Abstract
The Gram-positive bacterium Streptococcus pneumoniae causes life-threatening infections, especially among immunocompromised patients. The host's immune system senses S. pneumoniae via different families of pattern recognition receptors, in particular the Toll-like receptor (TLR) family that promotes immune cell activation. Yet, while single TLRs are dispensable for initiating inflammatory responses against S. pneumoniae, the central TLR adapter protein myeloid differentiation factor 88 (MyD88) is of vital importance, as MyD88-deficient mice succumb rapidly to infection. Since MyD88 is ubiquitously expressed in hematopoietic and non-hematopoietic cells, the extent to which MyD88 signaling is required in different cell types to control S. pneumoniae is unknown. Therefore, we used novel conditional knockin mice to investigate the necessity of MyD88 signaling in distinct lung-resident myeloid and epithelial cells for the initiation of a protective immune response against S. pneumoniae. Here, we show that MyD88 signaling in lysozyme M (LysM)– and CD11c-expressing myeloid cells, as well as in pulmonary epithelial cells, is critical to restore inflammatory cytokine and antimicrobial peptide production, leading to efficient neutrophil recruitment and enhanced bacterial clearance. Overall, we show a novel synergistic requirement of compartment-specific MyD88 signaling in S. pneumoniae immunity.
Introduction
Streptococcus pneumoniae is a Gram-positive bacterium that frequently causes invasive diseases such as acute pneumonia, sepsis, and meningitis in infants, the elderly, and immunocompromised individuals.1 An essential prerequisite for invasive pneumococcal infection of the lung involves the microaspiration of S. pneumoniae into the airway lumen. Upon entry into the lung, invading bacteria encounter the first line of defense, composed of the lung epithelium, alveolar macrophages (AMs), and dendritic cells (DCs). These cells recognize S. pneumoniae via pathogen recognition receptors like Toll-like receptors (TLRs).2 Bacterial cell wall and membrane components such as peptidoglycan, lipoproteins, and lipoteichoic acid serve as TLR2 ligands,3, 4 whereas intracellularly located unmethylated CpG-containing DNA is recognized by TLR9.5, 6 Moreover, S. pneumoniae–secreted toxins like the hemolytic pneumolysin, a key virulence factor, activate TLR4.7, 8
TLR activation in AMs induces several effector functions against S. pneumoniae infection to contain and eradicate bacteria, including phagocytosis, secretion of proinflammatory cytokines, and the induction of apoptosis.9 One of the first proinflammatory cytokines released upon S. pneumoniae infection is tumor necrosis factor α (TNFα), which acts in concert with the chemoattractants CXCL1 (KC, keratinocyte chemoattractant) and CXCL2 (MIP-2, macrophage inflammatory protein 2) to recruit polymorphonuclear neutrophils (PMNs) from the periphery to the lungs.10, 11, 12, 13 PMNs efficiently eradicate pneumococci, especially during acute pneumonia when increased bacterial proliferation supersedes the phagocytic capacity of AMs.14 To this end, PMNs engulf bacteria into the phagolysosome followed by the subsequent exposure to antimicrobial peptides.15 In contrast to AMs and PMNs, the phagocytic and bactericidal capability of DCs is reduced.16 During infection, resident DCs within the pulmonary interstitial spaces extend their protrusions through the lung epithelium into the alveolar lumen to sample antigens and pathogens.17 Upon antigen encounter, DC subsets such as CD103+ DCs preferentially migrate to lung-draining lymph nodes (LNs) in a CCR7-dependent manner. DCs pulsed with intact pneumococci are potent activators of the adaptive immune system.18
Besides innate immune cells, the lung epithelium consisting of several specialized cell types also expresses TLRs and contributes to innate immune responses.19 Club cells (CCs, formerly known as Clara cells) are lung epithelial cells expressing TLR4, among other pathogen recognition receptors, that line the bronchiolar airways down to the alveoli, where S. pneumoniae preferentially causes invasive disease.20 Hence, CCs can be triggered to secrete inflammatory cytokines along with antimicrobial peptides, but their role in pneumococcal defense in vivo remains elusive.21, 22 Surfactant protein D (SP-D), expressed by alveolar type II cells and CCs, is important for surfactant homeostasis and also serves as an antimicrobial peptide.23, 24, 25 Furthermore, patients with SP-D deficiency or genetic polymorphisms are more prone to recurrent pneumonia compared to control patients.26, 27
All these different cell types share common TLR expression in order to exert specific antibacterial functions. However, the activation of individual TLRs has a limited relevance in S. pneumoniae immunity as suggested by single TLR–deficient mice, which are able to clear the infection.8, 28, 29 Myeloid differentiation factor 88 (MyD88) is the central signal transduction protein for most TLRs, except for TLR3 and partially for TLR4, and is required for the activation and translocation of nuclear factor-κB into the nucleus and the induction of proinflammatory gene expression. In addition to TLR, interleukin-1 receptor (IL-1R) signaling is also mediated by MyD88 but similar to single TLR deficiency negligible in pneumococcal immunity.10, 30 Mice deficient in MyD88 (MyD88−/−) display impaired innate immune responses reflected by the absence or low levels of proinflammatory cytokines and phagocytic cells, and hence increased bacterial burden. In addition, MyD88−/− mice are highly susceptible to S. pneumoniae and die early after infection.31 Thus, during pneumococcal infection, innate signaling via MyD88 is essential. Yet, despite being the fact that complete deficiency of MyD88 strongly reduces antibacterial responses against S. pneumoniae, the precise contribution of cell type–specific MyD88 signaling provided by hematopoietic and lung epithelial cells in the control of pneumococci remains unclear. To date, the lack of transgenic mouse models for specifically targeting MyD88 in distinct cell subsets has constrained our understanding of MyD88 signaling in innate immune responses against S. pneumoniae and other pathogens.32
In this study, we made use of novel mouse models in which MyD88 expression is restricted to myeloid-derived or lung epithelial cells. We demonstrate that MyD88 signaling in AMs, DCs, and PMNs is crucial for initiating proinflammatory cytokine release and subsequent bacterial eradication, whereas MyD88 signaling in CCs is needed for the enhanced production of antimicrobial peptide to restrict bacterial outgrowth. Our results show that the concerted action of hematopoietic and lung epithelial cells, via MyD88 signaling, is essential for protective immune responses against S. pneumoniae.
RESULTS
Complete protection against pneumococcal infection requires MyD88 signaling in hematopoietic and non-hematopoietic cells
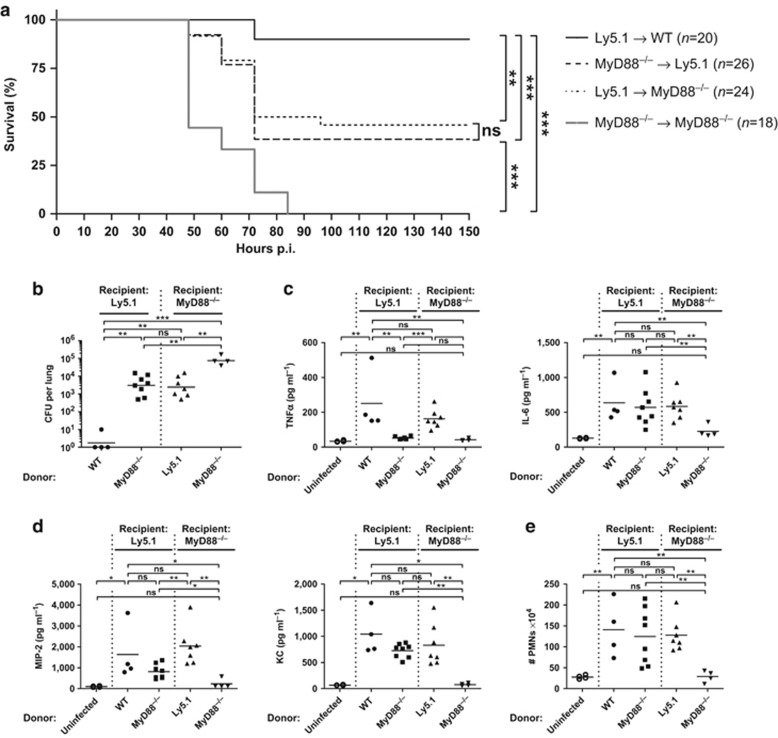
To address the precise role of MyD88-dependent immune responses derived from the hematopoietic and non-hematopoietic compartment, we first generated wild type (WT) and MyD88−/− bone marrow (BM) chimeric mice. The degree of chimerism after lethal irradiation and BM transfer was monitored (except for the MyD88 group) using WT mice expressing the congenic marker Ly5.1 either as BM donor (Ly5.1→MyD88−/−) or recipient (WT→Ly5.1, MyD88−/−→Ly5.1). First, we evaluated disease survival to determine whether compartment-specific MyD88 signaling is sufficient to rescue mice from S. pneumoniae infection. For this purpose, we used the S. pneumoniae TIGR4 strain, commonly found in patients with invasive infection.33 Furthermore, the kinetics of bacterial dissemination was followed using the isogenic luciferase–expressing TIGR4X strain31 and the in vivo imaging system (IVIS) Spectrum computer tomograph (CT) imager (Supplementary Figure S1 online). BM-reconstituted mice were inoculated intranasally with 0.2 × 106 colony-forming units (CFUs) of S. pneumoniae (termed low dose, since higher dosages caused death in >30% of WT mice; Supplementary Figure S2a), and survival was assessed for 336 h (14 days). Consistent with previous findings,31 our results show that 90% of the mice fully sufficient for MyD88 signaling (Ly5.1→WT) survived the infection, whereas all MyD88-deficient mice (MyD88−/−→MyD88−/−) succumbed before 85 h (Figure 1a and Supplementary Figure S1). Interestingly, mice expressing MyD88 only in hematopoietic (Ly5.1→MyD88−/−) or non-hematopoietic (MyD88−/−→Ly5.1) cells showed a survival rate of ∼40%, indicating that MyD88 signaling in either the hematopoietic or non-hematopoietic compartment critically contributes to the protective response against S. pneumoniae infection.
Figure 1.
