osteomyelitis in an orthopaedic model of implant infection.
METHODS
Phosphatidylcholine coatings loaded with 25% vancomycin were applied to washed and sterilized titanium wires 20 mm in length. A 10 mm segment was removed from rabbit radius (total = 9; 5 coated, 4 uncoated), and the segment was injected with 1 × 106 colony forming units (CFUs) of Staphylococcus aureus (UAMS-1 strain). Titanium wires were inserted through the intramedullary canal of the removed segment and into the proximal radial segment and the segment was placed back into the defect. After 7 d, limbs were removed, X-rayed, swabbed for tissue contamination. Wires were removed and processed to determine attached CFUs. Tissue was swabbed and streaked on agar plates to determine bacteriological score.
RESULTS
Antibiotic-loaded coatings resulted in significantly reduced biofilm formation (4.7 fold reduction in CFUs; P < 0.001) on titanium wires and reduced bacteriological score in surrounding tissue (4.0 ± 0 for uncoated, 1.25 ± 0.5 for coated; P = 0.01). Swelling and pus formation was evident in uncoated controls at the 7 d time point both visually and radiographically, but not in antibiotic-loaded coatings.
CONCLUSION
Active antibiotic was released from coated implants and significantly reduced signs of osteomyelitic symptoms. Implant coatings were well tolerated in bone. Further studies with additional control groups and longer time periods are warranted. Antibiotic-loaded phosphatidylcholine coatings applied at the point of care could prevent implant-associated infection in orthopaedic defects.
Keywords: Biofilm, Implant, Drug delivery coating, Antibiotic, Orthopaedic infection
Core tip: We report infection preventative results of a novel antibiotic-loaded coating in a severe contaminated model of orthopaedic infection. Phosphatidylcholine coatings loaded with 25% vancomycin, which can be applied to implants immediately prior to implantation, significantly reduced staphylococcal adherence to intramedullary titanium wires in rabbits. Reduction in bacterial load on implants and in tissue for antibiotic-loaded coatings accompanied reduction in swelling and pus formation. Mild inflammatory responses were noted with coated implants compared to uncoated infected controls. This preliminary short term study demonstrates the clinical potential of these broadly applicable coatings and the need for further characterization and development.
INTRODUCTION
Biofilm formation on implants continues to be a cause of orthopaedic infection[1,2]. While the rate of infection in some orthopaedic implants has been reported to range from 1%-10%, in some complex orthopaedic trauma requiring percutaneous devices the rate of infection may approach 30%[3-5]. Protection of orthopaedic implants from contamination may be more successful through prevention of biofilm formation. Because microbial cells within a biofilm are less metabolically active and shielded to some degree by exopolymeric substance, there is a high concentration of persister cells with resistance to commonly used antibiotics within biofilm[6-9]. Standard treatment practices of prophylactic systemic administration of antibiotics may not be sufficient to inhibit or treat biofilm-based infections partly due to poor vascularity in injured tissue and partly due to the increased concentrations of antimicrobials required for elimination of biofilm[7,10,11].
Local delivery devices have been developed to achieve high concentrations of antibiotic within the potentially contaminated tissue to target biofilm[12-14]. However, some have limitations in degradability, implant coverage, and antibiotic loading, including non-degradable polymethylmethacrylate bone cement beads[15], degradable calcium sulfate beads[16], sponge devices[17], and injectable hydrogels[18]. Local delivery coatings directly on the implant can protect the biomaterial from biofilm formation[19-21], but may require extensive prefabrication steps.
We previously described the biofilm inhibitory nature of phosphatidylcholine coatings loaded with antibiotics, both in vitro and in vivo[22]. When loaded with antibiotics, this coating applied at the point-of-care just prior to implantation was shown to reduce biofilm formation of Staphylococcus aureus (S. aureus) and Pseudomonas aeruginosa, as well as prevent biofilm formation in a polymicrobial model of contamination with both microorganisms. While proof of principle was established in a soft-tissue dorsal model of implant infection, for this study our primary question was whether antibiotic-loaded coatings could successfully prevent infection in a contaminated orthopaedic model.
