Abstract
Early environmental conditions are increasingly appreciated as critical in shaping behavior and cognition. Evidence suggests that stressful rearing environments can have an enduring impact on behaviors in adulthood, but few studies have explored the possibility that rearing environment could exacerbate genetic hyperactivity disorders. Uncovering a strong environmental influence on the transmission of hyperactivity could provide novel avenues for translational research. Recently we developed a selectively bred High-Active line of mice to model ADHD, providing a unique resource to address the question of environmental transmission. The High-Active line demonstrates transgenerational hyperactivity, but the influence of the postnatal environment (i.e. maternal care provided by dams) on hyperactivity had not been systemically quantified. This study employed a cross-fostering method to simultaneously address 1) whether High-Active and Control pups are provided with similar levels of care in the early environment, and 2) whether any differences in rearing environment influence hyperactive behavior. High-Active dams demonstrated impairment in all measures of maternal competence relative to Controls, which reduced survival rates and significantly reduced the body mass of offspring in early life and at weaning. While the deteriorated postnatal environment provided by High-Active dams was ultimately sufficient to depress Control activity, the hyperactivity of High-Active offspring remained unaffected by fostering condition. These data not only confirm the power of genetics to influence hyperactivity across generations, but also provide evidence that early rearing environments may not have a significant impact on the extreme end of hyperactive phenotypes.
Keywords: selective breeding, maternal care, pup retrieval, postnatal stress, genetic hyperactivity
1. Introduction
The power of environment to influence complex behavioral phenotypes has generated great interest over the past several decades [1]. In addition to the established influence of genetics (e.g. mutations altering protein expression/function) on phenotype, maternal environment (e.g. histone modification, DNA methylation) may also disrupt the trajectory of normal behavioral and psychological development in individuals predisposed to disease states. Multiple studies have demonstrated significant associations between adversive postnatal environments and the manifestation of clinical disorders later in life [2–4]. Modeling the effects of early life stressors in animals has largely corroborated such results; exposure to maternal separation or neglect heightens stress reactivity, aggression, and other features of clinical disorders such as depression and schizophrenia [5–8]. Accumulating evidence not only suggests that early life environment affects relevant genes via epigenetic modifications which may serve as biomarkers for susceptible populations [9, 10], but furthermore that environmentally-induced behavioral deficits may be transmitted to subsequent generations [11–13]. Determining the relative contribution of genes versus environment to the manifestation of a specific behavior is of critical importance for not only elucidating its etiology, but also guiding ongoing endeavors to identify relevant trait-specific biomarkers.
Over the past decade, our lab has maintained a line of mice selectively bred for an extreme hyperactivity phenotype which has shown promise as a model of Attention-deficit/Hyperactivity disorder (ADHD) in recent generations [14, 15]. ADHD demonstrates exceptionally high heritability estimates in the range of 70–90% [16, 17], therefore we propagated a highly genetically variable line of mice for hyperactive behavior across multiple generations. Additional studies have further validated the High-Active model by demonstrating that their hyperactivity is paradoxically ameliorated by low-dose amphetamine, a psychostimulant commonly used to treat ADHD [14]. However, an important remaining question is whether our assumption, that High-Active hyperactivity is driven purely through genetic factors, is correct. It is possible that some of the hyperactive phenotype is transmitted to offspring through extremely hyperactive dams creating a chaotic, stressful early rearing environment [18, 19]. This alternative explanation for the propagation of hyperactivity has never been systemically explored in our line, despite circumstantial evidence for its possibility; across multiple generations, the High-Active line shows impaired reproductive success [14] which has anecdotally been ascribed to High-Active dams providing poor maternal care by engaging in trampling and/or cannibalization behavior. Thus, in order to understand the transmission of hyperactivity in the High-Active line it is critical to 1) assess the quality of High-Active versus Control rearing environments, and 2) determine whether these environments influence the hyperactive phenotype. Evidence of a substantial environmental influence on hyperactivity would support studies suggesting adversive environments exacerbate ADHD-like behaviors [19, 20], while a predominantly genetic component to hyperactivity, minimally influenced by environment, would more closely support the construct validity of the High-Active line to model a disorder as highly influenced by genetics as ADHD [16].
In order to parse out the contributions of environment and genetics on home cage activity levels, we employed a cross-foster design to simultaneously address both aforementioned questions. Dams underwent maternal care observations and performed pup retrieval tasks with their assigned litters, which were comprised of a mixture of pups from both lines. This approach allowed us to operationalize the definition of poor maternal care. Evaluating maternal competency and its effect on hyperactivity are of critical importance, not only in understanding the transmission of hyperactivity in our model, but also in providing direct tangible evidence for or against the idea that postnatal environment affects hyperactivity in adulthood. Either outcome, that rearing environment does or does not influence hyperactivity, advances our understanding of the relative influence of genetic versus environmental factors that contribute to variation in physical activity.
2. Materials and Methods
2.1 Animals
2.1.1. Selective breeding and general husbandry
Our lab maintains two lines of outbred mice; a randomly bred, unselected Control line and a High-Active line selectively bred for increased distance traveled in the home cage [14, 15]. The starting population for each line was generated from the highly genetically variable Collaborative Cross mice [21]. At approximately postnatal day (PND) 60, mice of each line were phenotyped individually for home cage activity. Custom-made acrylic home cages (18.5 × 33.5 × 16 cm) with clear plastic lids allow for continuous video tracking by TopScan software (CleverSystems, Reston, VA, USA). Each cage individually housed 4 mice, with a wire mesh interaction zone that allowed for limited physical contact. This video coverage allowed for simultaneous tracking of a maximum of 64 individual mice over a 6-day test. After an extended habituation period of 4 days, the average distance traveled during days 5 and 6 was used as the selection criterion for the High-Active line. Controls were randomly bred with respect to distance traveled in the home cage. Selective pressure was not applied to the High-Active line in Generations 13 and 14 (Fig. 1C) due to a lack of resources and personnel; during these two generations, no mice were phenotyped and therefore the High-Active breeding protocol followed that of the Control line (mice from different families were bred randomly within each line).
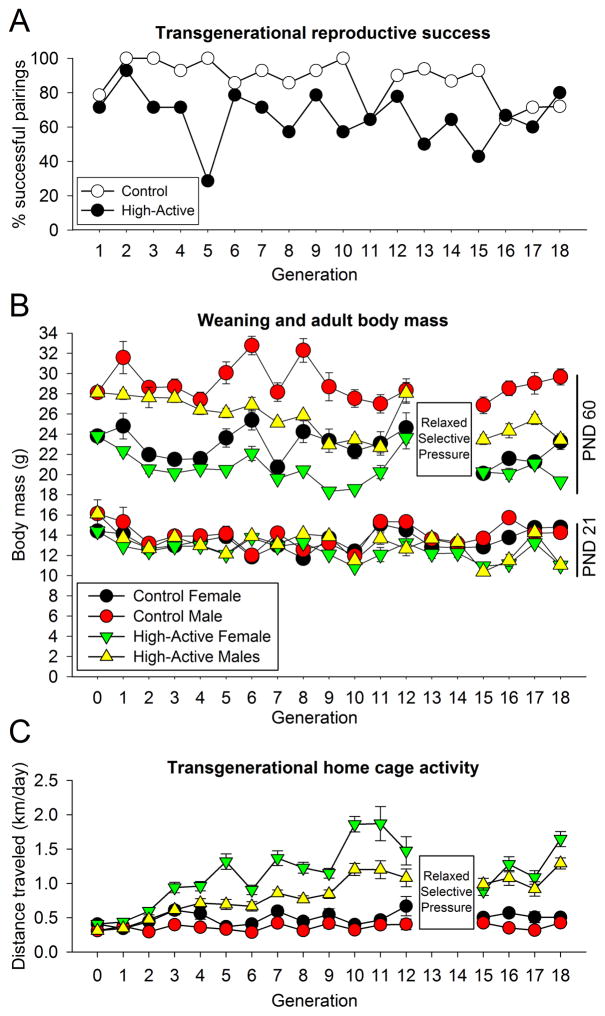
Figure 1. Transgenerational data.