Myeloid differentiation factor 88 (MyD88) signaling in hematopoietic and non-hematopoietic cells is necessary for survival and chemokine-dependent neutrophil recruitment. Lethally irradiated recipient Ly5.1 (wild type mice expressing the congenic marker Ly5.1) or MyD88−/− mice were reconstituted with bone marrow (BM) cells from donor wild type (WT), Ly5.1 or MyD88−/− mice. After 8 weeks of reconstitution, mice were intranasally inoculated with either a low dose (a) or a high dose (b–e) of Streptococcus pneumoniae. (a) Survival curve of S. pneumoniae–infected BM chimeric mice. Data are pooled from four individual experiments with three to eight mice per group. Statistics were calculated using log-rank (Mantel-Cox) test; **P<0.01 and ***P<0.001. (b) S. pneumoniae burden in total lung homogenates 18 h post infection (p.i.) (c) Tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and (d) macrophage inflammatory protein 2 (MIP-2) and keratinocyte chemoattractant (KC) levels in total lung homogenates 18 h p.i. measured by enzyme-linked immunosorbent assay (ELISA). (e) Fluorescence-activated cell sorting (FACS) analysis of CD11c− MHCII− CD11bhigh Ly6Cint neutrophils (PMNs; excluding CD11c− MHCII− CD11bint Ly6Chi monocytes). Shown are total cell numbers in lungs from mice 18 h p.i. (b–e) Data points depict individual mice. Bars indicate mean values. Data represent two pooled experiments with two to four mice per group. Statistics were calculated using Mann-Whitney test; *P<0.05, **P<0.01, ***P<0.001, and ns, not significant.
To further investigate the role of MyD88 signaling during S. pneumoniae infection, we analyzed inflammatory cytokine production as well as PMN infiltration of low-dose–infected mice18 h post infection (p.i.) WT and MyD88−/− mice showed no significant differences in lung TNFα, IL-6, or total PMN numbers, although at this time point bacterial burden was significantly increased in MyD88-deficient mice compared to WT mice (Supplementary Figure S2b). To examine if this phenotype was influenced by the dose of inoculum, BM chimeric mice were infected with 2 × 106 CFUs of S. pneumoniae (termed high dose), and the number of CFUs in lung homogenates was analyzed. Bacterial loads in WT→Ly5.1 mice were barely detectable, whereas in MyD88−/−→MyD88−/− mice, the number of bacteria was >104 CFUs per lung. Ly5.1→MyD88−/− and MyD88−/−→Ly5.1 mice also showed increased CFUs in comparison to the WT group, but significantly less than in MyD88-deficient mice (Figure 1b). Together, these data suggest that MyD88 signaling in both the hematopoietic and non-hematopoietic compartment is required for the control of bacterial replication in the lung.
Since decreased bacterial burden implies an initial functional innate immune response, we next analyzed pulmonary proinflammatory cytokine and chemokine expression. In MyD88-sufficient (WT→Ly5.1) mice, S. pneumoniae induced a robust TNFα and IL-6 release 18 h p.i. (Figure 1c). TNFα levels were also strongly enhanced in infected Ly5.1→MyD88−/− mice, whereas infected MyD88−/−→Ly5.1 and MyD88−/−→MyD88−/− mice showed levels comparable to the uninfected group, indicating that TNFα production is MyD88-dependent and restricted to the hematopoietic compartment, as previously suggested.34, 35 In contrast, lung IL-6 and to a lesser extent the PMN-attracting chemokines MIP-2 and KC increased upon infection in mice sufficient for MyD88 signaling in the hematopoietic, the non-hematopoietic, or both compartments, whereas MyD88−/−→MyD88−/− mice showed IL-6, MIP-2, and KC levels comparable to uninfected mice (Figure 1c,d). Furthermore, the total number of PMNs in the lungs of Ly5.1→MyD88−/− mice and MyD88−/−→Ly5.1 mice was comparable to WT→Ly5.1, whereas MyD88−/−→MyD88−/− mice completely lacked PMN recruitment (Figure 1e). Thus, MyD88 signaling either in hematopoietic or non-hematopoietic cells is crucial for IL-6, MIP-2, and KC production as well as PMN recruitment to the site of infection.
Together, these data suggest that even though MyD88 signaling in either the hematopoietic or non-hematopoietic compartment improves bacterial clearance, initiates proinflammatory cytokine release and prolongs survival, complete protection against S. pneumoniae infection requires MyD88 signaling in both immune and non-immune cells.
MyD88 signaling in CCs partially contributes to early innate responses against S. pneumoniae
We next asked which non-hematopoietic cells contribute to the innate immunity observed in BM chimeric mice during pneumococcal infection. CCs have been shown to produce proinflammatory cytokines and antimicrobial peptides in response to TLR ligation.21, 23, 24 Therefore, we made use of the MyD88stop (termed MyD88OFF) mouse model, which carries a disruptive stop cassette flanked by two loxP sites between exons 1 and 2 of the Myd88 locus, resulting in a complete loss of MyD88 protein expression.36 To assess whether MyD88 signaling in CCs contributes to the aforementioned immune responses, we crossed MyD88OFF mice to mice expressing the Cre recombinase under the control of the CC secretory protein (CCSP) promoter (CCSP–Cre mice).37 The resulting offspring (termed CCSP–MyD88ON mice) were homozygous for the stop cassette and transgenic for Cre, thus allowing only CCSP+ cells to induce Myd88 gene expression.
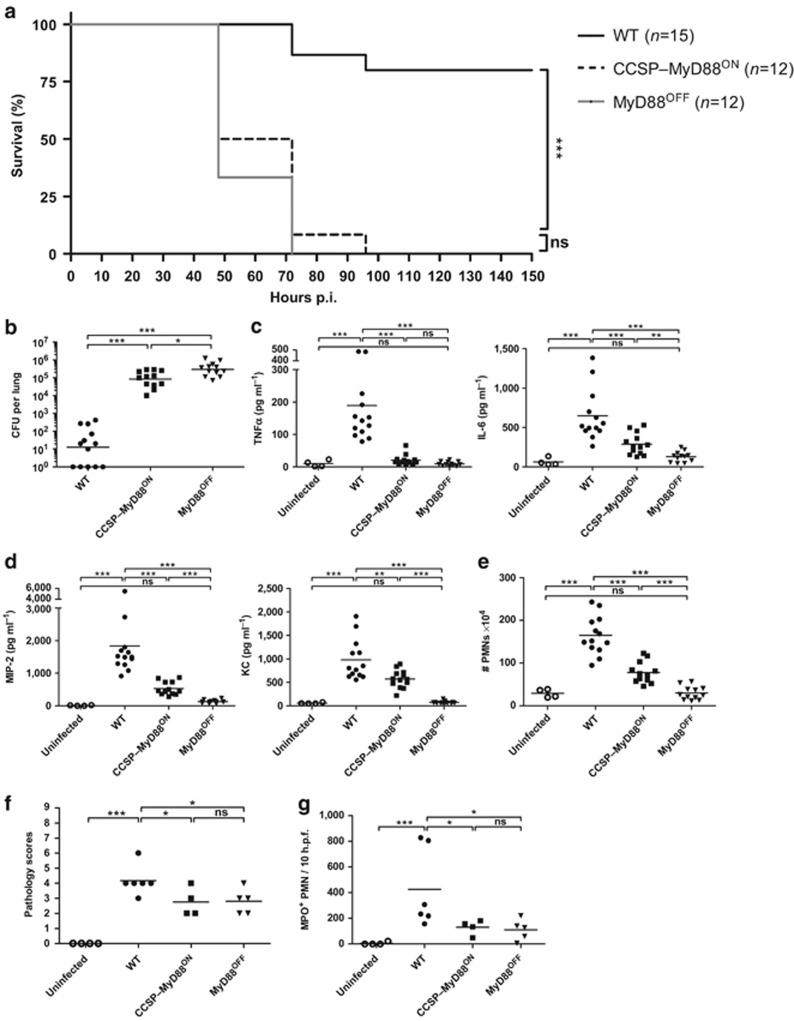
To directly test if MyD88 expression in CCs was sufficient to protect mice from S. pneumoniae infection, WT, CCSP–MyD88ON, and MyD88OFF mice were infected with a low dose of S. pneumoniae and survival was monitored for 14 days. WT mice showed a survival rate of 80% whereas all CCSP–MyD88ON and MyD88OFF mice succumbed to the infection within 96 h (Figure 2a). We next examined whether the impaired survival of CCSP–MyD88ON mice was due to a defect in bacterial clearance. WT, CCSP–MyD88ON, and MyD88OFF mice were infected with a high dose of S. pneumoniae, and bacterial burden was analyzed at 18 h p.i. by determining CFUs in lung homogenates and staining S. pneumoniae using immunohistochemistry. WT mice showed drastically reduced CFUs compared to CCSP–MyD88ON and MyD88OFF mice. However, reactivation of MyD88 signaling in CCSP–MyD88ON mice led to a small but significant reduction in bacterial burden compared to MyD88OFF mice (Figure 2b and Supplementary Figure S3a).
Figure 2.
Myeloid differentiation factor 88 (MyD88) signaling in club cells contributes to proinflammatory cytokine production and control of bacterial burden. Wild type (WT), club cell secretory protein (CCSP)–MyD88ON and MyD88OFF mice were intranasally inoculated with either a low dose (a) or a high dose (b–g) of Streptococcus pneumoniae. (a) Survival curve of S. pneumoniae–infected mice. Data show two pooled individual experiments with five to nine mice per group. Statistics were calculated using log-rank (Mantel-Cox) test; ***P<0.001 and ns, not significant. (b) S. pneumoniae burden in total lung homogenates 18 h post infection (p.i.). (c) Tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and (d) macrophage inflammatory protein 2 (MIP-2) and keratinocyte chemoattractant (KC) levels in total lung homogenates 18 h p.i. measured by enzyme-linked immunosorbent assay (ELISA). (e) Fluorescence-activated cell sorting (FACS) analysis of CD11c− MHCII− CD11bhigh Ly6Cint neutrophils (PMNs; excluding CD11c− MHCII− CD11bint Ly6Chi monocytes). Shown are total cell numbers in lungs from WT, CCSP–MyD88ON, and MyD88OFF mice 18 h p.i. Evaluation of (f) hematoxylin and eosin (H&E) and (g) myeloperoxidase (MPO) staining of lung tissue from uninfected and S. pneumoniae–infected mice 18 h p.i. Tissue sections were evaluated in a blinded manner. Dot plots depict (f) histopathological scoring and (g) MPO+ PMNs counted within 10 high power fields (h.p.f.). (b–e) Data represent three pooled individual experiments with three to five mice per group. (f,g) Data are pooled from two individual experiments with two to three mice per group. (b–g) Data points depict individual mice. Bars indicate mean value. Statistics were calculated using Mann-Whitney test; *P<0.05, **P<0.01, and ***P<0.001.