MATERIALS AND METHODS
Study design
Coatings were fabricated by mixing 6 g of purified phosphatidylcholine (Phospholipon 90G, Lipoid GMB, Germany) with 2 g of vancomycin through a previously described process of warming and kneading powdered antibiotics until the mixture was uniform[22]. The mixture was loaded into open-ended syringes and sterilized by low dose (25 kGy) gamma irradiation.
Titanium wire 0.81 mm in diameter (Mcmaster Carr) was trimmed to 15 cm in length. Titanium wire was cleaned by washing with dish detergent, after which it was soaked in 20% nitric acid for 1 h. Wire was rinsed thoroughly in ultrapure water, sonicated in soapy water, and rinsed again three times in ultrapure water. Implants were separately packaged and autoclaved at 121 °C for 20 min for sterilization.
Animal model
Animal care and use statement: Study protocols were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee and all appropriate measures were taken to minimize pain and discomfort.
Nine New Zealand white rabbits were anesthetized with 1-2 cc of a xyalzine/ketamine mixture intramuscularly for a dose range of 3-7 mg/kg xylazine and 30-40 mg/kg ketamine. Rabbits were maintained on 0.5%-3% isoflurane administered by nose cone to produce surgical anesthesia and monitored by a veterinary technician. The right forelimb of each rabbit was shaved and prepped using a betadine scrub and rinsed with 70% ethanol. An incision was made on the anterior surface of the right forelimb through the epidermis, musculature, and fascia until the radius was exposed. A 1 cm mid-radial segment was be excised from the right forelimb using a miniature saw blade (Exakt, Oklahoma City, OK). The excised segment was then infected by inoculation of S. aureus [10 μL of 107 colony forming units (CFUs)/mL; UAMS-1 strain] directly into the intramedullary canal.
Animals were divided into two treatment groups: Wire with 25% vancomycin-loaded coating (n = 5) or non-coated controls (n = 4). During surgical procedures titanium wires in the coated group were coated by direct manual application of coating through the syringe applicator. Coated or control wires were inserted into the medullary canal of the infected segment immediately after inoculation. Approximately 5 mm of wire extended through the proximal end of the segment and was inserted into the medullary canal of the intact proximal bone. The segment was then replaced in the same orientation and the wound was closed with sutures.
Rabbits were euthanized after 7 d. Forelimbs were removed and imaged by X-ray, noting signs of inflammation and swelling. A swab of the soft tissue and bone exposed was taken to determine bacteriological score of surrounding tissue. The segment was removed for retrieval of the wire and placed in 10% neutral buffered formalin for further processing. The wire was rinsed in sterile phosphate buffered saline (PBS) and sonicated in 5 mL of PBS for enumeration of viable CFUs attached as biofilm. The remaining forelimb was also trimmed and fixed in formalin for histological characterization. Fixed tissue was decalcified in ethylenediaminetetraacetic acid and stained with hematoxylin and eosin as well as a modified Gram stain[23]. Images taken at 4 × magnification were scored by three independent blinded reviewers for severity of inflammatory response on a scale of 0-4.
Statistical analysis
Mann-Whitney nonparametric t tests in Sigma Plot were used to determine whether there were statistically significant differences in colony counts in coated wires compared to uncoated control wires. Mann-Whitney tests were also used to determine statistical differences in bacteriological score and histological scores. Using results of previous studies determining log fold reduction of CFUs in response to antimicrobials[24], an a priori power analysis was performed using Sigma Plot. Assuming a standard deviation of 1 unit in log-transformed CFU counts, 4 animals per group are required to have 80% power to detect a difference of 2.5 units between the control and each of the 4 experimental treatment groups at a significance level of 5%. The statistical methods of this study were reviewed by Jessica Amber Jennings of the University of Memphis.
RESULTS
After the 7 d implantation period, animals with uncoated implants were noted to have characteristics indicative of inflammation such as swelling (Figure 1), redness, and increased temperature, compared to those with implants coated with vancomycin-loaded phosphatidylcholine. Pus formation was evident in muscle tissue of several animals with uncoated wires.
Figure 1.