A. Each data point reflects the percentage of paired mice each generation which successfully contributed offspring to propagate the lines. Typically 14 High-Active and 14 Control pairs were made during each breeding cycle. B. Average body mass in grams (±SEM) at PNDs 21 (weaning) and 60 (adulthood phenotyping) are represented for each generation. During Generations 13 and 14, mice were not phenotyped, therefore adulthood body mass was not recorded (as indicated by the boxed “relaxed selective pressure”). C. Data reflect the average locomotor activity in the home cage in km/day (±SEM) of adult High-Active and Control mice. Each data point reflects the phenotype of between 100–200 High-Active and Control mice per generation. Mice underwent distance tracking in the home cage for six consecutive days; the 24-hour activity levels on days 5 and 6 of a six-day test were averaged to assess phenotype.
Rooms were kept controlled for temperature (21 ± 1°C) and photo-period (12:12 light:dark; lights on at 7:30 PM and off at 7:30 AM). Food and water were provided ad libitum (Harlan Teklad 7004 for breeders in addition to 2g peanut butter daily while paired; Harlan Teklad 7012 for offspring). Corncob bedding (Harlan Teklad 7097, Madison, Wisconsin, USA) was provided in all standard shoebox cages. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines.
2.1.2. Generation 18 breeders
Male and nulliparous female mice from Generation 18 of the High-Active line (n=50/sex for 25 High-Active pairs) and Control line (n=50/sex for 25 Control pairs) were used. Mice were approximately 3 months at time of pairing, and represented 10 families from the Control line and 9 families from the High-Active line. Pairs were placed in standard clear shoebox cages, in order to non-invasively conduct behavioral observations following parturition. The pairs were left undisturbed, except for routine cage maintenance or peanut butter feeding. After 18 days, and prior to any litter births, males were removed from the cage.
2.1.3. Generation 19 offspring
Cross-fostering occurred on PND 0 only among litters born within 24 hours of each other. A dam and her litter were removed from the study if no other litters were born within the same 24 hour time window. Once a dam received a cross-fostered litter, no additional pups were crossed to that dam. Typically, half of each litter was given to a different dam of the same line, and the other half was given to a dam of the opposite line. In no case did a pup remained with its original mother. In the event that only 2 litters were born on a day, these litters were simply swapped between dams. Therefore, depending on the number of litters born on a particular day, each dam fostered 4–8 pups, from between 1–3 different families, from either one or both lines. Litter composition (i.e. mixed litters of High-Active and Control pups versus litters that were all of one line) did not influence maternal behavior or ultimate offspring phenotype. Before cross-fostering, all pups were tattooed with green animal ink (Ketchum Manufacturing Inc., Canada) on either the left or right hind paw, in order to easily identify original lineage at weaning age. All offspring were born within two weeks of each other.
2.2 Maternal Care
2.2.1. Pup-retrieval test
Pup retrieval was used to quantify the level of maternal competence displayed by the dams (adapted from Girard and colleagues [22]). Pup-retrieval tests were performed 2–5 hours into the dark cycle, one trial per day, from PND 3–6. Prior to each trial, the presence or absence of a nest (defined as a majority of bedding formed into a nest within one quadrant of the cage) was noted. The dam was temporarily removed from the cage, while her pups were gently placed in a pile in the opposite corner of the cage. The dam was immediately reintroduced to the empty nest, and a clear Plexiglas sheet was placed over the cage to prevent escape and distraction. Each trial lasted a maximum of 10 minutes, but the trial was stopped if a dam did not retrieve the first pup by the 5 minute mark. A video camera recorded the movements of the dam, and blinded video files were analyzed using the JWatcher program (JWatcher 1.0, Macquarie University, Sydney, Australia). Failures to retrieve were noted, and these dams were removed from subsequent analyses. Latency to retrieve each pup was recorded, in addition to a number of non-maternal behaviors such as approaching a pup and investigating it for one or more seconds without retrieval, digging by using paws and/or snout to displace bedding material in a directed fashion for one or more seconds, and trampling over one or more pups. Lower latency to retrieve pups and fewer non-maternal behaviors indicated better maternal care. Pup weights and survival rates were measured on the last retrieval test conducted on PND 6.
2.2.2. Maternal care observations
On PND 8, maternal observations were made approximately 1–3 hours into both the dark and light cycle (twice per day), while on PND 14 observations were made approximately 1–3 hours into the dark cycle only. Following the protocol from Girard and colleagues [22] a scan sampling technique was used as a non-invasive way to assess maternal care. An observer first watched a dam for 9 seconds in order to establish context for her behavior, and on the 10th second the action of the dam was recorded. Behaviors were broadly classified as maternal versus non-maternal in nature. Maternal behaviors included nursing, resting with pups, nest building, grooming pups, and carrying pups. Non-maternal behaviors included resting alone, grooming self, feeding, drinking, digging, climbing, walking or running, jumping, and inactivity. Importantly, “resting with pups” included both resting nearby the litter and passive nursing wherein the dam was asleep; “nursing” refers to an active process with an awake dam assuming the classic arched-back posturing. For each dam, 24 behavioral observations were made over the span of approximately 45 minutes.
2.3. Cross-Fostered Phenotype
On PND 21, pups were weighed, ear tagged, and weaned into groups of 4 by sex. During weaning, tattoos were used to compile information regarding genetic lineage. A total of eight groups (2 lines × 2 foster lines × 2 sexes) were generated. 158 pups comprised those eight groups: High-Active pups raised by High-Active dams (males=29, females=22), Control pups raised by High-Active dams (males=22, females=21), High-Active pups raised by Control dams (males=20, females=16), and Control pups raised by Control dams (males=13, females=15). During adulthood at PND 60, mice were phenotyped as detailed above.
2.4. Statistical Analysis
Data were analyzed with SAS (version 9.3) and R (3.0.2) statistical software. In all analyses, P≤0.05 was considered to be statistically significant, and P≤0.10 was considered a trend.
2.4.1. Transgenerational analysis
Reproductive success, defined as the percentage of pairings resulting in offspring which propagated the lines, was analyzed using a Fisher’s exact test. Body mass and home cage activity were analyzed per generation by 2-way analysis of variance (ANOVA), with sex and line (High-Active and Control) as factors.
2.4.2. Dam characteristics
Parental phenotype was analyzed using a two-way ANOVA with line and sex as factors. Litter sizes generated by each dam were compared using a student’s t-test. Cannibalization events were analyzed using a Fischer’s exact test.
2.4.3. Pup retrieval
The presence or absence of a nest across each day of pup retrieval testing was analyzed using Fischer’s exact test. Student’s t-tests were used to analyze all pup retrieval data, except for latency to retrieve pups across four days (repeated-measures ANOVA with line as between-subjects factor and day as the within-subjects factor). Post-hoc Tukey tests were used to determine significant line differences in retrieval time at each day.
2.4.4. Maternal care observations
Collapsed maternal observations were analyzed via a student’s t-test, while the frequency of maternal and non-maternal behaviors represented in the pie charts were analyzed using a Fischer’s exact test.
2.4.5. Cross-fostered phenotype
Cross-fostered phenotype data were first log transformed to improve homogeneity of variance, and then analyzed using a three-way ANOVA with sex, line, and foster-condition as factors. A t-test was used to compare the means of Control pups raised under either High-Active or Control conditions.
2.4.6. Correlated responses to selection
To determine whether the hyperactivity and body mass differences between High-Active and Control lines are likely caused by selection, variance expected from genetic drift was calculated by following previously established methods [14]. Briefly, in order to calculate standardized phenotypic differences between the lines (Dy), Control means were subtracted from High-Active means, and divided by the pooled estimates of the standard deviation. This Dy value was then compared to the 95% confidence interval for genetic drift, which was estimated using our inbreeding coefficient (“F”) generated by ASReml-R version 2.035, heritability (“h2”) values obtained from the literature [23], and the number of families used in the measurement (“n”). Absolute Dy values outside of the confidence interval indicate that the trait is likely a correlated response to selection.
