Abstract
The exponential rise in the number of functional brain connectivity studies, particularly those examining intrinsic functional connectivity (iFC) at rest, and the promises of this work for unraveling the ontogeny of functional neural systems motivate this review. Shortly before this explosion in functional connectivity research, developmental neuroscientists had proposed theories based on neural systems models to explain behavioral changes, particularly in adolescence. The current review presents recent advances in imaging in brain connectivity research, which provides a unique tool for the study of neural systems. Understanding the potential of neuroimaging for refining neurodevelopmental models of brain function requires a description of various functional connectivity approaches. In this review, we describe task-based and resting-state functional magnetic resonance imaging (fMRI) analytic strategies, but we focus on iFC findings from resting-state data to describe general developmental trajectories of brain network organization. Finally, we use the example of drug addiction to frame a discussion of psychopathology that emerges in adolescence.
Keywords: development, resting state, fMRI, networks, intrinsic functional connectivity
INTRODUCTION: ADOLESCENT BEHAVIORAL, PHYSIOLOGICAL, AND NEURAL DEVELOPMENT
In this section, we describe the complexity of one critical stage of development, adolescence, to set the stage for the rationale underlying the need to understand functional brain development, particularly during this time window. The field of functional brain connectivity is rapidly evolving but is still so young that it would be premature to address in a review the specific transition period of adolescence in terms of specifically changing networks. Nonetheless, we hope that this initial clinical description of adolescence will inspire researchers to use some of the techniques described below to further examine this pivotal period, which seems to be most vulnerable for the development of psychopathology. We end this review with a look at how changes in functional brain connectivity could contribute to a vulnerability to drug addiction in adolescence.
Adolescence is a transitional period linking childhood to adulthood. The definition of this period varies, but it is commonly anchored to the start of puberty (~9–11 years of age in girls and 11–13 in boys) and ends at the age of legal adulthood (Adkins 2013). Adolescence is characterized by considerable changes in multiple domains (i.e., physical, neurobiological, cognitive, emotional, motivational, and social). For example, goal-directed behaviors are executed faster in comparison with adults, motoric and cognitive inhibitions are facilitated, and working memory is improved (Bedard et al. 2002, Ernst & Mueller 2008, Gathercole et al. 2004, Munoz & Istvan 1998, Williams et al. 1999). Concurrently, impulsivity, emotional lability, and risk taking increase during this transition period before tapering down in adulthood (Arnett 1999, Buchanan et al. 1992, Dahl 2004, Ernst et al. 2006, Hardin & Ernst 2009). These latter changes, when extreme, can have potentially dramatic consequences, such as drug addiction, serious car accidents, sexually transmitted infections, and unplanned pregnancies (Kann et al. 2014). In addition, these adolescent changes are concomitant with the peak incidence of many psychiatric disorders, including mood and anxiety disorders (Angold & Costello 2006, Kessler et al. 2005).
Of particular relevance, these behavioral transformations have been considered in light of large structural brain reorganizations, which can be hormone dependent or hormone independent. Structural neuroimaging studies describe a prepubertal increase followed by a postpubertal decrease in total gray matter volume (e.g., Gogtay & Thompson 2010). These findings have been refined to show distinct trajectories across specific regions, with inflection points earliest in temporal cortex (11 years of age in boys and 11.8 years in girls), later in frontal cortex (12.1 years in boys and 11 years in girls), and latest in temporal cortex (16.5 years in boys and 16.7 years in girls). The prepubertal rise in gray matter volume has been associated with intense synaptogenesis (O’Muircheartaigh et al. 2013), and the postpubertal decline with synaptic pruning (e.g., Bourgeois & Rakic 1993). In parallel with these changes, structural studies also document linear increases in white matter volume and density across adolescence (Giedd et al. 1999, Lenroot et al. 2007), which are attributed to increases in axonal caliber and myelination (Mädler et al. 2008, Paus 2005, Snook et al. 2005). Finally, investigators measuring surface morphology have reported increases in brain gyrification, putatively reflecting axonal growth and biomechanical tensions during brain expansion (Blanton et al. 2001). These developmental shifts in brain structure converge to increase the efficiency and specificity of information exchange across the brain.