To further elucidate the relevance of MyD88 signaling in CCs upon S. pneumoniae infection, we next analyzed proinflammatory cytokine and chemokine expression. As expected from our results using BM chimeras, S. pneumoniae infection caused a significant increase in TNFα production in WT mice, while in CCSP–MyD88ON and MyD88OFF mice, TNFα levels were as low as in uninfected controls (Figure 2c). In contrast, in CCSP–MyD88ON mice, IL-6, MIP-2, and KC levels although lower than in WT controls were significantly higher than in MyD88OFF mice (Figure 2c,d). Similarly, WT and, to a lesser extent CCSP–MyD88ON mice, showed increased PMN numbers in the lung compared to MyD88OFF mice (Figure 2e).
Thus, our data suggest that while TNFα production in response to S. pneumoniae is strictly dependent on MyD88 expression in hematopoietic cells, IL-6, MIP-2, and KC secretion and thus, recruitment of PMNs to the lungs, is partially dependent on MyD88 expression in CCs.
In addition to the extensive PMN infiltration of the lungs, it has been described that S. pneumoniae infection is accompanied by transient immunopathology characterized by a marked inflammatory reaction, disruption of normal airway architecture, local bleeding, and alveolar exudates.31 Indeed, pulmonary tissue from WT mice stained with hematoxylin and eosin showed pronounced inflammatory cellular infiltration and local epithelial injury (Figure 2f and Supplementary Figure S4a). In contrast, the lungs of CCSP–MyD88ON and MyD88OFF mice displayed significantly reduced pathology scores. In addition, we tested for myeloperoxidase (MPO), a marker of activated PMNs with microbicidal activity.38 WT mice, but neither CCSP–MyD88ON nor MyD88OFF mice, showed extensive MPO+ PMN infiltration (Figure 2g and Supplementary Figure S4b). In summary, although reactivation of MyD88 signaling in CCs contributes to chemokine expression and PMN recruitment, it is insufficient to provide complete protection against pneumococcal infection.
CCs produce MIP-2, IL-6, and SP-D in a MyD88-dependent manner during pneumococcal infection
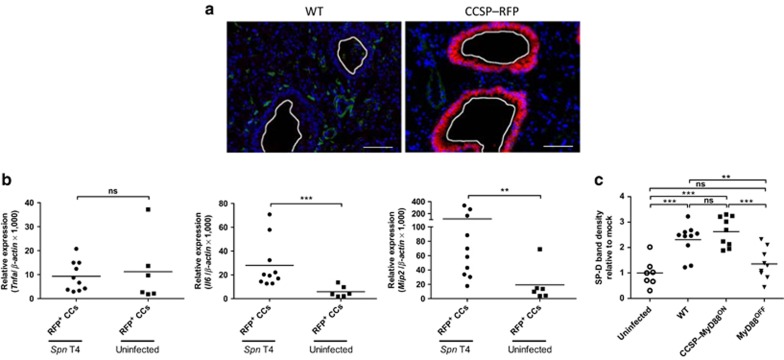
To determine whether intrinsic MyD88 signaling in CCs directly triggers the production of IL-6 and MIP-2 by these cells, or whether indirect effects on hematopoietic cells are responsible for the production of these inflammatory mediators, we made use of CCSP–Cre mice crossed to red fluorescent protein (RFP) reporter mice, thereby terminally labeling CCSP+ cells with RFP expression driven by the ubiquitous ROSA26 promoter.39 Lung sections from CCSP–RFP mice showed RFP signals only within cells located in the bronchiolar airways confirming specific RFP expression in CCs (Figure 3a). Subsequently, we infected CCSP–RFP mice and sorted RFP+ CCs 18 h p.i. RFP+ CCs from infected and uninfected CCSP–RFP mice showed equally low Tnfa levels, whereas increased Il6 and Mip2 transcript levels were detected in CCs from infected CCSP–RFP mice compared to uninfected controls (Figure 3b). We next sorted AMs and PMNs from infected WT, CCSP–MyD88ON, and MyD88OFF mice, and measured Tnfa, Il6, and Mip2 gene expression by quantitative PCR. In WT mice, Tnfa and Mip2 transcript levels were detectable in both AMs and PMNs, whereas Il6 was only detectable in AMs. Furthermore, expression of all the three genes in both AMs and PMNs were equally reduced in CCSP–MyD88ON and MyD88OFF compared to WT-derived cells. Of note, MyD88 expression was neither detectable in AMs or PMNs from CCSP–MyD88ON nor in MyD88OFF mice (Supplementary Figure S5). Thus, upon S. pneumoniae infection CCs produce IL-6 and MIP-2 in a MyD88-dependent cell–intrinsic manner.
Figure 3.
Club cells produce proinflammatory cytokines and surfactant protein D (SP-D) dependent on myeloid differentiation factor 88 (MyD88) signaling. (a) Cryosections from lungs of naive wild type (WT) and club cell secretory protein–red fluorescent protein (CCSP–RFP) mice were stained with anti-RFP antibody. Representative immunofluorescence pictures from one mouse of each group are shown. Bar = 50 μm. (b) CCSP–RFP mice were either intranasally inoculated with a high dose of Streptococcus pneumoniae (Spn T4) or treated with phosphate-buffered saline (PBS, uninfected). CCSP–RFP+ cells were fluorescence-activated cell sorting-(FACS) sorted and analyzed for Tnfa, Il6, and Mip2 gene expression levels by quantitative PCR. Gene expression is shown relative to β-actin expression. (c) WT, CCSP–MyD88ON, and MyD88OFF mice were intranasally inoculated with a high dose of S. pneumoniae and bronchoalveolar lavage (BAL) was taken from uninfected and infected mice 18 h post infection (p.i.) BAL proteins were analyzed by western blot for SP-D content. SP-D band densities were quantified with ImageJ gel analyzer and depicted relative to the mean value of SP-D levels of uninfected mice. (a) Data show one mouse out of two mice from each group from one experiment. (b) Data are pooled from three individual experiments with two to four mice per group. (c) Data represent three pooled individual experiments with one to four mice per group. (b,c) Data points depict individual mice. Bars indicate mean values. Statistics were calculated using Mann-Whitney test; **P<0.01, ***P<0.001, and ns, not significant.
Finally, we asked whether the decreased bacterial burden seen in CCSP–MyD88ON mice (Figure 2b) might be attributed to antimicrobial effects induced by CCs. The antimicrobial peptide SP-D can bind to several microbes including S. pneumoniae leading to enhanced phagocytosis of bacteria by PMNs.40 In addition, SP-D-deficient mice show an increased susceptibility to colonization and infection with S. pneumoniae even though recruitment of PMNs is not altered.41 Therefore, we analyzed SP-D levels within bronchoalveolar lavage (BAL) of infected and uninfected mice. Remarkably, WT and CCSP–MyD88ON mice had equally increased SP-D levels, whereas MyD88OFF mice showed no increase in SP-D compared to uninfected controls (Figure 3c and Supplementary Figure S6a).
Altogether, our data indicate that during S. pneumoniae infection intrinsic MyD88 signaling in CCs is required for IL-6, MIP-2, and SP-D production, but not for TNFα secretion.
MyD88 signaling in CD11c+ and LysM+ cells is required for early innate responses, but does not prevent bacterial dissemination
MyD88 signaling in lung epithelial CCs led to proinflammatory cytokine secretion and restored SP-D production. However, CCSP–MyD88ON mice succumbed to pneumococcal infection due to uncontrolled bacterial outgrowth. Early bacterial clearance is one major function of lung-resident hematopoietic cells, such as AMs and DCs, followed by PMNs. To define the role of MyD88 signaling in these cell populations, we crossed MyD88OFF mice to CD11c–Cre mice42 or LysM–Cre mice43 (expressing Cre recombinase under the control of Cd11c (Itgax) or the LysM promoter, respectively), only the offspring homozygous for MyD88OFF and transgenic for Cre (termed CD11c–MyD88ON and LysM–MyD88ON mice) will have functional Myd88 gene expression in CD11c+ or LysM+ cells. Because CD11c is considered as a crucial marker for DCs within the lungs but is also expressed in AMs,44 and LysM was shown to be expressed predominantly in AMs and PMNs,43 we first evaluated the targeting efficiency in the lungs using CD11c–Cre– and LysM–Cre–RFP reporter mice. In CD11c–RFP mice, 80% of AMs, 70% of DCs, and <5% of PMNs were RFP+. In contrast, LysM–RFP mice targeted ∼75% of AMs and only 20% of PMNs and DCs (Supplementary Figure S7a and b). Lung histology of CD11c–RFP and LysM–RFP mice showed RFP signals only within cells located in pulmonary parenchyma, thus confirming RFP expression exclusively in hematopoietic-derived cells (Supplementary Figure S7c).