3. Results
3.1. Transgenerational Demographics
The High-Active mice demonstrate a trend for reduced reproductive success as compared to the Control line (Fig. 1A); on average across all generations, 87% of paired Control mice produce offspring surviving to propagate future generations, while only 66% of High-Active mice successfully reproduce (Fischer’s exact test, P=0.053). Excluding the breeding success data from the first two generations, during which time there are no significant differences in activity between the lines, indicates there is a significant reduction in the reproductive success of the High-Active line (Fischer’s exact test, P=0.047). Weaning body mass (PND 21) does not differ consistently between the lines, yet in adulthood High-Active mice have significantly lower body mass across generations as compared to Control mice of the same sex, which becomes evident in Generation 5 (Fig. 1B). The High-Active line continues to respond to selection across 18 generations (Table 1), with females demonstrating higher levels of activity relative to males in both lines (Fig. 1C).
Table 1. Realized heritability (h2realized) estimates of home cage activity.
“S” is the selection differential (average phenotype of a generation’s most active mice used as breeders minus average phenotype of that generation), and “R” is response to selection (average phenotype of next generation minus average phenotype of current generation). Realized heritability (h2) is calculated as R/S. Only breeding pairs which successfully produced pups contributing to the next generation were used in generating the selection differential and therefore h2. Generations 13 and 14 were not phenotyped and therefore heritability estimates could not be produced. Superscript a indicates the actual value was negative, while superscript b indicates the actual value was above 1.0. The response to selection for Generation 18 was estimated using only High-Active mice raised under High-Active conditions, keeping in line with calculations for all previous generations
| Generation | S (km/day) | R (km/day) | h2 |
|---|---|---|---|
| 1 | 0.27 | 0.12 | 0.44 |
| 2 | 0.36 | 0.25 | 0.69 |
| 3 | 0.87 | 0.05 | 0.06 |
| 4 | 0.56 | 0.22 | 0.39 |
| 5 | 0.43 | 0a | 0a |
| 6 | 0.14 | 0.31 | 1b |
| 7 | 0.82 | 0a | 0a |
| 8 | 0.64 | 0.025 | 0.04 |
| 9 | 0.53 | 0.5 | 0.94 |
| 10 | 0.79 | 0a | 0a |
| 11 | 1.0 | 0.33 | 0.33 |
| 12 | 0.97 | - | - |
| 13 | - | - | - |
| 14 | - | - | - |
| 15 | 0.50 | 0.22 | 0.44 |
| 16 | 0.13 | 0a | 0 |
| 17 | 0.71 | 0.46 | 0.64 |
| 18 | 0.47 | 1b | 1b |
| Avg. | 0.57 | 0.23 | 0.40 |
3.2. Dam Demographics
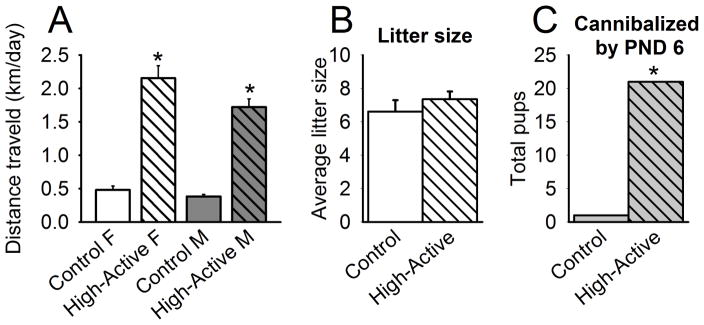
As expected, High-Active mice demonstrated hyperactivity relative to Controls (F1,72=120.95; P<0.0001), and dams exhibited higher activity levels relative to sires (F1,72=4.14; P=0.046; Fig. 2A). High-Active and Control dams gave birth to litters of similar size (t36=0.84, P=0.36; Fig. 2B), yet High-Active dams cannibalized more pups than Control dams after the first week (Fischer’s exact test, P<0.0001; Fig 2C). While we did not systematically record whether live or non-viable pups were cannibalized, we are confident the pups were alive or viable at the time of cannibalization due to the fact that 1) live cannibalizations were witnessed several times, and 2) every cross-fostered pup was first tattooed, and none were visibly lethargic or non-viable immediately prior to placement with their foster dam. Importantly, High-Active dams did not preferentially cannibalize pups of a specific line (62% were High-Active pups, 38% were Control pups; data not shown).
Figure 2. Generation 18 parental demographics.
An asterisk (*) denotes statistical significance (P≤0.05) between High-Active and Control groups. A. Data represent the average locomotor activity in the home cage in km/day (±SEM) of Generation 18 parents on days 5 and 6 of a six-day test. These phenotypic data reflect the genetic pressure contributed by dams and sires to their offspring. B. Data represent the average number of pups (±SEM) birthed by dams of the Control and High-Active lines. C. Data reflect the total number of pups cannibalized by PND 6. One Control dam cannibalized 1 pup, while five High-Active dams cannibalized a total of 21 pups.
3.3. Pup-Retrieval Test
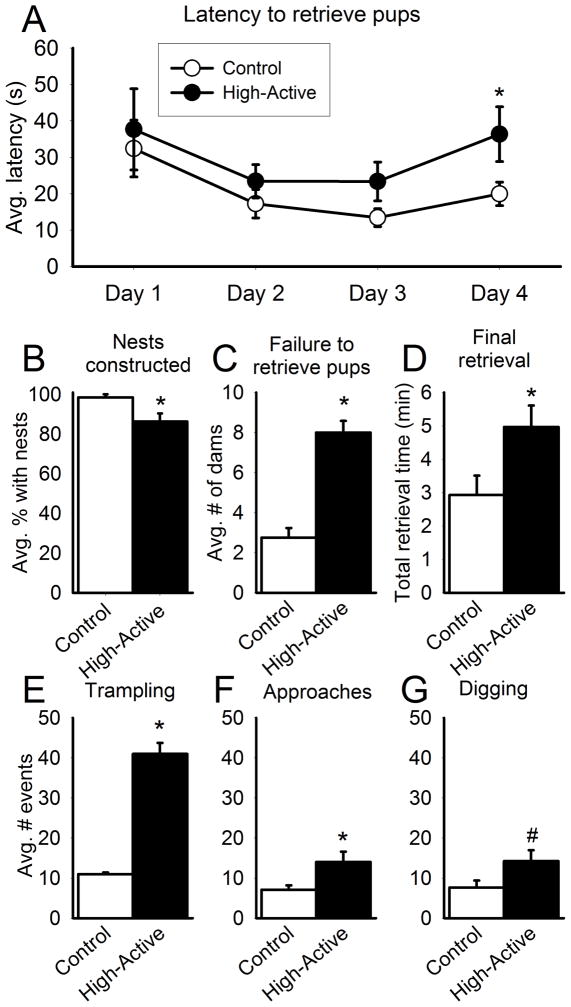
Prior to pup retrieval testing on each of the four days, the presence of absence of a nest was noted for each dam. Overall, in 99% of observations Control mice constructed nests while in only 86% of observations High-Active dams constructed nests for their pups (Fischer’s exact test, P=0.006; Fig 3B). Furthermore, High-Active mice were significantly less likely to even complete the retrieval task relative to Controls (t6=49.0; P=0.004; Fig 3C); i.e., significantly more High-Active dams did not pick up their first pup within the first 5 minutes, nor completed returning all pups to the nest in the allotted 10 minute trial. Analyses of those dams which did perform the task over 4 days indicate main effects of day (F3,65=3.10; P=0.033; Fig 3A) and a trend for line, suggesting High-Active dams retrieve pups more slowly (F1,33=3.97; P=0.055; Fig 3A). No interaction between line and day was detected. A Tukey post hoc test for Day 4 indicated a significant difference in average retrieval latencies for High-Active versus Control dams (P=0.032). Indeed, the total time to retrieve all pups on Day 4, when dams should be most experienced and efficient with the task, is significantly higher in High-Active relative to Control dams (t35=5.48; P=0.025; Fig 3D).