Aspects of these local changes can be captured at the gross spatial scale visible with human magnetic resonance imaging (MRI); they are also reflected in functional changes observable with human functional MRI (fMRI) studies (e.g., Rubia 2013). Activation studies identify brain regions involved in specific cognitive processes associated with the tasks that individuals perform in the scanner (e.g., Richards et al. 2013). Functional connectivity studies, however, extend these local findings to network changes by testing and exploring neural networks across which information is shared and integrated. These advances are particularly relevant in testing the neural systems models that have been proposed to describe neural changes underlying adolescent behavioral patterns, such as risk taking, novelty seeking, and increasing emotional intensity (Casey et al. 2011, Ernst et al. 2014, Ernst & Fudge 2009, Steinberg et al. 2008). These models posit a distinct functional equilibrium among two (Casey et al. 2011) or three (Ernst et al. 2006) distributed networks based on the relative degree of maturation of these networks. For example, Casey and colleagues (2011) describe a cognitive control system within the prefrontal cortex, which lacks efficiency to regulate the reward-related system centered within the striatum and which is particularly active in adolescence. The triadic model (Ernst et al. 2006) adds a third network to these two networks, the avoidance-related network, which codes preferentially negative emotions and is centered within the amygdala. The triadic model was developed to account not only for risk-taking behaviors but also for the emotional/social changes that strongly influence motivated behaviors. A number of task-based fMRI studies have tested these models (for a review, see Richards et al. 2013), but no studies as yet have focused on the relative connectivity and communication within and between these networks across age. Here, we present the tools available to examine these questions in the future and summarize some of the fundamental shifts that have been identified in the maturational patterns of brain networks in general.
Indeed, research in functional connectivity has witnessed an exponential growth, particularly with the development of sophisticated tools and an explosion of resting-state investigations (e.g., Friston 2011). Critically, these techniques—which are being applied to understand brain development in health and pathology—can provide important clues to vulnerability to psychopathology. We first review various functional connectivity methodologies before focusing on resting-state functional connectivity studies.
FUNCTIONAL MAGNETIC RESONANCE IMAGING METHODOLOGIES
Functional connectivity is operationally defined as statistical dependencies, such as correlations or coherence, among distinct neurophysiological events (Friston 2011). With fMRI, functional connectivity typically is measured by calculating correlations between time series of voxels or regional seeds, or both. The assumption is that high interregional correlations reflect neural communication between regions, i.e., that they are “working together.” The inferred nature of this communication largely varies depending on what subjects do during scanning. The following section is an effort to organize and make broadly comprehensible a variety of analysis methods that have grown from many questions over the past two decades. We do not take a historical perspective but instead a user’s perspective. We hope this section will orient readers to primary literature and help them select the design and analytical strategies appropriate to their specific research questions.
Task-based studies refer to the examination of blood-oxygen-level-dependent (BOLD) signals in response to a behavioral paradigm (e.g., Stroop task, face emotion identification task) that targets specific and experimentally controlled cognitive processes. In contrast, resting-state studies are so named because data are collected while subjects lie at rest in the scanner and are instructed to not fall asleep. Resting-state fMRI analyses are designed to probe general properties of brain organization that relate, most commonly by inference, to specific cognitive processes. Therefore, different analyses typically accompany these two broad types of neuroimaging data (task-based versus resting-state), which offer different but overlapping pictures of network organization.
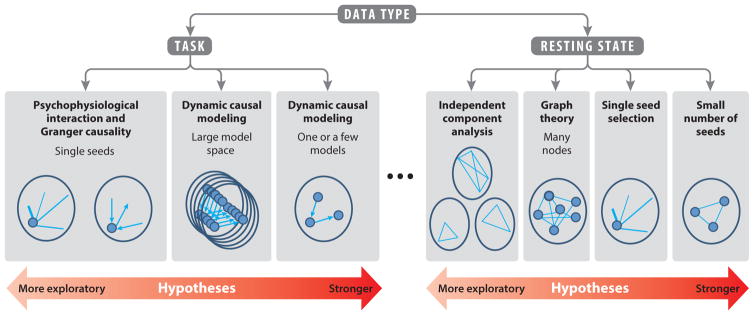
Two additional factors broadly characterize the nature of the information gained about brain organization from connectivity studies. The first factor is whether the analysis is model driven (e.g., a strong hypothesis about the connectivity of a particular region or group of regions) or data driven (e.g., an algorithm blindly separates network features within the data). The second factor is whether the analysis provides insights into the direction of information flow (e.g., from region A to region B; its effective connectivity) (Friston et al. 2003, Roebroeck et al. 2005) or not (i.e., its functional connectivity). Although these two features of connectivity analyses (model driven versus data driven and directional versus nondirectional) theoretically could be applied to both task-based and resting-state connectivity studies, the current state of development of these techniques is not equally applicable to both types of studies. The data-driven analyses are directed most commonly to resting-state studies, whereas the directional analyses currently are usually accessed with task-based studies. For orientation, Figure 1 schematically summarizes these analyses along a hypothesis-driven to data-driven gradient as they apply to the two major types of fMRI data currently being collected. The next section addresses coactivation and connectivity analyses used in task-based studies, followed by connectivity analyses used in resting-state studies.