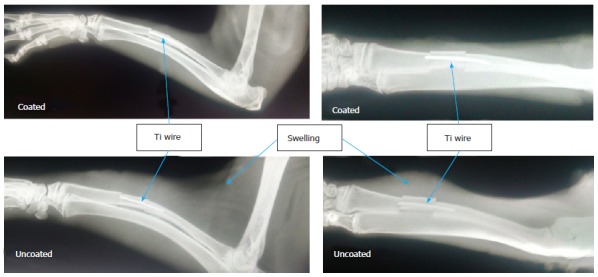
Representative radiographs from implant-associated osteomyelitis model after 7 d in antibiotic-coated group (top) and uncoated control group (bottom). Location of Ti wires and swelling in tissue surrounding implants are noted by arrows.
Bacteriological scores confirmed that there were statistically significant reductions in bacteria recovered from surrounding tissue (P = 0.01) (Figure 2). CFU counts of S. aureus remaining as biofilm on Ti implants revealed that there were statistically fewer colonies retrieved from coated implants, 20 ± 21 CFUs vs 6.0 × 105 CFUs in the control group (P < 0.001) (Figure 3). Complete clearance of attached S. aureus biofilm was achieved in 40% of the coated wires vs 0% in the control group.
Figure 2.
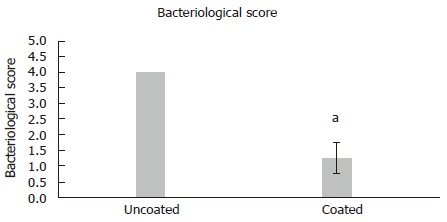
Graphical representation of bacteriological scores from surrounding tissue on a 0-4 point scale. Data is represented as mean ± SD. Statistically significant difference between groups was determined by Mann-Whitney Rank Sum test (aP < 0.05).
Figure 3.

Graphical representation of log colony forming units retrieved from Ti wires. Data is represented as mean ± SD. Statistically significant difference between groups was determined by Mann-Whitney Rank Sum test (aP < 0.05). CFUs: Colony forming units.
In histological specimens, evidence of inflammatory cell infiltration was evident in many of the control groups with confirmed bacterial presence, while inflammatory cell presence was minimal in the groups with vancomycin coating (P < 0.001) (Figure 4). Representative histological slides show severe infiltration of inflammatory cells in uncoated groups and evidence of Gram-positive staphylococci in the tissue surrounding the implant and in the cortical bone (Figure 5). Although some evidence of Gram-positive staphylococci was observed in the cortical bone of animals with antibiotic coated implants, the tissue surrounding the implant had minimal evidence of bacteria.
Figure 4.
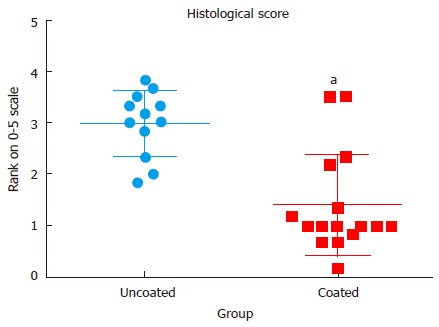
Plot of histological scores for uncoated and coated groups. Statistically significant difference between groups was determined by Mann-Whitney Rank Sum test (aP < 0.05).
Figure 5.
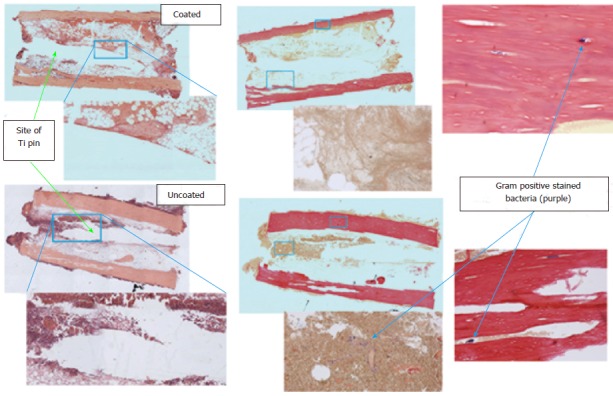
Photomicrographs of sections of decalcified bone stained with hematoxylin and eosin and the modified Gram stain at 4 × and 10 ×. Approximate areas of higher magnification are denoted by blue boxes. Areas where pin was removed and areas of visible staphylococci are marked with arrows.