Figure 3. Pup retrieval testing.
An asterisk (*) denotes statistical significance (P≤0.05) between High-Active and Control groups. A. The average latency in seconds (±SEM) for dams to retrieve a displaced pup from the corner and return it to the nest across the four days of retrieval sessions. B. The percentage (±SEM) of dams with nests constructed in the home cage across the four days of pup retrieval tests. C. The average number of Control vs High-Active dams (±SEM) which failed to retrieve pups across the four days. These dams were removed from all subsequent pup retrieval analyses. D. On the final day of pup retrieval testing (Day 4), the total time in minutes (±SEM) for dams to return all pups back into the nest. Litter sizes were not significantly different between Control and High-Active dams. E. Average number of trampling events (±SEM) during pup retrieval across all four days. A trampling event was defined as the dam running over one or more pups. F. Average number of approaches without retrieving pups (±SEM) across all four days. Approaches were defined as a dam investigating a distressed pup with its nose for one or more seconds, but not returning it to the nest. G. Average number of digging events (±SEM) performed during pup retrieval across all four days. A digging event was defined as focused bedding displacement for one or more seconds.
Behaviors other than the expected retrievals were observed in both lines of mice and hence quantified. High-Active dams trampled pups more than Controls (t35=5.0; P=0.032; Fig 3E) and made significantly more approaches to distressed pups without retrieving them (t35=5.56; P=0.024; Fig 3F). Furthermore, there is a trend of High-Active dams performing more digging/tunneling throughout the home cage compared to Control dams (t35=4.1; P=0.051; Fig 3G).
3.4. Maternal Care Observations
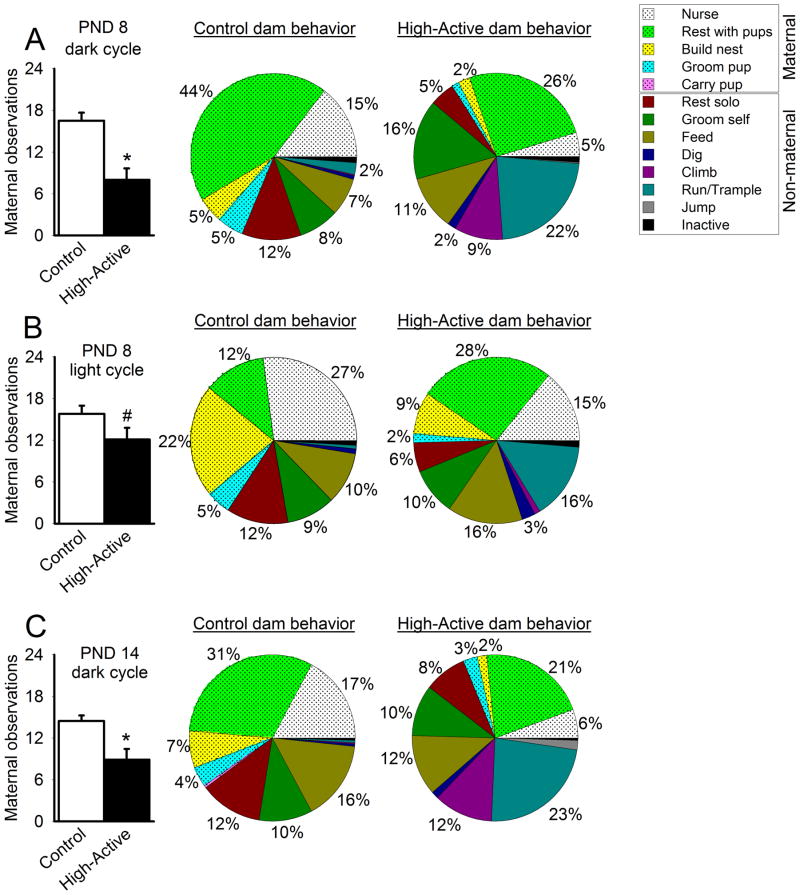
The number of maternal behaviors demonstrated by the dams was assessed at 3 different time points, on PND 8 (during the dark and light cycle), and PND 14 (during the dark cycle). The first observation on PND 8 during the active phase (dark cycle) showed significantly fewer maternal behaviors being performed by High-Active dams relative to Controls (t35=17.7; P=0.0002; Fig 4A). Observations 12 hours later into their inactive phase (light cycle) held this pattern, albeit as a trend for reduced maternal behaviors performed by High-Active mice (t35=3.15; P=0.085; Fig 4B). One week later, on PND 14 during the active phase, High-Active dams still performed fewer routine maternal care behaviors for their pups, similar to the PND 8 time point (t35=10.1; P=0.0031; Fig 4C).
Figure 4. Maternal and non-maternal dam behaviors.
A total of 24 observations were made during each session, and dam activities were classified broadly into maternal versus non-maternal behaviors. Bar graph data represent the average number of maternally-related behaviors performed by Control versus High-Active dams (±SEM). An asterisk (*) denotes statistical significance (P≤0.05) between High-Active and Control groups. Pie charts summarize dam behavior both in detail and more broadly, as maternal behavior is shaded while non-maternal behavior is unshaded. Activities performed less than 1% of the time were not labeled with a percentage in pie charts. A. Maternal and non-maternal behavior recorded for dams 2–3 hours into the dark cycle (active phase) on pups’ PND 8. B. Maternal and non-maternal behavior recorded for dams 2–3 hours into the light cycle (inactive phase) on pups’ PND 8. C. Maternal and non-maternal behavior recorded for dams 2–3 hours into the dark cycle (active phase) on pups’ PND 14.
The frequency of key behaviors (e.g. nursing, resting with pups, trampling) represented in the pie charts significantly differ between High-Active and Control dams at each observation period (Fischer’s exact test, P<0.05). Only low frequency behaviors (occurring at a rate of 3% or less) did not demonstrate significant line differences (e.g. carrying pup, feeding, time spent inactive).
3.5. Cross-Fostered Phenotype
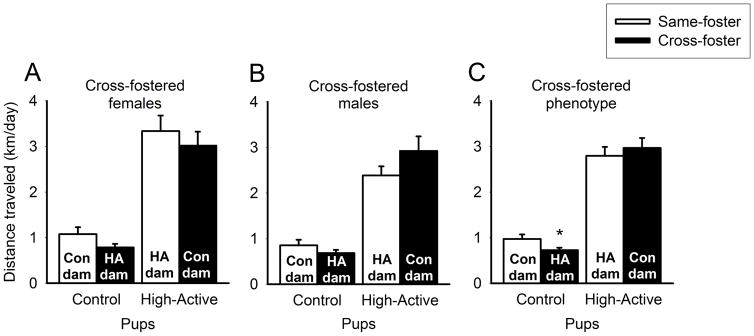
Genetic line had the strongest impact on the levels of activity displayed by Control and High-Active mice (F1,150=242.8; P<0.0001; Fig. 5C). Fostering environment showed a trend for an effect on activity (F1,150=2.8; P=0.098), driven by the reduced activity of Control pups raised by High-Active dams (P=0.025). There was no significant interaction of line and fostering environment.
Figure 5. Environmental impact on phenotypic home cage activity.
Data represent the average home cage locomotor activity in km/day (±SEM) of Generation 19 cross-fostered offspring during adulthood. An asterisk (*) denotes statistical significance (P≤0.05) between Control pups raised by High-Active versus Control dams. A. Phenotype of female mice. B. Phenotype of male mice. C. Phenotype of all mice collapsed across sex.
Females demonstrated increased levels of home cage activity relative to males (F1,150=7.1; P=0.008), but sex did not interact with any other main effects (Figs. 5A and B).
3.6. Correlated responses to selection
The difference in activity levels between High-Active and Control mice falls outside of the rate of genetic drift predicted by population parameters, indicating that between-line differences in activity levels are likely due to selective pressure (Table 2). This holds true for both Generation 18 mice as well as Generation 19 mice raised by Control dams.
Table 2. Traits across the lifespan and their correlation with selection for home cage hyperactivity.