Figure 1.
A heuristic organization of popular functional magnetic resonance imaging (fMRI)-based connectivity methods. A gradient between fully data-driven, exploratory analyses and very strongly hypothesis-driven analyses is proposed. Small filled circles indicate functionally or anatomically defined nodes. Light blue lines represent functional connectivity, and arrows represent effective connectivity. Lines and arrows not pointing to nodes indicate whole-brain, voxel-wise analyses. Ellipsis indicates the as-yet not fully understood relation between task and rest.
Task-Based Studies
Coactivation as connectivity clue
Some investigators have considered that the concomitant activations of regions in response to a specific task, or coactivation, constitute a network engaged in a particular process. For example, Knutson et al. (2001) used a monetary reward task and reported activation of a set of regions that included the ventral striatum, caudate, amygdala, mesial prefrontal cortex, and anterior cingulate cortex. A subsequent meta-analysis based on 142 neuroimaging activation studies echoed these findings, reporting that this same set of regions was consistently activated in response to reward processes (Liu et al. 2011). The meta-analysis project NeuroSynth.org offers similar insight across a wide variety of processes (Yarkoni et al. 2011). However, although an activation study (or meta-analysis) can reveal the areas involved in a cognitive process, the coactivation approach is limited because it does not directly test network properties and does not describe the strength or signs (positive or negative) of specific region-to-region couplings.
Task-based functional connectivity
In contrast to the qualitative inference of networks from coactivation maps, a statistical approach called psychophysiological interaction (PPI) analysis regresses the activity of a seed region with activity across the rest of the brain, which tests whether connection strengths vary as a function of the experimental or behavioral manipulation (Friston et al. 1997). The idea underlying PPI is that brain regions exhibit a level of baseline communication that may be modulated (i.e., strengthened or weakened) by a particular cognitive process. In one study, for example, the task of viewing fearful faces versus neutral faces boosted connectivity strength between the right amygdala and fusiform gyrus (Das et al. 2005).
PPI analyses are usually driven by an a priori hypothesis regarding the connectivity pattern of a particular seed region, and the technique is usually applied such that task-induced couplings are then shown across the rest of the brain. This is an advantage when one focuses on a specific cognitive function known to change with age or to be differentially affected by a disease process.
Despite the benefits and popularity of the PPI technique, it remains subject to some of the same weaknesses as any task-based study. Task-based studies can be problematic when used to compare populations with systematic biases in performance. For example, young children or patients may use different strategies or experience distinct difficulties when performing certain tasks, relative to older or healthy individuals, and thus it is difficult to specify whether group differences are age or disease related or simply reflect a general difference in performance. This issue of variability in task performance is the source of continual debate in the literature and should be considered when deciding on any task-based analysis (e.g., Snyder et al. 2011).
Another potential disadvantage of PPI analysis lies with the fact that a hypothesis about the connectivity of a particular brain region may bias findings by restricting the analysis to seeding one region at the expense of other potentially important loci. Finally, PPI, which is regression based, does not allow for inferences on the direction of information flow. For these inferences, effective connectivity techniques have been developed.