DISCUSSION
Successful function of orthopaedic implants can be threatened by infections, which are not always prevented by systemic antibiotic treatment[25-27]. Implant coatings are among the local drug delivery strategies that aim to prevent or treat colonization of implants with biofilm bacteria[21,28,29]. The naturally-derived material phosphatidylcholine has not been approved by the Food and Drug Administration (FDA) as a carrier of antibiotics, but is a component of FDA approved matrices for demineralized bone graft materials[30]. Confirming results of previous preliminary studies[22], this initial study of a novel antibiotic loaded coating further recommends the potential of the coating to prevent implant-associated osteomyelitis.
This study demonstrates significant reduction in contamination and progression of disease, though some bacteria were recovered from the cortical bone and observed in the Gram stains. Any remaining bacteria could potentially rebound and lead to osteomyelitis. We chose vancomycin as the first antibiotic to evaluate in this coated implant model due to the known susceptibility of this strain of bacteria[24]. We have demonstrated previously that the coating can be loaded with different antibiotics or combinations of antibiotics such as amikacin, although the release profile may vary based on solubility or other chemical properties of the antimicrobial[22]. In the context of preventing biofilm, vancomycin has lower efficacy than antibiotics such as daptomycin[24] though it is commonly used as an adjunctive therapy in local drug delivery devices or even sprinkled into a surgical site after implantation of orthopaedic devices[31-34]. An advantage of this coating is that it may provide clinicians with a choice of antibiotic-or antimicrobial-loaded coating based on patient assessment and risk evaluation. Recently, investigations into biofilm inhibitors such as cis 2-decenoic acid, D-amino acids, and farnesol have offered potential therapeutic options to specifically target biofilm[35-37]. The phosphatidylcholine coating could serve as delivery system for combined therapy with antibiotics and biofilm inhibitors, since additive and synergistic prevention of biofilm and bacterial growth have been demonstrated in vitro[38-42].
The results of this study confirm that the results of our previous in vitro and soft-tissue in vivo studies of this coating are applicable within an orthopaedic implant context. Inflammatory response was typical of normal infiltration of inflammatory cells during the healing process[43] and was reduced compared to when active infection was present. During implantation procedures used in this study, the manual application of coating was sufficient to retain antibiotic for local release. In vitro simulations of implant scenarios or alternative orthopaedic models[44-46] may be useful in determining coating retention during intra-cortical implantation procedures such as fixation nails or bone screws. The lipid-based coating can be applied to different surfaces, though differences in adhesion to the surface and elution from different surfaces have not been fully characterized. Future studies will evaluate the properties of the coatings on different surfaces other than metal commonly used in orthopaedic applications, such as polyether ether ketone polymer or hydroxyapatite ceramic biomaterials.
Our results should be interpreted in light of the limitations of this preliminary study. A short duration with a small number of samples in each group was selected for this initial evaluation of only 7 d. For this pilot study, we powered the study to detect large differences in remaining bacteria, but this study should be repeated for robust results. While this model has been used previously and confirmed to lead to osteomyelitic infection without an implant[47,48], previous studies have monitored disease progression and treatment of existing infection after 3 or more weeks post-inoculation. Since the ulnar bone is still intact, the implant does not provide stability to the defect but provides a surface on which biofilm can form, so that animals return to normal activity hours after recovery without the need for fixation devices. Since the focus of this study was prevention of osteomyelitis in an implant-associated infection model, we selected an early time point at which histological and microbiological differences could still be observed[49]. Further studies expanding on these preliminary results should include increased animal numbers and longer durations of implantation, as well as non-contaminated control groups for comparison.
The development of this model has been refined so the strain of S. aureus and the amount of CFUs in the inoculum results in significant evidence of infection in more than 75% of rabbits[49]. Although this provides consistent results for evaluation of anti-infection therapy, it may not be representative of the clinical scenario where infection occurs at much lower rates and presumably with fewer contaminating bacteria. The evidence in this study demonstrating that the antibiotic coating does prevent bacterial growth even in this model of high levels of contamination indicates the clinical potential of this coating. Further, while we did observe histology for uncoated and coated groups to note any potential inflammatory responses, there were no non- antibiotic loaded coatings used as controls or defects without contamination to monitor inflammatory response in this preliminary study. Since this material has been used as a component of bone graft substitutes without issues of severe inflammation[30], it is expected that a degradable thin coating on the implant surface would not lead to negative tissue response and may even stimulate bone growth and healing around the implant. Further studies expanding on these preliminary results should include longer durations of implantation as well as non-contaminated control groups for comparison.