Mean values (±SEM) of each line (“Control” and “High-Active”) are analyzed to determine whether line differences may be attributed to selective breeding for hyperactivity. The statistical significance of line differences is expressed in standardized phenotypic units, which are compared to their 95% confidence interval; absolute values which fall outside of this interval indicate the difference is a correlated genetic response. “M”=male, “F”= female, “MF”=collapsed by sex, “HA”=High-Active, “CON”=Control; abbreviations separated by a colon indicate mice of a genetic line raised by a line (e.g., HA:CON indicates High-Active mice raised by Control dams). “P-value” refers to a standard pair-wise comparison of the High-Active versus Control (for Generation 19 weaning and adult body mass and activity levels, lines raised by Control dams are compared and emphasized in bold); a heritability (“h2”) reference was used for analyses of body mass at PNDs 21 and 60 (as the heritability estimate was calculated at PND 49 [23], we considered its application to PNDs 0 and 6 inappropriate) as well as our calculated heritability for activity (Table 1); “F” refers to the inbreeding coefficient; “n” refers to the number of families represented in the cohort; “95% CI” refers to the 95% confidence interval for Dy expected by genetic drift; “Dy” refers to the difference between lines in standardized phenotypic SD units, and those values in bold are considered correlated responses to selection.
| Gen | Trait | PND | Sex | High-Active line | Control line | P-value | h2 | F | n | 95% CI | Dy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18 | Mass | 21 | M | 10.3 (±0.5) | 14.7 (±0.6) | 0.0001 | 0.31 | 0.06 | 19 | 1.05 | −1.73 | |||
| F | 11.4 (±0.5) | 14.2 (±0.7) | 0.001 | 0.31 | 0.06 | 19 | 1.05 | −1.01 | ||||||
| 18 | Mass | 60 | M | 22.7 (±0.5) | 28.7 (±1.6) | 0.0001 | 0.31 | 0.06 | 19 | 1.05 | −1.24 | |||
| F | 19.5 (±0.5) | 23.1 (±1.0) | 0.01 | 0.31 | 0.06 | 19 | 1.05 | −1.04 | ||||||
| 18 | Activity | 60 | M | 1.72 (±0.1) | 0.33 (±0.02) | 0.0001 | 0.40 | 0.06 | 19 | 1.09 | 3.22 | |||
| F | 2.14 (±0.2) | 0.48 (±0.07) | 0.0001 | 0.40 | 0.06 | 19 | 1.09 | 2.26 | ||||||
| 19 | Mass | 0 | MF | 1.54 (±0.06) | 1.68 (±0.06) | 0.11 | - | - | - | - | - | |||
| 19 | Mass | 6 | MF | 4.38 (±0.2) | 4.88 (±0.1) | 0.04 | - | - | - | - | - | |||
| HA:HA | HA:CON | CON:CON | CON:HA | |||||||||||
| 19 | Mass | 21 | M | 14.1 (±0.3) | 14.4 (±0.3) | 15.7 (±0.3) | 14.7 (±0.5) | 0.003 | 0.31 | 0.06 | 37 | 0.84 | −0.79 | |
| F | 13.5 (±0.4) | 14.2 (±0.3) | 14.3 (±0.4) | 14.1 (±0.4) | NS | 0.31 | 0.06 | 37 | 0.84 | −0.04 | ||||
| 19 | Mass | 60 | M | 26.3 (±0.5) | 24.9 (±0.6) | 27.5 (±1.1) | 27.7 (±0.9) | 0.03 | 0.31 | 0.06 | 37 | 0.84 | −0.78 | |
| F | 20.6 (±0.6) | 21.1 (±0.6) | 20.6 (±0.8) | 21.4 (±0.9) | NS | 0.31 | 0.06 | 37 | 0.84 | −0.17 | ||||
| 19 | Activity | 60 | M | 2.38 (±0.2) | 2.9 (±0.3) | 0.85 (±0.1) | 0.68 | (±0.1) | 0.0001 | 0.40 | 0.06 | 37 | 0.89 | 1.69 |
| F | 3.33 (±0.3) | 3.0 (±0.3) | 1.08 (±0.2) | 0.78 | (±0.1) | 0.0001 | 0.40 | 0.06 | 37 | 0.89 | 1.70 | |||
Furthermore, this cross-fostering study provides context and insight as to whether genetic hyperactivity disorders are associated with increased or decreased body mass (Table 2). The use of the High-Active line to correlate body mass and hyperactivity circumvents some issues identified in clinical studies, such as the confounding influence of socioeconomic status [24].
Firstly body mass means were compared using standard t-test analyses. Generation 18 High-Active mice were smaller at PND 21 weaning (F1,72=34.7; P<0.0001), and no sex differences were detected (Table 2). During adulthood (PND 60–65) High-Active mice overall maintain a smaller body mass (F1,72=24.8; P<0.0001) with males of both High-Active and Control lines heavier than females (F1,72=80.9; P<0.0001). The interaction between sex and line was not significant.
At birth (PND 0), Generation 19 High-Active and Control pups did not differ in body mass (t36=2.7; P=0.11), yet 6 days of High-Active fostering significantly reduced the body mass (t35=4.6; P=0.04) of those pups which survived High-Active rearing conditions (Fig. 3C). At weaning (PND 21), the dominant influence on body mass was fostering condition (F1,178=6.8; P=0.010) and not line of origin (F1,178=3.6; P=0.061). Females had a smaller body mass relative to males (F1,178=6.3; P=0.013) but this effect did not interact with any other main effects. However, by adulthood (PND 60–65) mice of High-Active origin demonstrated a reduced body mass (F1,152=4.4; P=0.04) which is not influenced by foster condition. Females displayed smaller body masses relative to males (F1,152=122.3; P<0.0001), with High-Active mice tending to demonstrate a smaller sex difference than Controls (interaction of line and sex F1,152=3.3; P=0.07); on average, High-Active males are 4.8 grams heavier than High-Active females, while Control males are 6.6 grams heavier than Control females.
Table 2 indicates that the reduced body mass of High-Active males from Generation 18 is likely associated with selection for hyperactivity, but drift cannot be ruled out for the smaller body mass of High-Active females. Generation 19 cross-fostered pups further clarify this observation. Comparisons were made between High-Active and Control lines both raised by Control dams (“HA:CON” and “CON:CON”; Table 2), thereby removing the impact of environment on mass. While pairwise comparisons between High-Active and Control males (raised Control) at PND 21 and 60 indicate significant body mass differences (P=0.003 and P=0.03, respectively), these differences do not survive the test for drift. These data suggest that severely adverse rearing conditions reduce body mass in hyperactive mice.
4. Discussion
This study makes use of a novel genetic model of ADHD to provide converging evidence for the role of environment versus genetics on the transmission of locomotor hyperactivity. The High-Active line displays impaired maternal competence as evidenced by poor performance in the pup-retrieval task as well as during maternal care observations. The deteriorated maternal care provided by High-Active dams was sufficient to reduce survival rates of pups, as well as the body mass of surviving pups during both early life and weaning. Yet ultimately this environment did not influence the hyperactivity of High-Active offspring. Alternatively, these adversive conditions significantly depressed the locomotor activity of Control offspring. The finding that home cage hyperactivity is entirely genetically mediated not only resolves a significant question in the breeding of the High-Active model, but also within genetic hyperactivity literature; early postnatal environment was insufficient to impact the trajectory of a heritable hyperactivity phenotype.