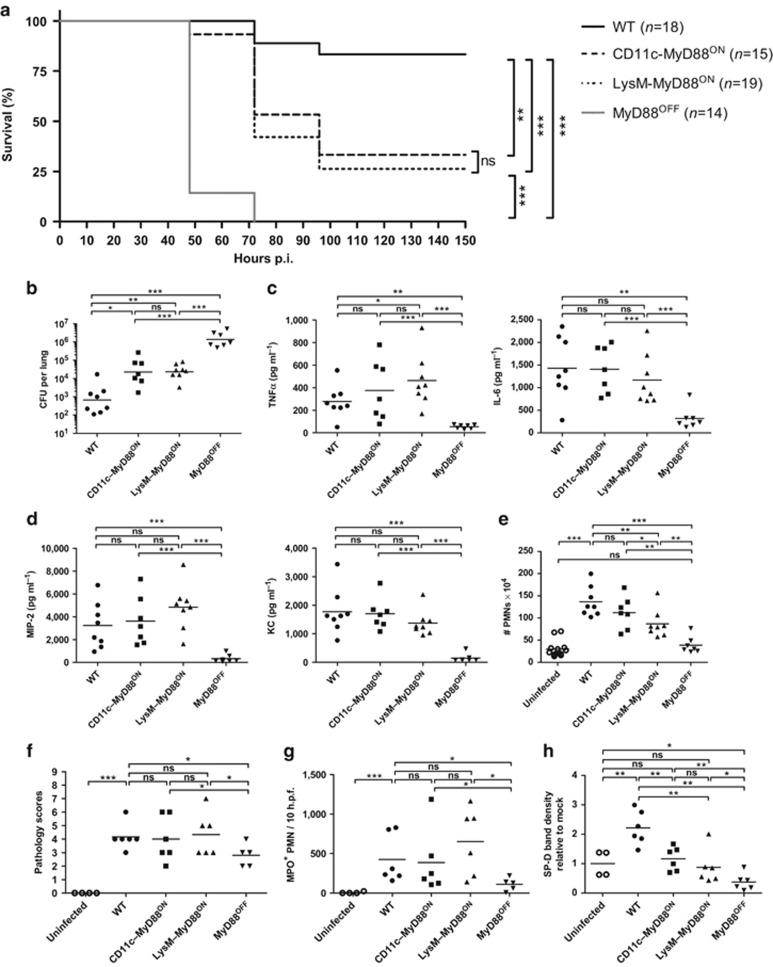
To test whether MyD88 reactivation in CD11c+ or LysM+ cells is sufficient for survival, we infected WT, CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice with a low dose of S. pneumoniae TIGR4, and assessed survival over a period of 14 days. WT mice showed a survival rate of 80%, whereas all MyD88OFF mice succumbed to infection by 72 h. In contrast, ∼25% of CD11c–MyD88ON and LysM–MyD88ON mice survived the infection, suggesting that MyD88 reactivation in either CD11c+ or LysM+ cells is partially required to control the infection. Of note, mice from the WT, CD11c–MyD88ON, or LysM–MyD88ON group succumbed to the infection only after 72 h, implying that MyD88 signaling in these cell populations is crucial in the early phase of infection (Figure 4a). Indeed, the bioluminescence in CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice, infected with a low dose of TIGR4X, was significantly higher than in WT mice until 28 h p.i. However, the signal decreased by 43 h within CD11c–MyD88ON and LysM–MyD88ON mice in comparison to MyD88OFF mice, demonstrating that MyD88 signaling in CD11c+ or LysM+ cells is important for the early containment of bacterial outgrowth (Supplementary Figure S8a). Computed tomography scanning at 67 h p.i. showed that S. pneumoniae disseminated across the whole lungs in CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice (Supplementary Figure S8b).
Figure 4.
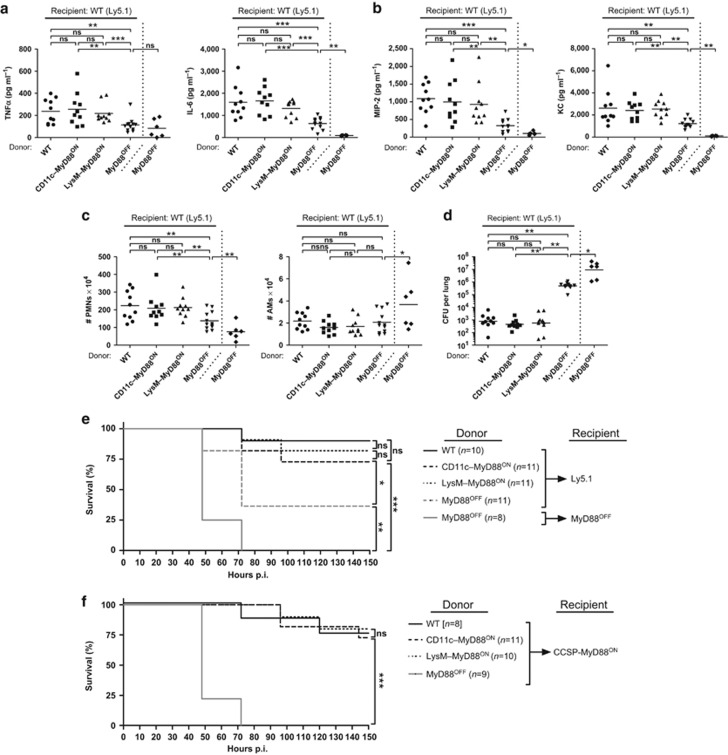
Myeloid differentiation factor 88 (MyD88) reactivation in CD11c or lysozyme M (LysM)–positive cells initiates early innate immune responses leading to improved survival and reduced bacterial burden. Wild type (WT), CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice were intranasally inoculated with either a low dose (a) or a high dose (b–h) of Streptococcus pneumoniae. (a) Survival curve of S. pneumoniae–infected mice. Data show two pooled individual experiments with 6–10 mice per group. Statistics were calculated using log-rank (Mantel-Cox) test; **P<0.01 and ***P<0.001. (b) S. pneumoniae burden in total lung homogenates 18 h post infection (p.i.). (c) Tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and (d) macrophage inflammatory protein 2 (MIP-2) and keratinocyte chemoattractant (KC) levels in total lung homogenates 18 h p.i. measured by enzyme-linked immunosorbent assay (ELISA). (e) Fluorescence-activated cell sorting (FACS) analysis of CD11c− MHCII− CD11bhigh Ly6Cint neutrophils (PMNs; excluding CD11c− MHCII− CD11bint Ly6Chi monocytes). Shown are total cell numbers in lungs from mice 18 h p.i. Uninfected group represents pooled data from three to four phosphate-buffered saline (PBS)–treated mice from each mouse line: WT, CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF. Evaluation of (f) hematoxylin and eosin (H&E) and (g) myeloperoxidase (MPO) staining of lung tissue from uninfected and S. pneumoniae–infected mice 18 h p.i. Tissue sections were evaluated in a blinded manner. Dot plots depict (f) histopathological scoring and (g) MPO+ PMNs counted within 10 high power fields (h.p.f.). (h) Bronchoalveolar lavage (BAL) was taken from uninfected and infected mice 18 h p.i. BAL proteins were analyzed by western blot for surfactant protein D (SP-D) content. SP-D band densities were quantified with ImageJ gel analyzer and depicted relative to the mean value of SP-D levels of uninfected mice. (b–e) Data represent two pooled individual experiments with three to four mice per group. (f,g) Data are pooled from two individual experiments with two to four mice per group. (h) Data show two pooled individual experiments with one to three mice per group. (b–h) Data points depict individual mice. Bars indicate mean values. Statistics were calculated using Mann-Whitney test; *P<0.05, **P<0.01, ***P<0.001, and ns, not significant.
To further investigate the MyD88-dependent contribution of CD11c+ and LysM+ cells in the early phase of infection, we infected mice with a high dose of S. pneumoniae. Consistent with the IVIS data, bacterial burden 18 h p.i. was significantly reduced in the lungs of WT mice compared to CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice. However, CD11c–MyD88ON and LysM–MyD88ON mice showed significantly less CFUs than MyD88OFF mice, demonstrating that MyD88 signaling in CD11c+ and LysM+ cells partially controls early bacterial burden (Figure 4b and Supplementary Figure S3b).
Reactivation of MyD88 signaling in CD11c+ or LysM+ cells was sufficient to restore total lung TNFα, IL-6, MIP-2, and KC to WT levels, whereas MyD88OFF mice produced significantly less inflammatory cytokines (Figure 4c,d). Moreover, sorted AMs from CD11c–MyD88ON and LysM–MyD88ON mice expressed Myd88, Tnfa, Mip2, and Il6 transcript levels comparable or even higher than WT cells and significantly increased in comparison to MyD88OFF mice (Supplementary Figure S9a). In turn, the levels of Myd88, Tnfa, and Mip2 in the PMN population were similar between LysM–MyD88ON and WT mice, but reduced in the CD11c–MyD88ON group. However, WT, CD11c–MyD88ON, and, to a lesser extent, LysM–MyD88ON mice showed significantly more PMNs than MyD88OFF mice, demonstrating that MyD88 signaling in CD11c+ or LysM+ cells is sufficient for the initiation of PMN recruitment to the site of infection (Figure 4e). Hematoxylin and eosin staining of WT, CD11c–MyD88ON, and LysM–MyD88ON pulmonary tissue showed a pronounced inflammatory cellular infiltration and local epithelial injury, whereas the lungs of MyD88OFF mice showed significantly reduced pathology scores in comparison to the aforementioned groups (Figure 4f and Supplementary Figure S4c). In addition, WT, CD11c–MyD88ON, and LysM–MyD88ON mice showed an extensive MPO+ PMN infiltration in comparison to MyD88OFF mice (Figure 4g and Supplementary Figure S4d). Thus, MyD88 reactivation in CD11c+ and LysM+ cells is sufficient to restore early innate responses, but not for complete bacterial control.
Apoptosis of AMs has been proposed as a crucial mechanism contributing to the clearance of pneumococcal infection.45 Inflammatory PMNs participate in this process by increasing TNF-related apoptosis–inducing ligand (TRAIL) expression, thus leading to caspase-3 activation in AMs.45 Consequently, we evaluated whether this apoptotic pathway was related to the impaired bacterial control observed in CD11c–MyD88ON and LysM–MyD88ON mice. However, WT, CD11c–MyD88ON, and LysM–MyD88ON mice had significantly reduced AM numbers in comparison to MyD88OFF mice (Supplementary Figure S9b). Moreover, AMs and PMNs isolated from WT, CD11c–MyD88ON, and LysM–MyD88ON mice, showed significantly higher caspase-3 and Trail transcript levels, respectively, than MyD88OFF cells (Supplementary Figure S9a), suggesting that this mechanism is intact in CD11c–MyD88ON and LysM–MyD88ON mice.