In conclusion, vancomycin-loaded phosphatidylcholine coatings effectively reduced bacterial biofilm formation in an orthopaedic implant-associated model of infection. Local release of antibiotic inhibits bacterial growth and inhibits biofilm formation on the implant. These easily-applied coatings can be used at the time of surgery to prevent orthopaedic infection and improve patient outcomes.
ACKNOWLEDGMENTS
The authors wish to thank Michael Harris, Elysia Masters, Allen Mamaril, and Jonathan Tapp for assistance with histological imaging and scoring.
COMMENTS
Background
Infections associated with medical implants can pose severe threats to patient health. Methods to prevent attachment of bacteria and the formation of biofilm on implants could prevent life-threatening musculoskeletal infections. Phosphatidylcholine coatings can be loaded with antibiotics to protect the implant and surrounding tissue from pathogenic microorganisms.
Research frontiers
Phosphatidylcholine is a naturally-derived lipophilic material that forms a thin layer when manually applied to an implant surface. The point-of-care application provides versatility for use on various implant types and with many different types of antimicrobial molecules.
Innovations and breakthroughs
Compared to systemic administration of antibiotics, local delivery provides high concentrations of therapeutic molecules at the site of injury and potential contamination. Phosphatidylcholine provides a short-term degradable drug carrier matrix that can be directly coated on implants. Complete degradability, as well as implant coverage and biocompatibility, are advantages over other common local drug delivery devices such as polymethylmethacrylate beads or calcium sulfate.
Applications
Results suggest that vancomycin-loaded coatings significantly inhibited staphylococcal growth in a model of implant-associated osteomyelitis, providing prophylactic drug release to prevent infection.
Terminology
Phosphatidylcholine is an amphiphilic molecule consisting of a polar head group and fatty acid tails that when purified can be fabricated into a coating material that leaves a waxy residue on the surface of a biomaterial. Biofilm forms when bacteria or other microorganisms attach to a surface, such as a metal implant or damaged tissue, and causes infection that is highly resistant to antibiotics or immune system clearance. Staphylococcus aureus is among the common pathogens that cause infections in the musculoskeletal system and can lead to osteomyelitis, which is infection of bone.
Peer-review
Interesting paper. This is a basic science study with a useful clinical application.
Footnotes
Supported by Institutional support from Biomet, LLC.
Institutional animal care and use committee statement:Animal studies were approved by the Institutional Review Board at the University of Arkansas for Medical Sciences (IACUC protocol #3540) and followed the IACUC guidelines.
Animal care and use statement: Study protocols were approved by the University of Arkansas for Medical Sciences IACUC and all appropriate measures were taken to minimize pain and discomfort.
Conflict-of-interest statement: Studies were funded and by Biomet, LLC. Karen S Troxel, PhD, is an employee of Zimmer Biomet.
Data sharing statement: Technical appendix, statistical analysis, and dataset available from the corresponding author at jjnnings@memphis.edu.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: March 27, 2016
First decision: May 13, 2016
Article in press: June 29, 2016
P- Reviewer: Elgafy H, Papachristou GC, Vulcano E S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 2.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 3.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med. 2014;276:111–119. doi: 10.1111/joim.12233. [DOI] [PubMed] [Google Scholar]
- 5.Shirwaiker RA, Springer BD, Spangehl MJ, Garrigues GE, Lowenberg DW, Garras DN, Yoo JU, Pottinger PS. A clinical perspective on musculoskeletal infection treatment strategies and challenges. J Am Acad Orthop Surg. 2015;23 Suppl:S44–S54. doi: 10.5435/JAAOS-D-14-00379. [DOI] [PubMed] [Google Scholar]
- 6.Percival SL, Hill KE, Malic S, Thomas DW, Williams DW. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 2011;19:1–9. doi: 10.1111/j.1524-475X.2010.00651.x. [DOI] [PubMed] [Google Scholar]