Breeding for hyperactivity in the home cage produced secondary deficits in maternal competence. While there was no evidence for reduced fertility (Fig. 2B), it was clear High-Active dams provided a hostile early environment which results in significantly more pup deaths (Fig. 2C) and ultimately contributes to reduced reproductive success (Fig. 1A). This harsh environment includes a baseline lack of brood nest building (Fig. 3B) and impaired maternal competence during both light and dark cycles (Fig. 4). High-Active dams are less likely to retrieve pups (Fig. 3C) despite recognizing their displacement and/or distress (Fig. 3F), and those dams which do complete the task tend to be slower (Figs. 3A and D). Instead of tempering hyperactive behavior and engaging in instinctual pup retrievals, High-Active dams manifest hyperactivity via trampling and digging behaviors (Figs. 3E and G). Such digging or burying behavior has been suggested to reflect an obsessive/compulsive-like tendency for repetitive movement that is unrelated to anxiety or the construction of a new nest [25]. Unobtrusive home cage observations confirmed the neglectful and injurious High-Active dam behavior; less nursing and more trampling were recorded across multiple sessions prior to weaning on PND 21 (Fig. 4). The only period of reprieve for pups under High-Active care is during the light cycle (inactive phase), during which time pups take advantage of dam inactivity and nurse passively (Fig. 4B). Impaired dark cycle maternal care and passive nursing during the light cycle is also observed in lines of rats bred for exaggerated novelty-induced locomotion [26]. The impaired maternal competence of the High-Active line effectively reduces the sampling pool used to estimate activity means per generation and therefore also reduces the potential for a strong selection differential during breeding; ultimately the data indicate that impoverished maternal care may set a selection limit for hyperactivity.
The severity of maternal impairment of the High-Active line is unique, as selective breeding for increased levels of physical activity do not necessarily beget secondary deficits in maternal care. Pup retrieval and maternal observation protocols used in this study were adapted from analyses of replicate lines of mice bred for high levels of wheel running. In those studies, no differences in pup retrieval or maternal observations were identified between Control and High-Runner lines [22]. However High-Runner dams were not given access to wheels in this study, in keeping with standard breeding protocol of the High-Runner lines. Therefore it is possible that wheel access might reduce the quantity, if not necessarily the quality, of maternal care. Furthermore, dams of the hyperactive SHR model show evidence of enhanced maternal care relative to controls; SHR dams spend more time with pups and engage in a higher frequency of nursing and licking behavior compared to WKY controls [27]. Even dams from a line of mice bred for inter-male aggression are not deficient in maternal care; dams from the aggressive line displayed less trampling and higher pup retrieval rates compared to non-aggressive lines [28, 29]. The extent of impairment in maternal competence of the High-Active line is truly unique to selective breeding for home cage hyperactivity.
The High-Active foster environment may therefore be considered a severe rodent model of abuse or neglect in early life. To our knowledge, no pre-weanling rodents have been exposed to conditions as extreme as those consistently maintained by High-Active dams. The extent of physical harm from a behavior such as repeated trampling (Fig. 3E) is unexplored in rodent literature. The reduced nursing and time spent with pups (Fig. 4) leading to pup death may be interpreted as maternal neglect. One model of naturally-occurring maternal neglect arises from selective breeding for maternal aggression, yet these neglectful dams, defined as litters lost by PND 5, do not engage in trampling or cannibalization behavior [30]. Another interpretation is that High-Active behavior reflects maternal separation procedures used to induce early life stress. However, most maternal separation protocols tend to be less severe relative to the observed High-Active conditions. Maternal separations range from a few hours per day over one or more days, and still produce significant effects on stress reactivity, locomotor behavior, and regional brain activation [7]. Compounding any potential (unmeasured) effects on pups are those induced by the lack of a well-constructed nest (Fig. 3B). Nest building is considered an ethologically-relevant “activity of daily living” for healthy rodents [31] which is positively correlated to fitness [32]. Increasing activity levels via pharmacological treatment or selective breeding also reduces nest building behavior [33, 34]. However, failure to build sleeping nests differs from the failure of pregnant dams to build brooding nests. Intentionally restricting access to nesting or bedding material on PNDs 2–9 not only stresses the dam but also reduces pup body weight, decreases the licking/grooming experienced by pups, and alters pup hypothalamic-pituitary-adrenal responsivity [35–37]. Taken together, the level of maternal neglect experienced by pups raised in High-Active conditions is well beyond published behaviors or stress techniques employed in rodent studies. The fact that Control or High-Active pups survive these conditions at all may be due to the heterosis of the lines.
Given both the exceptionally adversive rearing environment and simultaneous strength of genetic predisposition to hyperactivity, the intractability of High-Active home cage behavior to environment was not only remarkable but difficult to predict (Fig. 5). Previous studies have demonstrated that early life stress may elicit depressive-like behavior [5], which was observed in the activity levels of Control pups raised by High-Active dams (Fig. 5C). Yet the response of a line selectively bred for extreme habituated hyperactivity was wholly unexplored. Some SHR studies have been conducted on the subject of environment and hyperactivity, but are limited to assessing environmental enrichment interventions during post-weaning periods [38, 39], not negative experiences in early life. Two previous cross-fostering studies of SHRs provide tentative hypotheses for the outcome of the present study [40, 41]. In both cases, cross-fostering SHR pups to either WKY or Sprague-Dawley control dams did not influence activity levels, consistent with our findings. Yet these studies preclude foresight of our outcome, due to the fact that 1) WKY maternal care is not an improvement over SHR maternal care [27], and 2) these studies employ a different definition of hyperactivity. Our selective breeding protocol defines hyperactivity as an average activity level over several days in a home cage setting, well after an extended habituated period [14, 15]; SHR hyperactivity in these studies was defined as activity across one fifteen-minute open field test [41] or activity during a ten-minute social-interaction test [40]. Therefore the present study not only corroborates previous findings in a related model of genetic hyperactivity, but also expands on them by first quantifying the vast differences in maternal behavior of both High-Active and Control dams, and then measuring the habituated hyperactivity of large samples of both male and female cross-fostered pups.
It is important to acknowledge that the increased activity demonstrated by the High-Active line is likely due to selective pressure for hyperactivity, as opposed to effects unrelated to selection. After several generations of selective breeding, multiple genetic events occur and coalesce to produce trait differences between lines. Therefore it is critical to determine whether any measured line differences are due to applied selective pressure or random events related to founder effects or genetic drift [42, 43]. The ideal resolution for this limitation is the inclusion of replicate lines, which provide converging evidence for the correlated nature of the trait if it is observed across replications of the selectively bred lines but not across replications of the control lines. However, in the absence of replicate lines a statistical approach was used to estimate whether line differences are due to selection or drift. This analysis indicated that the hyperactivity of the High-Active line resulted from selective pressure [14] (Table 2). We also used this method to uncover sex-specific effects of body mass as it correlates to selection for hyperactivity. Twin and adoption studies indicate a strong genetic component to body mass index [44, 45], and analyses of High-Active mice from Generation 10 suggested decreased body mass is correlated with hyperactivity [14]. In Generation 18, male High-Active mice show reduced body mass which survives the test for drift, but decreased body mass in females does not (Table 2). Male mice selectively bred for increased wheel-running behavior also demonstrate a negative association with body mass, but females do not [23]. However, raising both lines under Control dams eradicates the significant selection-related differences at PNDs 21 and 60, suggesting that rearing environment is the primary factor reducing the body mass of High-Active male mice (Table 2). This data is further supported by the finding that a standard comparison of PND 21 body masses (High-Active males raised High-Active and Control males raised Control) does survive the test for drift (data not shown). It may be that a lack of appropriate nest building contributes to this decrease in body weight (Fig. 3B), as previous studies have demonstrated that limiting nesting material between PNDs 2–9 significantly reduces pup weight gain [36]. Taken together, these data suggest that fostering condition affects growth and development of pups; therefore, future studies using the High-Active line should exercise precaution and cross-foster both lines to Control dams prior to behavioral testing in cognitive and behavioral domains outside of gross locomotor activity [7, 46]. These body mass findings also have implications for the High-Active line as a developmental model of ADHD. Smaller body masses at birth are observed in ADHD populations [47, 48], but there was only a marginally non-significant reduction of High-Active pup weight at birth (Table 2). Line differences appeared at PND 6, likely in response to the poor care afforded by High-Active dams. The finding that body mass was not significantly associated with genetic hyperactivity (after removing the rearing confound) supports studies that show ADHD not associated with weight [24] but not others which find a positive correlation between ADHD and weight gain [49, 50].