In contrast to phagocytic AMs and PMNs, DCs have less antibacterial activity and rather link the innate and adaptive immune system by presenting processed antigens from the lung lumen to T-cells in the peripheral LNs. Particularly, CD103+ DCs migrate to lung-draining LNs upon antigen encounter.46 Hence, we analyzed if cell-specific reactivation of MyD88 signaling caused alterations in CD103+ DC numbers in the lungs from infected mice. WT, CD11c–MyD88ON, and LysM–MyD88ON mice had significantly decreased CD103+ DC numbers in comparison to MyD88OFF mice (Supplementary Figure S10a). Since migration of DCs to lung-draining LNs was shown to be CCR7-dependent,46 we tested for Ccr7 gene expression in sorted pulmonary CD103+ DCs from infected mice. CD103+ DCs from WT, CD11c–MyD88ON, and LysM–MyD88ON mice showed significantly increased Ccr7 transcript levels in comparison to CD103+ DCs from MyD88OFF mice (Supplementary Figure S10b). Thus, functional MyD88 signaling in PMNs, AMs, and CD103+ DCs restored innate immune responses comparable to WT, but CD11c–MyD88ON and LysM–MyD88ON mice still showed elevated CFU numbers. Therefore, we measured SP-D levels in the BAL of CD11c–MyD88ON and LysM–MyD88ON mice. WT mice had significantly increased SP-D levels, whereas CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice showed SP-D levels comparable to uninfected mice, providing an explanation for higher pneumococcal outgrowth in these mice (Figure 4h and Supplementary Figure S6b).
In conclusion, reactivation of MyD88 signaling in CD11c+ or LysM+ cells is sufficient to restore proinflammatory cytokine responses and to recruit PMNs to the site of infection. Likewise, MyD88 signaling is required for the induction of AM apoptosis by initiating Trail and caspase-3 induction. However, CD11c–MyD88ON and LysM–MyD88ON mice have an impaired pneumococcal clearance leading to delayed bacterial outgrowth and increased mortality, which might be at least in part due to diminished upregulation of epithelial-derived antimicrobial peptides.
Lung epithelium, AMs, and PMNs coordinate innate immunity via MyD88 signaling to control pneumococcal infection
Cell-specific reactivation of MyD88 signaling in AMs, PMNs, or CCs contributes to early innate immune responses by the release of proinflammatory cytokines and chemokines and antimicrobial peptides. However, the immune response from either the hematopoietic or lung epithelial compartment alone is not sufficient to rescue mice from S. pneumoniae infection. To test if MyD88 signals derived from the lung epithelium and hematopoietic cells act synergistically to resolve S. pneumoniae infection, we again generated BM chimeric mice. Here, lethally irradiated WT mice were reconstituted with BM from donor CD11c–MyD88ON, LysM–MyD88ON, or MyD88OFF mice. We also included control chimeras consisting of WT→WT and MyD88OFF→MyD88OFF mice. To confirm the early proinflammatory cytokine response and PMN recruitment seen in mouse lines described earlier in this study, we infected reconstituted mice with a high dose of S. pneumoniae and analyzed 18 h p.i. for TNFα, IL-6, MIP-2, and KC levels. Our results showed that in CD11c–MyD88ON→WT and LysM–MyD88ON→WT mice no significant differences in cytokine levels were observed in comparison to WT→WT mice, whereas MyD88OFF→WT and MyD88OFF→MyD88OFF mice had significantly decreased cytokine levels compared to the other groups (Figure 5a,b). Moreover, PMN and AM numbers in WT→WT mice were comparable to CD11c–MyD88ON→WT and LysM–MyD88ON→WT mice. In contrast, correlating with the decreased chemokine levels MyD88OFF→WT mice had decreased PMN numbers in comparison to WT→WT mice (Figure 5c).
Figure 5.
Lung epithelium, alveolar macrophages (AMs), and neutrophils (PMNs) act synergistically to clear pneumococcal infection in a myeloid differentiation factor 88 (MyD88)–dependent manner. Lethally irradiated recipient (a–e) wild type (WT) or (f) club cell secretory protein (CCSP)–MyD88ON mice were reconstituted with bone marrow (BM) from donor CD11c–MyD88ON, LysM–MyD88ON, or MyD88OFF mice. After 8 weeks of reconstitution, mice were intranasally inoculated with either a high dose (a–d) or low dose (e,f) of Streptococcus pneumoniae. (a) Tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and (b) macrophage inflammatory protein 2 (MIP-2) and keratinocyte chemoattractant (KC) levels in total lung homogenates 18 h post infection (p.i.) measured by enzyme-linked immunosorbent assay (ELISA). (c) Fluorescence-activated cell sorting (FACS) analysis of CD11c− MHCII− CD11bhigh Ly6Cint neutrophils (PMNs; excluding CD11c− MHCII− CD11bint Ly6Chi monocytes) and autofluorescence+ MHCIIint CD11c+ SiglecF+ AMs. Shown are total cell numbers in lungs from mice 18 h p.i. (d) S. pneumoniae burden in total lung homogenates 18 h p.i. (e,f) Survival curve of S. pneumoniae–infected BM chimeric mice. (a–d) Data represent two pooled individual experiments with two to six mice per group. Data points depict individual mice. Bars indicate mean values. Statistics were calculated using Mann-Whitney test; *P<0.05, **P<0.01, and ***P<0.001. (e,f) Data are pooled from two individual experiments with three to six mice per group. Statistics were calculated using log-rank (Mantel-Cox) test; *P<0.05, **P<0.01, ***P<0.001, and ns, not significant.
Since MyD88-dependent antimicrobial peptide production in lung epithelial CCs critically contributed to S. pneumoniae containment, we analyzed lung bacterial burdens in BM chimeric mice. CFUs in WT→WT mice were comparable between CD11c–MyD88ON→WT and LysM–MyD88ON→WT mice. Contrary to this, MyD88OFF→WT mice had strongly increased CFUs in comparison to WT→WT mice but still significantly less bacterial burden than MyD88OFF→MyD88OFF mice (Figure 5d). Therefore, MyD88 signaling in lung epithelial cells, AMs and PMNs is required to efficiently eradicate bacteria.
Furthermore, we infected the mice with a low dose of S. pneumoniae and monitored their survival over 14 days. Consistent with the results obtained in Figure 1a, all MyD88OFF→MyD88OFF mice succumbed to infection, whereas 90% and 36% of WT→WT and MyD88OFF→WT mice survived the infection, respectively. In line with efficient reduction of CFUs, 73% of CD11c–MyD88ON→WT mice and 82% of LysM–MyD88ON→WT mice survived the infection (Figure 5e). Finally, to rule out MyD88 signaling within other pulmonary epithelial cells than CCs, we generated BM chimeric mice consisting of lethally irradiated CCSP–MyD88ON mice reconstituted with BM from donor WT, CD11c–MyD88ON, LysM–MyD88ON, or MyD88OFF mice. WT→CCSP–MyD88ON, CD11c–MyD88ON→CCSP–MyD88ON, and LysM–MyD88ON→CCSP–MyD88ON mice showed ⩾80% survival, whereas all MyD88OFF→CCSP–MyD88ON mice succumbed to infection (Figure 5f), demonstrating that MyD88 signaling by CCs and CD11c+ or LysM+ cells is critically needed within the early phase of infection to rescue mice from an otherwise lethal outcome.
In summary, our data suggest that MyD88 mediated proinflammatory responses in lung epithelium, AMs, and PMNs are required and act in concert to control and clear S. pneumoniae infection.
DISCUSSION
The central importance of MyD88 signaling during S. pneumoniae infection for the induction of a protective immune response is demonstrated by the high susceptibility and early death of MyD88-deficient mice.31 In humans, polymorphisms in genes encoding TLRs have been associated with enhanced susceptibility to recurrent infection with pyogenic bacteria, in particular with S. pneumoniae.47 Mice with individual TLR or IL-1R knockouts were shown to have defects in early proinflammatory responses such as reduced cytokine and chemokine levels, delayed PMN recruitment, or less phagocytic activity within the first hours after S. pneumoniae infection.8, 10, 28, 29 Nevertheless, these impaired initial responses had only partial consequences in establishing functional protection, since the majority of mutant mice survived the infection. Hence, single TLR or IL-1R deficiency has a limited importance in protection against S. pneumoniae. In contrast, MyD88, the central adapter protein for most TLRs and the IL-1R, is of utmost relevance during pneumococcal immunity, suggesting a certain degree of redundancy of pathogen recognition receptors.30, 31
A variety of cell types of hematopoietic and non-hematopoietic origin express MyD88 to induce different inflammatory responses against several pathogens.35, 36, 48, 49, 50, 51 Until now, the cell type–specific role of MyD88 signaling in control and eradication of pneumococci in the respiratory tract remains largely unexplored. In this study, we dissect MyD88 signaling from epithelial and hematopoietic cells during S. pneumoniae infection. To this end, we used BM chimeric mice, as well as conditional MyD88 knockin mouse lines, to gain further insights into the cell-specific contribution of MyD88 in initiating protective immune responses against pneumococcal infection. We demonstrate that both, the hematopoietic and non-hematopoietic compartment are critically dependent on MyD88 for sufficient control and eradication of lung invading S. pneumoniae.
PMN recruitment to the site of infection is a central prerequisite for bacterial eradication, since mice devoid of PMNs, by either using antibody depletion or MIP-2 neutralisation, display increased bacterial outgrowth and mortality upon S. pneumoniae or Klebsiella pneumoniae infection.12, 52 Our study shows that functional MyD88 signaling in either the hematopoietic or the non-hematopoietic compartment has a critical role for the recruitment of PMNs by the release of PMN-attracting chemokines MIP-2 and KC. Airway epithelial cells have been shown to contribute to this process in a variety of experimental systems in vivo.22, 34, 50, 53, 54 In line with our data, pulmonary Pseudomonas aeruginosa infection or lipopolysaccharide (LPS) challenge using MyD88 BM chimeras, showed that lung epithelial cells expressed MIP-2 and/or KC to promote PMN recruitment in a MyD88-dependent manner.34, 53 However, these experiments did not define which specific lung cell subsets are the most relevant for the chemokine secretion. Recently, it has been shown that the transformed murine CC cell line, C22, as well as ex vivo–isolated CCs can be stimulated to induce chemotactic cytokine production upon LPS treatment.21 Similarly, MyD88-dependent activation of CCs in airway inflammation with live P. aeruginosa was demonstrated.35 The authors used a MyD88 cDNA under the control of the rat CCSP promoter expressed in MyD88-deficient mice. P. aeruginosa–infected transgenic mice produced sufficient chemokines to recruit PMNs and to clear the infection.35 In contrast, our data show that upon S. pneumoniae infection, MyD88 signaling in CCs induces MIP-2 and KC production as well as PMN recruitment, albeit to a lower extent than in WT mice. Furthermore, CCSP–MyD88ON mice failed to clear the infection. Differences between the two studies could be attributed to the different nature of the pathogens used. P. aeruginosa, a Gram-negative bacterium, activates a different set of pathogen recognition receptors than the Gram-positive S. pneumoniae. In addition, Mijares et al. used mice on a mixed genetic background not fully backcrossed onto the C57Bl/6J. In addition, in their study, Myd88 expression was under the control of the rat CCSP promoter, unlike our model, which uses the endogenous Myd88 promoter elements and is therefore subjected to more physiological regulation.32 Besides CCs, other lung epithelial cells such as alveolar type I and II cells have been shown to contribute to PMN recruitment.54 We observed greater PMN recruitment in BM chimeric mice expressing MyD88 within the whole lung epithelium compared to CCSP–MyD88ON mice, suggesting that chemokine secretion by several epithelial cells is needed for efficient chemotaxis. A certain level of redundancy might, however, exist between the non-hematopoietic and the hematopoietic compartment, as MyD88 signaling in CD11c+ or LysM+ cells was sufficient to restore chemokine levels and thus PMN numbers to WT levels.