Task-based effective connectivity
Effective connectivity analyses for fMRI are sophisticated attempts to find degrees of evidence for causal or directional influences among brain regions. The most common directional analyses are the Granger causality (GC) and the dynamic causal modeling (DCM) techniques. In the late 1960s, economist Clive Granger developed a way to infer causal relations among time series based on lag; more recently, this approach was adapted to time series in neuroimaging data (Roebroeck et al. 2005). DCM was originally developed by neuroscientists who used dynamic system modeling to infer causal influences in a subset of regions (Friston et al. 2003, Stephan et al. 2010). Like PPI, DCM essentially looks for task-based changes in coupling, but in contrast to PPI, DCM focuses on a subset of regions (generally fewer than eight). The investigator specifies models of how these regions may causally interact, and, with a very strong hypothesis, tests which of the models the data support best (Penny et al. 2004). Advances with this technique also allow the testing of a large number of models if the available knowledge of a particular network is insufficient to support very specific hypotheses (Penny et al. 2010, Torrisi et al. 2013b).
Over the past decade, researchers have applied GC and DCM to both activation studies and resting-state studies (see below) (Friston et al. 2014, Kahan & Foltynie 2013, Liao et al. 2010), although their use with resting-state data is much less common. Finally, these analyses remain difficult to conduct and are not without controversy (David et al. 2008, Lohmann et al. 2012, Ramsey et al. 2010, Smith et al. 2011a, Webb et al. 2013).
Functional Connectivity at Rest
Resting-state functional connectivity analyses assess synchronous activity between brain regions when subjects are awake but resting. A great advantage of such data over those involving psychological tasks is that they can be collected from a wide variety of populations because the resting-state condition involves minimal instructions and low demands on attention. Thus, most patient populations (Zhang & Raichle 2010) and children of all ages (Thomason et al. 2013) can participate in resting-state studies. Another advantage is the ability to perform wholly exploratory or data-driven analyses, less biased by our present knowledge of brain dynamics and possibly more likely to lead to the discovery of new principles of brain organization.
Because activity during resting state is spontaneous and uncontrolled, intrinsic functional connectivity (iFC) analyses do not focus on particular cognitive processes per se but rather aim at elucidating the dynamics and components of general networks. These general or intrinsic networks are not necessarily identical to those activated during targeted psychological tasks and are instead thought to reflect genetically guided structural and experience-dependent functional substrates underlying normal cognitive processing (Sporns 2013). Despite this division, brain networks elicited from task-based fMRI studies appear to be qualitatively and quantitatively similar to those elicited from resting data, and some researchers are actively trying to clarify this relationship (Barnes et al. 2009, Rehme et al. 2013, Schultz et al. 2012, Smith et al. 2009).
Resting-state studies have thus revealed a number of active canonical networks, defined by distinct sets of correlations among discrete regions. Many of these resting state networks have been identified using both model-based and model-independent analyses. Furthermore, they are associated with specific sensory functions (e.g., auditory, vision, and motor control networks) or cognitive functions (e.g., salience, attention, and default mode networks). The interrelationships of these networks themselves (not simply nodes within networks) have also been a source of investigation that has opened a new window into a higher level of brain organization (Fox et al. 2005, Sridharan et al. 2008).
The advantages of the absence of tasks in resting-state studies can also represent a disadvantage. Specifically, without a task-induced process, which provides unique timing of events, resting-state correlations can fall victim to spurious and nonneuronal sources of covariation, including scanner artifacts, cardiac pulsations, respiration, and head motion (Jo et al. 2010). These nonneuronal physiological events can produce false-positive or highly skewed results. Therefore, careful and sophisticated preprocessing approaches must be employed to eliminate the impact of such systematic biological noise (Murphy et al. 2013, Saad et al. 2012). A second disadvantage is directly linked to the complexity of the analyses, which, in recent years, has given rise to an increasing number of techniques resulting in a general lack of replication. However, we expect this limitation to be resolved in the near future with improved technology and consensus on the most valid and reliable analyses. A third disadvantage, as alluded to above, is that we do not fully understand the relationship between task and resting-state data, and the relationship between, for example, the higher frequencies of activity usually studied with tasks and the lower spontaneous frequencies analyzed with rest. In the next section, we describe the three techniques most commonly used to analyze the latter.