Ultimately these findings inform the construct validity and utility of the High-Active line to model the hyperactivity associated with ADHD. Keeping in line with published etiological studies of ADHD [16, 51] this cross-fostering experiment unambiguously supports the conclusion that genetics mediate hyperactive behavior in the High-Active line (Fig. 5; Table 1). This finding confirms the construct validity of the High-Active model to recapitulate genetic hyperactivity-associated disorders. Furthermore, the dysregulation of monoaminergic neurotransmission is a recurring theme not only in ADHD literature and animal modeling of increased physical activity [52–55] but also in the field of maternal stress and neglect [19, 30]. The convergence of both innate hyperactivity and maternal neglect in the High-Active line strongly implicates monoaminergic dysregulation in this model. This confluence of traits is rarely observed to such an extent in other animal models, but is found in the well-studied dopamine transporter knockout (DAT-KO) mouse model of ADHD [53, 56]. High-Active mice and DAT-KO dams share key traits; both lines are fertile, demonstrate hyperactivity, therapeutic responsivity to psychostimulant administration, and are also significantly impaired on the pup retrieval task [14, 53, 56]. Moreover both DAT-KO and High-Active dams are less likely to retrieve displaced pups; if pups are retrieved, they demonstrate a longer latency to complete the task and are more likely to become preoccupied with grooming and digging behaviors during retrieval tests [56]. Taken together these data suggest that High-Active maternal behavior is on par with the behavior of mice exposed to strong chemical disruptions of dopamine. However, current literature suggests that such extreme disruptions of genes related to a single pathway are unlikely to accurately reflect clinical populations [57, 58]. Nevertheless, the interpretation that extreme monoamine dysfunction mediates disparate facets of High-Active behavior suggests the line may be well-suited for exploring a theoretical extreme of the ADHD continuum. Ultimately the High-Active line can be used to identify exaggerated variants in gene networks underlying hyperactive behavior which may provide novel targets for biomarkers of populations displaying hyperactivity in habituated environments.
4.1 Conclusions
The High-Active model serves as a unique platform to address important questions regarding the transmission of hyperactive behavior. High-Active dams largely neglect their pups, creating a rearing environment which ranges from stressful to lethal. Exposure to this environment is ultimately sufficient to significantly depress the locomotor activity of the robust, heterogeneous Control line. Yet this powerful environmental effect was not observed in High-Active locomotor activity. The discovery that the High-Active line itself is predominantly influenced by genetic factors, despite the severity of its early rearing environment which effectively reduces body mass and survival rates, validates the power of raw genetics in mediating hyperactivity. Moreover this finding that hyperactivity is unaffected by environment provides construct validity for the High-Active line in modeling a highly heritable disorder such as ADHD [16, 17]. Taken together, these data suggest that future endeavors to identify biomarkers for extreme genetic hyperactivity-associated disorders may benefit from focusing on genetic, as opposed to epigenetic, targets.
Highlights.
Selective breeding for hyperactivity severely impairs maternal competence.
Severely adverse rearing conditions do not influence genetic hyperactivity.
Severely adverse rearing conditions significantly depress Control activity.
Severely adverse rearing conditions reduce body mass in hyperactive mice.
Acknowledgments
This work was supported by NIH grants MH083807 and DA027487. Elizabeth Grogan was supported over the summer of 2015 by the Janssen Family Undergraduate Research Award. The authors extend their sincere thanks to the Janssen Family for their generosity, which made this unique undergraduate research opportunity possible. The authors gratefully acknowledge the expert technical support and advice provided by Pul Park and Tushar K. Bhattacharya throughout the cross-fostering experiment. We sincerely thank the Beckman Institute Animal care staff, especially Dack M. Shearer and Danial W. Branson, for their consistently attentive and high-quality animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental neurology. 2012;233(1):102–11. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Dudley KJ, Li X, Kobor MS, Kippin TE, Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neuroscience and biobehavioral reviews. 2011;35(7):1544–51. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–46. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Maguire SA, Williams B, Naughton AM, Cowley LE, Tempest V, Mann MK, Teague M, Kemp AM. A systematic review of the emotional, behavioural and cognitive features exhibited by school-aged children experiencing neglect or emotional abuse. Child: care, health and development. 2015;41(5):641–53. doi: 10.1111/cch.12227. [DOI] [PubMed] [Google Scholar]
- 5.Bian Y, Yang L, Wang Z, Wang Q, Zeng L, Xu G. Repeated Three-Hour Maternal Separation Induces Depression-Like Behavior and Affects the Expression of Hippocampal Plasticity-Related Proteins in C57BL/6N Mice. Neural plasticity. 2015;2015:627837. doi: 10.1155/2015/627837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, Simen AA. Maternal separation with early weaning: a rodent model providing novel insights into neglect associated developmental deficits. Development and psychopathology. 2012;24(4):1401–16. doi: 10.1017/S095457941200079X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishi M, Horii-Hayashi N, Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Frontiers in neuroscience. 2014;8:166. doi: 10.3389/fnins.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pena CJ, Bagot RC, Labonte B, Nestler EJ. Epigenetic signaling in psychiatric disorders. Journal of molecular biology. 2014;426(20):3389–412. doi: 10.1016/j.jmb.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M, Loeschner A, Gonik M, Mercer KB, Bradley B, Muller-Myhsok B, Ressler KJ, Binder EB. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(20):8302–7. doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uddin M, Galea S, Chang SC, Koenen KC, Goldmann E, Wildman DE, Aiello AE. Epigenetic signatures may explain the relationship between socioeconomic position and risk of mental illness: preliminary findings from an urban community-based sample. Biodemography and social biology. 2013;59(1):68–84. doi: 10.1080/19485565.2013.774627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O Babenko, I Kovalchuk, GA Metz. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neuroscience and biobehavioral reviews. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biological psychiatry. 2010;68(5):408–15. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Lee KP, Spencer TJ, Biederman J, Bhide PG. Transgenerational transmission of hyperactivity in a mouse model of ADHD. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(8):2768–73. doi: 10.1523/JNEUROSCI.4402-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majdak P, Bucko PJ, Holloway AL, Bhattacharya TK, DeYoung EK, Kilby CN, Zombeck JA, Rhodes JS. Behavioral and pharmacological evaluation of a selectively bred mouse model of home cage hyperactivity. Behavior genetics. 2014;44(5):516–34. doi: 10.1007/s10519-014-9667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zombeck JA, Deyoung EK, Brzezinska WJ, Rhodes JS. Selective breeding for increased home cage physical activity in collaborative cross and Hsd:ICR mice. Behavior genetics. 2011;41(4):571–82. doi: 10.1007/s10519-010-9425-2. [DOI] [PubMed] [Google Scholar]
- 16.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological psychiatry. 2005;57(11):1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 17.McLoughlin G, Ronald A, Kuntsi J, Asherson P, Plomin R. Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive-impulsive components. Journal of abnormal child psychology. 2007;35(6):999–1008. doi: 10.1007/s10802-007-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak HR, Lee JW, Kwon KJ, Kang CD, Cheong IY, Chun W, Kim SS, Lee HJ. Maternal social separation of adolescent rats induces hyperactivity and anxiolytic behavior. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2009;13(2):79–83. doi: 10.4196/kjpp.2009.13.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son GH, Chung S, Geum D, Kang SS, Choi WS, Kim K, Choi S. Hyperactivity and alteration of the midbrain dopaminergic system in maternally stressed male mice offspring. Biochemical and biophysical research communications. 2007;352(3):823–9. doi: 10.1016/j.bbrc.2006.11.104. [DOI] [PubMed] [Google Scholar]