Proinflammatory cytokines, such as IL-6 and TNFα promote not only the recruitment but also PMN activation.12, 55, 56 IL-6 plays an important role in the regulation of TNFα, interferon-γ, and MPO secretion during pneumococcal infection, and deficiency in IL-6 leads to higher susceptibility and lethality.56 TNFα has been shown to be essential for the initiation of S. pneumoniae immunity, since depletion of TNFα prior to infection increases the susceptibility and mortality rate.10, 11, 13 In addition, TNFα has been shown to compensate for host IL-1 type I receptor deficiency, demonstrating that TNFα is more relevant for host defense against S. pneumoniae than IL-1.10 PMN-derived interferon-γ was postulated to be critical in S. pneumoniae immunity by partially regulating the formation of neutrophil extracellular traps and inducing apoptosis, both contributing to bacterial clearance.57 However, we could not observe any MyD88-dependent differences in interferon-γ production. Upon pneumococcal infection WT, CD11c–MyD88ON, LysM–MyD88ON, and MyD88OFF mice showed equally elevated interferon-γ levels (data not shown). Here, we show that upon pneumococcal infection, secretion of TNFα unlike IL-6, is critically dependent on intrinsic MyD88 signaling in the myeloid cell compartment, as previously postulated for other bacterial infections.34, 35
Recruited PMNs in CCSP–MyD88ON mice showed no activation in terms of Tnfa, Il6, or Mip2 transcription. Moreover, low MPO+ PMN numbers were observed in these mice. In contrast, PMNs from CD11c–MyD88ON and LysM–MyD88ON mice had increased transcript levels of proinflammatory cytokine genes along with high MPO+ PMN numbers, suggesting the requirement of MyD88 signaling in myeloid cells for full PMN activation. In particular, since MyD88 reactivation in AMs, but not in PMNs, was comparable between CD11c–MyD88ON and LysM–MyD88ON mice, we cannot exclude indirect cytokine-mediated activation of PMNs independent of intrinsic MyD88 signaling, for example, via AM-derived IL-6. New and more specific Cre lines will therefore be needed to dissect the direct vs. indirect contribution of MyD88 signaling in the different myeloid cell populations.
Isolated primary AMs from MyD88−/− mice have been shown to have a reduced uptake of pneumococci, providing an explanation as to why pneumococcal outgrowth and systemic spread is strongly enhanced in mice deficient for MyD88 signaling in hematopoietic cells.28 In addition, upon S. pneumoniae infection, AMs have been shown to undergo apoptosis. This represents a critical host response that contributes to bacterial eradication and to the resolution of inflammation in the lungs.14, 58 Caspase-3 is a frequently activated protease in cell apoptosis and was shown to be relevant in S. pneumoniae–induced apoptosis in AMs in cooperation with TRAIL expression in PMNs.45 Our results show that MyD88 signaling in AMs critically contributes to the induction of caspase-3 expression and TRAIL might be involved in this process in a MyD88-dependent manner.
The key finding in our study, that MyD88 signaling solely in hematopoietic or non-hematopoietic cells was not sufficient to eradicate bacteria as in WT mice reveals the crucial interplay of both compartments and draws the focus on epithelial cell–derived mediators in pneumococcal clearance. Besides the release of chemokines and cytokines, lung epithelial cells secrete a large array of bacteriostatic or bactericidal molecules. These antimicrobial peptides protect from invading pathogens by several mechanisms, including opsonization, pore formation, or direct bacterial killing.59 Lung epithelial CCs secret several antimicrobial peptides, of which SP-D is consistently reported to be crucially involved in S. pneumoniae immunity. SP-D efficiently binds and aggregates pneumococci in vitro.40 Furthermore, SP-D-deficient mice as well as humans have an increased susceptibility to S. pneumoniae infection.26, 27, 41 Here, we demonstrate that MyD88 signaling in CCs, but not in myeloid cells, is required for the enhanced production of SP-D in response to S. pneumoniae infection. This may contribute to the clearance of pneumococci, as illustrated by BM chimeric mice with functional MyD88 signaling in lung epithelial cells and either CD11c+ or LysM+ cells, where the bacterial burden was comparable to WT mice. Further studies will be necessary to analyze the contribution of other antimicrobial peptides such as serum amyloid A or surfactant protein A, which both have been shown to participate in antimicrobial defense of Gram-positive and -negative bacteria.40, 50, 60
Overall, our results suggest that AMs and PMNs provide essential chemokines and proinflammatory cytokines to recruit phagocytic immune cells, which are in turn partially dependent on epithelial cell–derived SP-D to entirely eradicate pulmonary pneumococci. We propose that AMs, PMNs, and the lung epithelium are critically dependent on MyD88 signaling and act in concert to coordinate innate immunity against S. pneumoniae. This might have direct implications for other lung infection models such as Staphylococcus aureus, P. aeruginosa, and Haemophilus influenzae, in which the bacterial burden is also critically controlled by myeloid and epithelial cells.
Methods
Mice. All animal experiments were performed in compliance with the German animal protection law (TierSchG BGBI. I S. 1105; 25.05.1998), and approved by the Lower Saxony Committee on the Ethics of Animal Experiments as well as the responsible state office (Lower Saxony State Office of Consumer Protection and Food Safety). Mice were housed and handled in accordance with good animal practice as defined by the Federation of European Laboratory Animal Science Associations (FELASA) and the national animal welfare body Society of Laboratory Animals (GV-SOLAS). Mice were bred at the Twincore and Helmholtz Centre for Infection Research (HZI) animal facilities (Hannover and Braunschweig, Lower Saxony, Germany) (Hannover/Braunschweig) under specific pathogen-free conditions.
MyD88−/− mice were kindly provided by Shizuo Akira;61 MyD88OFF mice were provided by Bernhard Holzmann;36 RFP mice were provided by Hans Jörg Fehling;39 CD11c–Cre mice were provided by Boris Reizis;42 LysM–Cre mice were provided by Irmgard Förster;43 and CCSP–Cre mice were provided by Francesco J DeMayo.37 All the mice used in the experiments were on the C57Bl/6J background, sex- and agematched and of 6–16 weeks of age. Mice denoted as WT mice in Figure 1 were C57Bl/6 mice and in Figures 2, 3, 4, 5 were from the CD11c–Cre or CCSP–Cre breeding.
To generate BM chimeras, mice were lethally irradiated with 9 Gy. Donor BM cells were transferred intravenous (i.v.) the next day. Engraftment was assessed prior to the experiments 7–8 weeks later in the blood and during the experiment from total lung cells. After 8 weeks of reconstitution mice were taken for infection experiments.
Antibodies. The following antibodies were purchased from eBioscience (Heidelberg, Germany): APC-conjugated anti-mouse CD3ɛ (clone: 145-2C11), APC-conjugated anti-mouse CD11b (clone: M1/70), APC eFluor 780–conjugated anti-mouse CD11b (clone: M1/70), eFluor 450–conjugated anti-mouse CD11b (clone: M1/70), APC eFluor 780–conjugated anti-mouse CD11c (clone: N418), Alexa Fluor 647–conjugated anti-mouse CD19 (clone: 1D3), PE-conjugated anti-mouse CD45.1 (clone: A20), APC-conjugated anti-mouse CD45.2 (clone: 104), PE-conjugated anti-mouse CD103 (clone: 2E7), PE Cyan 7–conjugated anti-mouse CD45R/B220 (clone: RA3-6B2), Alexa Fluor 647–conjugated anti-mouse Gr-1 (clone: RB6-8C5), FITC-conjugated anti-mouse MHCII (clone: M5/114.15.2), APC-conjugated anti-mouse MHCII (clone: M5/114.15.2), and APC-conjugated anti-mouse NK1.1 (clone: PK136); the following antibodies were purchased from BioLegend (Fell, Germany): Pacific Blue–conjugated anti-mouse CD103 (clone: 2E7), PE Cyan 7–conjugated anti-mouse Ly-6C (clone: HK1.4), and PE Cyan 7–conjugated anti-mouse Ly-6G (clone: 1A8); the following antibody was purchased from BD Pharmingen (Heidelberg, Germany): Brilliant Violet 421–conjugated anti-mouse Siglec-F (clone: E50-2440); the following antibodies were purchased from R&D Systems (Wiesbaden, Germany): sheep anti-mouse SP-D and donkey horseradish peroxidase–conjugated anti-sheep IgG; the following antibody was purchased from Statens Serum Institut (Hillerod, Denmark): Neufeld pneumococcal antiserum type 4; the following antibody was purchased from Dako (Hamburg, Germany): polyclonal anti-MPO; the following antibodies were purchased from Dianova (Hamburg, Germany): biotinylated donkey anti-rabbit and donkey serum; the following antibody was purchased from antibodies online (Aachen, Germany): polyclonal anti-RFP; the following antibody was purchased from Life Technologies (Darmstadt, Germany): Alexa Fluor 555–conjugated donkey anti-rabbit.