- 7.Olson ME, Ceri H, Morck DW, Buret AG, Read RR. Biofilm bacteria: formation and comparative susceptibility to antibiotics. Can J Vet Res. 2002;66:86–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999;11:217–221; discussion 237-239. doi: 10.1016/s0924-8579(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 9.Ranall MV, Butler MS, Blaskovich MA, Cooper MA. Resolving biofilm infections: current therapy and drug discovery strategies. Curr Drug Targets. 2012;13:1375–1385. doi: 10.2174/138945012803530251. [DOI] [PubMed] [Google Scholar]
- 10.Tabbaa SM, Horton CO, Jeray KJ, Burg KJ. Role of vascularity for successful bone formation and repair. Crit Rev Biomed Eng. 2014;42:319–348. doi: 10.1615/critrevbiomedeng.2014011662. [DOI] [PubMed] [Google Scholar]
- 11.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46:S47–S52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 12.McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res B Appl Biomater. 2015;103:870–877. doi: 10.1002/jbm.b.33247. [DOI] [PubMed] [Google Scholar]
- 13.Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23:100–107. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanssen AD. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res. 2005;(437):91–96. doi: 10.1097/01.blo.0000175713.30506.77. [DOI] [PubMed] [Google Scholar]
- 15.McLaren AC, McLaren SG, Hickmon MK. Sucrose, xylitol, and erythritol increase PMMA permeability for depot antibiotics. Clin Orthop Relat Res. 2007;461:60–63. doi: 10.1097/BLO.0b013e3181123e90. [DOI] [PubMed] [Google Scholar]
- 16.Orellana BR, Hilt JZ, Puleo DA. Drug release from calcium sulfate-based composites. J Biomed Mater Res B Appl Biomater. 2015;103:135–142. doi: 10.1002/jbm.b.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JK, Moshref AR, Jennings JA, Courtney HS, Haggard WO. Chitosan sponges for local synergistic infection therapy: a pilot study. Clin Orthop Relat Res. 2013;471:3158–3164. doi: 10.1007/s11999-013-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overstreet D, McLaren A, Calara F, Vernon B, McLemore R. Local gentamicin delivery from resorbable viscous hydrogels is therapeutically effective. Clin Orthop Relat Res. 2015;473:337–347. doi: 10.1007/s11999-014-3935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene AH, Bumgardner JD, Yang Y, Moseley J, Haggard WO. Chitosan-coated stainless steel screws for fixation in contaminated fractures. Clin Orthop Relat Res. 2008;466:1699–1704. doi: 10.1007/s11999-008-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketonis C, Parvizi J, Jones LC. Evolving strategies to prevent implant-associated infections. J Am Acad Orthop Surg. 2012;20:478–480. doi: 10.5435/JAAOS-20-07-478. [DOI] [PubMed] [Google Scholar]
- 21.Norowski PA, Courtney HS, Babu J, Haggard WO, Bumgardner JD. Chitosan coatings deliver antimicrobials from titanium implants: a preliminary study. Implant Dent. 2011;20:56–67. doi: 10.1097/ID.0b013e3182087ac4. [DOI] [PubMed] [Google Scholar]
- 22.Jennings JA, Carpenter DP, Troxel KS, Beenken KE, Smeltzer MS, Courtney HS, Haggard WO. Novel Antibiotic-loaded Point-of-care Implant Coating Inhibits Biofilm. Clin Orthop Relat Res. 2015;473:2270–2282. doi: 10.1007/s11999-014-4130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner RA, Hickmon SG, Nelson CL, Germer RA. Modified stain for identification of staphylococcus-aureus in osteomyelitis. J Histotechnol. 1992;15:303–306. [Google Scholar]
- 24.Weiss EC, Spencer HJ, Daily SJ, Weiss BD, Smeltzer MS. Impact of sarA on antibiotic susceptibility of Staphylococcus aureus in a catheter-associated in vitro model of biofilm formation. Antimicrob Agents Chemother. 2009;53:2475–2482. doi: 10.1128/AAC.01432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabath LD, Garner C, Wilcox C, Finland M. Susceptibility of Staphylococcus aureus and Staphylococcus epidermidis to 65 antibiotics. Antimicrob Agents Chemother. 1976;9:962–969. doi: 10.1128/aac.9.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–2339. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 27.McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M, Kathju S, Stoodley P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9:987–1007. doi: 10.2217/fmb.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ketonis C, Barr S, Adams CS, Shapiro IM, Parvizi J, Hickok NJ. Vancomycin bonded to bone grafts prevents bacterial colonization. Antimicrob Agents Chemother. 2011;55:487–494. doi: 10.1128/AAC.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams CS, Antoci V, Harrison G, Patal P, Freeman TA, Shapiro IM, Parvizi J, Hickok NJ, Radin S, Ducheyne P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J Orthop Res. 2009;27:701–709. doi: 10.1002/jor.20815. [DOI] [PubMed] [Google Scholar]