- 20.Nigg J, Nikolas M, Burt SA. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):863–73. doi: 10.1016/j.jaac.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesler EJ, Miller DR, Branstetter LR, Galloway LD, Jackson BL, Philip VM, Voy BH, Culiat CT, Threadgill DW, Williams RW, Churchill GA, Johnson DK, Manly KF. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mammalian genome: official journal of the International Mammalian Genome Society. 2008;19(6):382–9. doi: 10.1007/s00335-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard I, Swallow JG, Carter PA, Koteja P, Rhodes JS, Garland T. Maternal-care behavior and life-history traits in house mice (Mus domesticus) artificially selected for high voluntary wheel-running activity. Behavioural processes. 2002;57(1):37–50. doi: 10.1016/s0376-6357(01)00206-6. [DOI] [PubMed] [Google Scholar]
- 23.Swallow JG, Koteja P, Carter PA, Garland T. Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. The Journal of experimental biology. 1999;202(Pt 18):2513–20. doi: 10.1242/jeb.202.18.2513. [DOI] [PubMed] [Google Scholar]
- 24.van Egmond-Frohlich AW, Widhalm K, de Zwaan M. Association of symptoms of attention-deficit/hyperactivity disorder with childhood overweight adjusted for confounding parental variables. Int J Obes (Lond) 2012;36(7):963–8. doi: 10.1038/ijo.2012.78. [DOI] [PubMed] [Google Scholar]
- 25.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–73. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinton SM, Bedrosian TA, Abraham AD, Watson SJ, Akil H. Neural and environmental factors impacting maternal behavior differences in high- versus low-novelty-seeking rats. Hormones and behavior. 2010;57(4–5):463–73. doi: 10.1016/j.yhbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cierpial MA, Shasby DE, McCarty R. Patterns of maternal behavior in the spontaneously hypertensive rat. Physiology & behavior. 1987;39(5):633–7. doi: 10.1016/0031-9384(87)90165-x. [DOI] [PubMed] [Google Scholar]
- 28.Lagerspetz K, Wuorinen K. A Cross Fostering Experiment with Mice Selectively Bred for Aggressiveness and Non-agressiveness. Institute of Psychology University of Turki; 1965. [Google Scholar]
- 29.Sandnabba NK. Selective breeding for isolation-induced intermale aggression in mice: associated responses and environmental influences. Behavior genetics. 1996;26(5):477–88. doi: 10.1007/BF02359752. [DOI] [PubMed] [Google Scholar]
- 30.Gammie SC, Edelmann MN, Mandel-Brehm C, D’Anna KL, Auger AP, Stevenson SA. Altered dopamine signaling in naturally occurring maternal neglect. PloS one. 2008;3(4):e1974. doi: 10.1371/journal.pone.0001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. Journal of neuroscience methods. 2014;234:139–46. doi: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Bult A, Lynch CB. Multiple selection responses in house mice bidirectionally selected for thermoregulatory nest-building behavior: crosses of replicate lines. Behavior genetics. 1996;26(4):439–46. doi: 10.1007/BF02359488. [DOI] [PubMed] [Google Scholar]
- 33.Carter PA, Swallow JG, Davis SJ, Garland T., Jr Nesting behavior of house mice (Mus domesticus) selected for increased wheel-running activity. Behavior genetics. 2000;30(2):85–94. doi: 10.1023/a:1001967019229. [DOI] [PubMed] [Google Scholar]
- 34.G Pathak, BA Ibrahim, SA McCarthy, K Baker, MP Kelly. Amphetamine sensitization in mice is sufficient to produce both manic- and depressive-related behaviors as well as changes in the functional connectivity of corticolimbic structures. Neuropharmacology. 2015;95:434–47. doi: 10.1016/j.neuropharm.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–42. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naninck EF, Hoeijmakers L, Kakava-Georgiadou N, Meesters A, Lazic SE, Lucassen PJ, Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25(3):309–28. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- 37.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botanas CJ, Lee H, de la Pena JB, Dela Pena IJ, Woo T, Kim HJ, Han DH, Kim BN, Cheong JH. Rearing in an enriched environment attenuated hyperactivity and inattention in the Spontaneously Hypertensive Rats, an animal model of Attention-Deficit Hyperactivity Disorder. Physiology & behavior. 2016;155:30–7. doi: 10.1016/j.physbeh.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 39.Pamplona FA, Pandolfo P, Savoldi R, Prediger RD, Takahashi RN. Environmental enrichment improves cognitive deficits in Spontaneously Hypertensive Rats (SHR): relevance for Attention Deficit/Hyperactivity Disorder (ADHD) Progress in neuro-psychopharmacology & biological psychiatry. 2009;33(7):1153–60. doi: 10.1016/j.pnpbp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Gauthier AC, DeAngeli NE, Bucci DJ. Cross-fostering differentially affects ADHD-related behaviors in spontaneously hypertensive rats. Developmental psychobiology. 2015;57(2):226–36. doi: 10.1002/dev.21286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howells FM, Bindewald L, Russell VA. Cross-fostering does not alter the neurochemistry or behavior of spontaneously hypertensive rats. Behavioral and brain functions: BBF. 2009;5:24. doi: 10.1186/1744-9081-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garland T, Adolph SC. Why not to do two-species comparative studies: limitations on inferring adaptation. Physiological Zoology. 1994;67(4):797–828. [Google Scholar]
- 43.Swallow JG, Garland T. Selection experiments as a tool in evolutionary and comparative physiology: insights into complex traits—an introduction to the symposium. Integrative and comparative biology. 2005;45(3):387–390. doi: 10.1093/icb/45.3.387. [DOI] [PubMed] [Google Scholar]
- 44.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behavior genetics. 1997;27(4):325–51. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 45.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. The New England journal of medicine. 1990;322(21):1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 46.Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behavioural processes. 2006;71(1):51–8. doi: 10.1016/j.beproc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Lahti J, Raikkonen K, Kajantie E, Heinonen K, Pesonen AK, Jarvenpaa AL, Strandberg T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. Journal of child psychology and psychiatry, and allied disciplines. 2006;47(11):1167–74. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- 48.Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH, Henriksen TB. Gestational age, birth weight, and the risk of hyperkinetic disorder. Archives of disease in childhood. 2006;91(8):655–60. doi: 10.1136/adc.2005.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanc T, Slopien A, Wolanczyk T, Szwed A, Czapla Z, Durda M, Dmitrzak-Weglarz M, Ratajczak J. Attention-Deficit/Hyperactivity Disorder is Related to Decreased Weight in the Preschool Period and to Increased Rate of Overweight in School-Age Boys. Journal of child and adolescent psychopharmacology. 2015;25(9):691–700. doi: 10.1089/cap.2014.0157. [DOI] [PubMed] [Google Scholar]
- 50.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122(1):e1–6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 51.Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):737–44. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. The Journal of clinical psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- 53.Gainetdinov RR. Strengths and limitations of genetic models of ADHD. Attention deficit and hyperactivity disorders. 2010;2(1):21–30. doi: 10.1007/s12402-010-0021-3. [DOI] [PubMed] [Google Scholar]
- 54.Rhodes JS, Garland T. Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology. 2003;167(3):242–50. doi: 10.1007/s00213-003-1399-9. [DOI] [PubMed] [Google Scholar]
- 55.Sagvolden T, Xu T. l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD) Behavioral and brain functions: BBF. 2008;4:3. doi: 10.1186/1744-9081-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behavioural pharmacology. 2000;11(3–4):279–90. doi: 10.1097/00008877-200006000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flint J, Munafo MR. Candidate and non-candidate genes in behavior genetics. Current opinion in neurobiology. 2013;23(1):57–61. doi: 10.1016/j.conb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neale BM, Medland SE, Ripke S, Asherson P, Franke B, Lesch KP, Faraone SV, Nguyen TT, Schafer H, Holmans P, Daly M, Steinhausen HC, Freitag C, Reif A, Renner TJ, Romanos M, Romanos J, Walitza S, Warnke A, Meyer J, Palmason H, Buitelaar J, Vasquez AA, Lambregts-Rommelse N, Gill M, Anney RJ, Langely K, O’Donovan M, Williams N, Owen M, Thapar A, Kent L, Sergeant J, Roeyers H, Mick E, Biederman J, Doyle A, Smalley S, Loo S, Hakonarson H, Elia J, Todorov A, Miranda A, Mulas F, Ebstein RP, Rothenberger A, Banaschewski T, Oades RD, Sonuga-Barke E, McGough J, Nisenbaum L, Middleton F, Hu X, Nelson S. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(9):884–97. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]