Bacterial culture and infection. For infection studies, the S. pneumoniae TIGR4 strain and the luciferase-expressing S. pneumoniae T4X strain31 (kindly provided by B Henriques-Normark) were used. S. pneumoniae was grown overnight from frozen bead stocks on blood agar plates at 37 °C and 5% CO2. The next day single colonies were inoculated into Todd Hewitt broth supplemented with 0.5% yeast extract and grown to mid-logarithmic phase (OD600=0.3) resulting in ∼1 × 108 CFUs ml−1. Appropriate dilutions were made to obtain the desired infectious dose of 0.2 × 106 CFUs for low-dose infections and 2 × 106 CFUs for high-dose infections. CFUs were confirmed by log10 serial dilutions of the inoculum cultured overnight on blood agar plates. Mice were anesthetized with ketamine (Bela-Pharm, Vechta, Germany)/ xylazine (CP-Pharma, Burgdorf, Germany), and a total of 20 μl containing the desired CFU of S. pneumoniae in phosphate-buffered saline (PBS) were intranasally inoculated.
Isolation of lung cells and determination of bacterial burden. Mice were killed by CO2 asphyxiation, and perfused with 5 ml ice-cold PBS. Lung lobes were separated from the trachea, chopped, and incubated in Roswell Park Memorial Institute (RPMI) 1640 GlutaMAX medium (Life/Gibco, Darmstadt, Germany) supplemented with 5% fetal calf serum (Biochrom, Berlin, Germany), containing 2.2 mg ml−1 collagenase D (Sigma-Aldrich, Seelze, Germany) and 0.055 mg ml−1 DNase I (Roche, Mannheim, Germany) for 45 min at 37 °C. Digestion was stopped by addition of 10 mM ethylenediaminetetraacetic acid (EDTA, pH 7.5), and the lung cell suspension was passed through a 70-μm cell strainer. Aliquots were taken for CFU determination, fluorescence-activated cell sorting (FACS) analysis, and enzyme-linked immunosorbent assay (ELISA) of cytokines.
CFU determination was performed by log10 serial dilution of the aliquot and incubation on blood agar plates overnight at 37 °C and 5% CO2. For FACS analysis, the aliquot was pelleted and red blood lysis (150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA at pH 7.2–7.4) was performed for 2 min at room temperature and stopped by adding phosphate-bovine-azide (PBA) (2 mM EDTA, 0.5% bovine serum albumin, and 0.02% sodium azide in PBS). Lung cells were washed with PBA, and then used for FACS staining. For ELISA, one volume of lung lysis buffer (600 mM NaCl, 4 mM MgCl2 6H2O, 4 mM CaCl2 2H2O, 60 mM TRIS, 2% Triton X-100 (Sigma-Aldrich, München, Germany), and complete protease inhibitor cocktail (Roche) at pH 7.4) was added to the aliquot, and incubated for 20 min at 4 °C. Cell debris was pelleted, and the supernatant was used for ELISA. The concentrations of cytokines in lung homogenates were analyzed using the DuoSet ELISA kits (R&D Systems) according to the manufacturer's instructions.
Flow cytometry. For cell surface marker staining, cells were preincubated with 1% Fc-block in PBA for 10 min on ice, and then washed and incubated with the indicated monoclonal antibody conjugates for 20 min on ice in a total volume of 50 μl of PBA. Dead cells were excluded by 4′,6-diamidin-2-phenylindol (DAPI; Sigma-Aldrich) or ethidium bromide monoazide (EMA; Sigma-Aldrich) staining, and cellular aggregates were excluded by side scatter pulse width. After the staining procedure cells were fixed in 200 μl PBS containing 2% paraformaldehyde for 15 min on ice. After fixation cells were washed and resuspended in PBA and stored at 4 °C until FACS analysis. Samples were acquired on a LSRII (BD Biosciences, Heidelberg, Germany), and analyzed with FlowJo software (Tree Star, Ashland, OR).
Immunohistochemistry. Formalin-fixed lungs were embedded in paraffin, and 1–2 μm sections were cut. Paraffin sections were deparaffinized and subjected to a heat-induced epitope retrieval step. Slides were rinsed in cool running water and washed in Tris-buffered saline (pH 7.4) prior to incubation with polyclonal anti-MPO (Dako) or with Neufeld antiserum (Statens Serum Institut) for 30 min at room temperature and overnight at 4 °C, respectively. After rinsing, sections stained for MPO were incubated for 30 min at room temperature with biotinylated donkey anti-rabbit (Dianova). For detection, Dako REAL Detection System, alkaline phosphatase/Red (Dako) was used. Alkaline phosphatase was revealed by Fast Red (Dako) as chromogen for 30 min at room temperature. For detection of pneumococci, Dako EnVision+ System-HRP labelled polymer (Dako) was used. Horseradish peroxidase was revealed by diaminobenzidine as chromogen for 5 min at room temperature. Nuclei were counterstained with hematoxylin and slides were mounted with gelatine (Merck Millipore, Darmstadt, Germany).
For detection of RFP, 5 μm sections of cryopreserved lungs were fixed in acetone and incubated with serum-free protein block (Dako). After tapping off the blocking solution, sections were incubated with polyclonal anti-RFP (antibodies online, Aachen) overnight at 4 °C. Slides were rinsed, blocked with donkey serum (Dianova), and incubated with Alexa Fluor 555–conjugated donkey anti-rabbit (Life Technologies). Nuclei were counterstained with DAPI (Sigma-Aldrich), and slides were mounted with Fluoromount-G (eBioscience).
Negative controls were performed omitting primary antibodies. Images were acquired using an Axio Imager Z1 microscope (Carl Zeiss MicroImaging, Jena, Germany). Positive cells were quantified in 10 high power fields (h.p.f.; 1 h.p.f.=0.237 mm2).
For evaluation of histomorphology, hematoxylin and eosin–stained paraffin sections were scored as follows. The perivascular, peribronchial, and interstitial infiltration of leukocytes was individually scored with 1 as minimal, 2 as mild, and 3 as severe infiltration. The overall inflammation score is the sum of the individual scores. All immunohistochemical evaluations were performed in a blinded manner.
In vivo imaging. Mice were anesthetized with isoflurane carried in 2% O2 and placed in a supine position in a chamber for imaging with the IVIS Spectrum CT (PerkinElmer, Waltham, MA). For detection of three-dimensional bioluminescent signal, anesthetized mice were first scanned by computed tomography and then S. pneumoniae–derived bioluminescence was detected by the charge-coupled device camera. Analysis was performed with Living Image 4.4 software (PerkinElmer).
BAL and western blot. Mice were killed by CO2 asphyxiation and BAL was collected by gently lavaging the lung two times with 0.7 ml ice-cold PBS. BAL was centrifuged and the cell-free supernatant was stored at −80 °C until western blot analysis. Total protein concentration in BAL was measured by bicinchoninic acid assay (Life Technologies). Equal amounts of total protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane (Merck Millipore). Immunoblotting was performed using sheep anti-mouse SP-D and donkey horseradish peroxidase–conjugated anti-sheep IgG, and detected using ECL prime (GE Healthcare, Freiburg, Germany). Chemoluminescence was detected with the ChemoStar Imager (Intas, Göttingen, Germany), and analyzed with ImageJ software (National Institutes of Health, Bethesda, MD).
RNA isolation and quantitative real-time PCR. Total RNA was isolated from FACS-sorted lung cells using the RNeasy Micro kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA was generated using SuperScript III reverse transcriptase with oligo(dT)12–18 primer in the presence of RNaseOUT recombinant ribonuclease inhibitor (all Invitrogen, Darmstadt, Germany). Real-time PCR was carried out with iQ SYBR green supermix (Bio-Rad, Munich, Germany). Following primers were used: β-actin forward strand 5′-TGTTACCAACTGGGACGACA-3′, reverse strand 5′-GGGGTGTTGAAGGTCTCAAA-3′ Myd88 forward strand 5′-AGAGCTGCTGGCCTTGTTAG-3′, reverse strand 5′-TTCTCGGACTCCTGGTTCTG-3′ Trail forward strand 5′-GAAGACCTCAGAAAGTGGC-3′, reverse strand 5′-GACCAGCTCTCCATTCCTA-3′ Ccr7 forward strand 5′-GTGTGCTTCTGCCAAGATGA-3′, reverse strand 5′-CCACGAAGCAGATGACAGAA-3′ Casp3 forward strand 5′-TCAGAGGCGACTACTGCCGGA-3′, reverse strand 5′-CCACCGGTATCTTCTGGCAAGCC-3′ Tnfa forward strand 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, reverse strand 5′-TGGGAGTAGACAAGGTACAACCC-3′ Mip2 forward strand 5′-AGACAGAAGTCATAGCCACTCTCAAG-3′, reverse strand 5′-CCTCCTTTCCAGGTCAGTTAGC-3′ Il6 forward strand 5′-TGTCTATACCACTTCACAAGTCGGAG-3′, reverse strand 5′-GCACAACTCTTTTCTCATTTCCAC-3′ (all primers were obtained from Eurofins MWG Operon, Hamburg, Germany). Samples were analyzed on a LightCycler 480 II (Roche). Relative gene expression was calculated as 2−Ct(target)/2−Ct(β-actin).
Statistics. For survival curves, a log-rank (Mantel-Cox) test was performed. All other statistical analyses were performed using Mann-Whitney test unless additionally indicated. Analysis was performed using GraphPad Prism software (GraphPad software, La Jolla, CA); *P<0.05, **P<0.01, and ***P<0.001.
Ethics statement. All animal experiments were approved by the Lower Saxony Committee on the Ethics of Animal Experiments as well as the responsible state office (Lower Saxony State Office of Consumer Protection and Food Safety) under the permit numbers 33.9-42502-04-09/1785 and 33.12-42502-04-12/0910.
Acknowledgments
We thank M Swallow and P Ghorbani for critical reading of the manuscript, and F Kruse, M Swallow, M Gohmert, and M Thiele for their excellent technical help. We also acknowledge the assistance of the Cell Sorting Core Facility of the Hannover Medical School supported in part by Braukmann-Wittenberg-Herz-Stiftung and Deutsche Forschungsgemeinschaft. MD was supported by the International Research Training Group 1273 funded by the German Research Foundation (DFG).
The authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
Supplementary Material
References
- Bogaert, D., De Groot, R. & Hermans, P.W. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4, 144–154 (2004). [DOI] [PubMed] [Google Scholar]
- Kadioglu, A. & Andrew, P.W. The innate immune response to pneumococcal lung infection: the untold story. Trends Immunol. 25, 143–149 (2004). [DOI] [PubMed] [Google Scholar]
- Aliprantis, A.O. et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739 (1999). [DOI] [PubMed] [Google Scholar]
- Schwandner, R., Dziarski, R., Wesche, H., Rothe, M. & Kirschning, C.J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J. Biol. Chem. 274, 17406–17409 (1999). [DOI] [PubMed] [Google Scholar]
- Heeg, K., Sparwasser, T., Lipford, G.B., Hacker, H., Zimmermann, S. & Wagner, H. Bacterial DNA as an evolutionary conserved ligand signalling danger of infection to immune cells. Eur. J. Clin. Microbiol. Infect. Dis. 17, 464–469 (1998). [DOI] [PubMed] [Google Scholar]
- Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000). [DOI] [PubMed] [Google Scholar]
- Molloy, S. Pneumolysin: stimulating protection. Nat. Rev. Microbiol. 9, 4 (2011). [DOI] [PubMed] [Google Scholar]
- Malley, R. et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100, 1966–1971 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberdein, J.D., Cole, J., Bewley, M.A., Marriott, H.M. & Dockrell, D.H. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin. Exp. Immunol. 174, 193–202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijneveld, A.W., Florquin, S., Branger, J., Speelman, P., Van Deventer, S.J. & van der Poll, T. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J. Immunol. 167, 5240–5246 (2001). [DOI] [PubMed] [Google Scholar]
- O'Brien, D.P., Briles, D.E., Szalai, A.J., Tu, A.H., Sanz, I. & Nahm, M.H. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect. Immun. 67, 595–601 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger, M.J et al. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 173, 159–165 (1996). [DOI] [PubMed] [Google Scholar]
- van der Poll, T., Keogh, C.V., Buurman, W.A. & Lowry, S.F. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 155, 603–608 (1997). [DOI] [PubMed] [Google Scholar]
- Knapp, S. et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167, 171–179 (2003). [DOI] [PubMed] [Google Scholar]
- Standish, A.J. & Weiser, J.N. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J. Immunol. 183, 2602–2609 (2009). [DOI] [PubMed] [Google Scholar]
- Guilliams, M., Lambrecht, B.N. & Hammad, H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 6, 464–473 (2013). [DOI] [PubMed] [Google Scholar]
- Sung, S.S., Fu, S.M., Rose, C.E. Jr, Gaskin, F., Ju, S.T. & Beaty, S.R. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176, 2161–2172 (2006). [DOI] [PubMed] [Google Scholar]
- Colino, J., Shen, Y. & Snapper, C.M. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J. Exp. Med. 195, 1–13 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenstiel, S., Opitz, B., Schmeck, B. & Suttorp, N. Lung epithelium as a sentinel and effector system in pneumonia—molecular mechanisms of pathogen recognition and signal transduction. Respir. Res. 7, 97 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita, T., Sakurai, Y., Ishibashi, S. & Maru, Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene 30, 3429–3439 (2011). [DOI] [PubMed] [Google Scholar]
- Elizur, A., Adair-Kirk, T.L., Kelley, D.G., Griffin, G.L., deMello, D.E. & Senior, R.M. Clara cells impact the pulmonary innate immune response to LPS. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L383–L392 (2007). [DOI] [PubMed] [Google Scholar]
- Poynter, M.E., Irvin, C.G. & Janssen-Heininger, Y.M. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J. Immunol. 170, 6257–6265 (2003). [DOI] [PubMed] [Google Scholar]
- Crouch, E., Parghi, D., Kuan, S.F. & Persson, A. Surfactant protein D: subcellular localization in nonciliated bronchiolar epithelial cells. Am. J. Physiol. 263, L60–L66 (1992). [DOI] [PubMed] [Google Scholar]
- Wong, C.J., Akiyama, J., Allen, L. & Hawgood, S. Localization and developmental expression of surfactant proteins D and A in the respiratory tract of the mouse. Pediatr. Res. 39, 930–937 (1996). [DOI] [PubMed] [Google Scholar]
- Botas, C. et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc. Natl. Acad. Sci. USA 95, 11869–11874 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese, M., Steinecker, M., Schumacher, S., Braun, A., Lohse, P. & Heinrich, S. Children with absent surfactant protein D in bronchoalveolar lavage have more frequently pneumonia. Pediatr. Allergy Immunol. 19, 639–647 (2008). [DOI] [PubMed] [Google Scholar]
- Lingappa, J.R. et al. Identifying host genetic risk factors in the context of public health surveillance for invasive pneumococcal disease. PLoS One 6, e23413 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albiger, B. et al. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell. Microbiol. 9, 633–644 (2007). [DOI] [PubMed] [Google Scholar]
- Knapp, S. et al. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 172, 3132–3138 (2004). [DOI] [PubMed] [Google Scholar]
- Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004). [DOI] [PubMed] [Google Scholar]
- Albiger, B. et al. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell. Microbiol. 7, 1603–1615 (2005). [DOI] [PubMed] [Google Scholar]
- Arnold-Schrauf, C., Berod, L. & Sparwasser, T. Dendritic cell specific targeting of MyD88 signalling pathways in vivo. Eur. J. Immunol. 45, 32–39 (2015). [DOI] [PubMed] [Google Scholar]
- Sandgren, A., Albiger, B., Orihuela, C.J., Tuomanen, E., Normark, S. & Henriques-Normark, B. Virulence in mice of pneumococcal clonal types with known invasive disease potential in humans. J. Infect. Dis. 192, 791–800 (2005). [DOI] [PubMed] [Google Scholar]
- Hajjar, A.M., Harowicz, H., Liggitt, H.D., Fink, P.J., Wilson, C.B. & Skerrett, S.J. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am. J. Respir. Cell Mol. Biol. 33, 470–475 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijares, L.A., Wangdi, T., Sokol, C., Homer, R., Medzhitov, R. & Kazmierczak, B.I. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J. Immunol. 186, 7080–7088 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais, P. et al. Cutting edge: divergent cell-specific functions of MyD88 for inflammatory responses and organ injury in septic peritonitis. J. Immunol. 188, 5833–5837 (2012). [DOI] [PubMed] [Google Scholar]
- Li, H., Cho, S.N., Evans, C.M., Dickey, B.F., Jeong, J.W. & DeMayo, F.J. Cre-mediated recombination in mouse Clara cells. Genesis 46, 300–307 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, D. et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. USA 102, 431–436 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luche, H., Weber, O., Nageswara Rao, T., Blum, C. & Fehling, H.J. Faithful activation of an extra-bright red fluorescent protein in “knock-in” Cre-reporter mice ideally suited for lineage tracing studies. Eur. J. Immunol. 37, 43–53 (2007). [DOI] [PubMed] [Google Scholar]
- Hartshorn, K.L. et al. Pulmonary surfactant proteins A and D enhance neutrophil uptake of bacteria. Am. J. Physiol. 274, L958–L969 (1998). [DOI] [PubMed] [Google Scholar]
- Jounblat, R., Clark, H., Eggleton, P., Hawgood, S., Andrew, P.W. & Kadioglu, A. The role of surfactant protein D in the colonisation of the respiratory tract and onset of bacteraemia during pneumococcal pneumonia. Respir. Res. 6, 126 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton, M.L., Smith-Raska, M.R. & Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, B.E., Burkhardt, C., Reith, W., Renkawitz, R. & Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999). [DOI] [PubMed] [Google Scholar]
- Vermaelen, K. & Pauwels, R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 61, 170–177 (2004). [DOI] [PubMed] [Google Scholar]
- Steinwede, K. et al. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J. Exp. Med. 209, 1937–1952 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, H. et al. Migratory properties of pulmonary dendritic cells are determined by their developmental lineage. Mucosal Immunol. 6, 678–691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea, M.G., Wijmenga, C. & O'Neill, L.A. Genetic variation in Toll-like receptors and disease susceptibility. Nat. Immunol. 13, 535–542 (2012). [DOI] [PubMed] [Google Scholar]
- Arnold-Schrauf, C. et al. Dendritic cells coordinate innate immunity via MyD88 signaling to control Listeria monocytogenes infection. Cell Rep. 6, 698–708 (2014). [DOI] [PubMed] [Google Scholar]
- Berod, L. et al. MyD88 signalling in myeloid cells is sufficient to prevent chronic mycobacterial infection. Eur. J. Immunol. 44, 1399–1409 (2014). [DOI] [PubMed] [Google Scholar]
- Cleaver, J.O. et al. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol. 7, 78–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad, H., Chieppa, M., Perros, F., Willart, M.A., Germain, R.N. & Lambrecht, B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias, K.A., Roche, A.M., Standish, A.J., Shchepetov, M. & Weiser, J.N. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J. Immunol. 180, 6246–6254 (2008). [DOI] [PubMed] [Google Scholar]
- Noulin, N. et al. Both hemopoietic and resident cells are required for MyD88-dependent pulmonary inflammatory response to inhaled endotoxin. J. Immunol. 175, 6861–6869 (2005). [DOI] [PubMed] [Google Scholar]
- Yamamoto, K. et al. Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia. Am. J. Respir. Cell Mol. Biol. 50, 253–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs, N.W., Strieter, R.M., Chensue, S.W., Widmer, M. & Kunkel, S.L. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J. Immunol. 154, 5411–5417 (1995). [PubMed] [Google Scholar]
- van der Poll, T., Keogh, C.V., Guirao, X., Buurman, W.A., Kopf, M. & Lowry, S.F. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176, 439–444 (1997). [DOI] [PubMed] [Google Scholar]
- Gomez, J.C. et al. Mechanisms of interferon-gamma production by neutrophils and its function during Streptococcus pneumoniae pneumonia. Am. J. Respir. Cell Mol. Biol. 52, 349–364 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell, D.H. et al. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171, 5380–5388 (2003). [DOI] [PubMed] [Google Scholar]
- Bals, R. & Hiemstra, P.S. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur. Respir. J. 23, 327–333 (2004). [DOI] [PubMed] [Google Scholar]
- Shah, C., Hari-Dass, R. & Raynes, J.G. Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood 108, 1751–1757 (2006). [DOI] [PubMed] [Google Scholar]
- Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.