- 30.Han B, Tang B, Nimni ME. Combined effects of phosphatidylcholine and demineralized bone matrix on bone induction. Connect Tissue Res. 2003;44:160–166. doi: 10.1080/03008200390215863. [DOI] [PubMed] [Google Scholar]
- 31.Molinari RW, Khera OA, Molinari WJ. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21 Suppl 4:S476–S482. doi: 10.1007/s00586-011-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giavaresi G, Bertazzoni Minelli E, Sartori M, Benini A, Della Bora T, Sambri V, Gaibani P, Borsari V, Salamanna F, Martini L, et al. Microbiological and pharmacological tests on new antibiotic-loaded PMMA-based composites for the treatment of osteomyelitis. J Orthop Res. 2012;30:348–355. doi: 10.1002/jor.21531. [DOI] [PubMed] [Google Scholar]
- 33.Penn-Barwell JG, Murray CK, Wenke JC. Local antibiotic delivery by a bioabsorbable gel is superior to PMMA bead depot in reducing infection in an open fracture model. J Orthop Trauma. 2014;28:370–375. doi: 10.1097/BOT.0b013e3182a7739e. [DOI] [PubMed] [Google Scholar]
- 34.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong) 2002;10:53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 35.Davies DG, Marques CN. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol. 2009;191:1393–1403. doi: 10.1128/JB.01214-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J Bacteriol. 2011;193:5616–5622. doi: 10.1128/JB.05534-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 38.Jennings JA, Courtney HS, Haggard WO. Cis-2-decenoic acid inhibits S. aureus growth and biofilm in vitro: a pilot study. Clin Orthop Relat Res. 2012;470:2663–2670. doi: 10.1007/s11999-012-2388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahmani-Badi A, Sepehr S, Mohammadi P, Soudi MR, Babaie-Naiej H, Fallahi H. A combination of cis-2-decenoic acid and antibiotics eradicates pre-established catheter-associated biofilms. J Med Microbiol. 2014;63:1509–1516. doi: 10.1099/jmm.0.075374-0. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez CJ, Akers KS, Romano DR, Woodbury RL, Hardy SK, Murray CK, Wenke JC. D-amino acids enhance the activity of antimicrobials against biofilms of clinical wound isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58:4353–4361. doi: 10.1128/AAC.02468-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 2006;50:1463–1469. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estrela AB, Abraham WR. Combining biofilm-controlling compounds and antibiotics as a promising new way to control biofilm infections. Pharmaceuticals. 2010;3:1374–1393. doi: 10.3390/ph3051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010;5:e12580. doi: 10.1371/journal.pone.0012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun. 2011;79:1789–1796. doi: 10.1128/IAI.01386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazzarini L, Overgaard KA, Conti E, Shirtliff ME. Experimental osteomyelitis: what have we learned from animal studies about the systemic treatment of osteomyelitis? J Chemother. 2006;18:451–460. doi: 10.1179/joc.2006.18.5.451. [DOI] [PubMed] [Google Scholar]
- 47.Blevins JS, Elasri MO, Allmendinger SD, Beenken KE, Skinner RA, Thomas JR, Smeltzer MS. Role of sarA in the pathogenesis of Staphylococcus aureus musculoskeletal infection. Infect Immun. 2003;71:516–523. doi: 10.1128/IAI.71.1.516-523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beenken KE, Smith JK, Skinner RA, Mclaren SG, Bellamy W, Gruenwald MJ, Spencer HJ, Jennings JA, Haggard WO, Smeltzer MS. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J Biomater Appl. 2014;29:514–523. doi: 10.1177/0885328214535452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeltzer MS, Thomas JR, Hickmon SG, Skinner RA, Nelson CL, Griffith D, Parr TR, Evans RP. Characterization of a rabbit model of staphylococcal osteomyelitis. J Orthop Res. 1997;15:414–421. doi: 10.1002/jor.1100150314. [DOI] [PubMed] [Google Scholar]