Seed-based connectivity at rest
Analogous to the PPI technique used for assessing task-based functional connectivity, the most common analytic approach for resting-state connectivity requires selecting a seed region and examining the pattern of connectivity, via correlations, from this seed to the rest of the voxels in the brain (Biswal et al. 1995, van den Heuvel & Hulshoff Pol 2010). For example, a number of studies have examined how key functional regions communicate with the rest of the brain in health and disease, such as the amygdala in anxiety (e.g., Roy et al. 2013). Alternatively, more constrained hypotheses can be tested by correlating the time series of a small number of regions with one another (Torrisi et al. 2013a). This approach requires a stronger rationale and basis on how to select the regions of interest, but it benefits from fewer multiple comparisons. In contrast to these relatively simple analytic strategies that query known discrete networks, more complex analytic schemes can be applied to examine metrics of global brain organization, using graph theory techniques, which we describe in the next section.
Graph analyses
Graph theory provides a number of metrics that characterize networks, such as social networks, for which they were originally developed. Graph analyses have been applied to many fields to describe global and local characteristics of networks (Strogatz 2001), and they are best used to characterize large networks with many nodes. In the brain, these metrics compute descriptive measures of pairwise connections (“edges”) between dozens or hundreds of regions (nodes) to reveal both between- and within-network connectivity structure. Terms such as path length, motifs, hubs, global efficiency, centrality, and small-worldness describe the measures of a rich variety of global connectivity properties (Rubinov & Sporns 2010).
However, similar to seed-based analyses, graph analyses of brain function still depend on the functional relevance of the nodes selected for analysis, and recent work suggests that functionally defined parcellations provide better network estimates than do structural atlases or arbitrarily defined sampling grids (Craddock et al. 2012, Sepulcre et al. 2012, Smith et al. 2011b). A larger issue with graph theory, however, lies in the difficulty to specifically connect its metrics and global descriptive power with underlying biological phenomena. Fully hypothesis-free analytic approaches are described in the next section.
Model-independent methods
A model-independent analysis approach called independent component analysis (ICA) is a multivariate decomposition method that blindly separates spatial signals across the brain on the basis of their temporal characteristics (Beckmann et al. 2005). This technique is optimal for discovery findings. Without the necessity of a priori node selections, ICA can be used to identify complex interactions among regions that might otherwise go undetected (McKeown et al. 2003). In addition, it can be used to reveal the multifunctionality of nodes (i.e., nonmodularity) that are involved in multiple networks by separating independent signals from the same, often functionally heterogeneous, region (Beckmann et al. 2005). One difficulty with ICA, however, is that the optimal number of components to extract can be unclear, and different algorithms calculating this number often produce different results (Calhoun & Adali 2012). This is especially problematic when extracting separate ICAs for different populations that need to be compared (e.g., children versus adults). Another difficulty is how to classify which components are neural networks and which are not, such as noise. In sum, ICA is strong as an exploratory technique and can be used as a later guide for hypothesis-driven analyses.
Summary
For well over a decade, these fMRI-based connectivity analyses have led an emerging picture of network-level properties of brain function. Some of these properties undergo substantial changes during development. These ontogenic changes need to be carefully delineated to understand the various neural mechanisms underlying improvement of brain functionality; as a moving target, they critically warrant replication. However, fMRI task-based connectivity studies are specific to the task employed, which can greatly vary from study to study. Because of task specificity and differences in networks studied, PPI findings have often not been replicated (e.g., Rubia 2013), making it currently difficult to integrate task-based connectivity results into a coherent review of connectivity in development. Therefore, the next section focuses selectively on resting-state studies, which do not suffer as strongly from this methodological issue.
RESTING-STATE FUNCTIONAL CONNECTIVITY ACROSS DEVELOPMENT
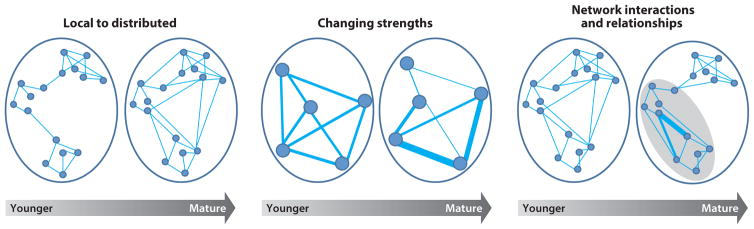
In this section, we review a selected number of landmark studies on ontogenic changes in brain connectivity. At present, no longitudinal studies of intrinsic connectivity in typically developing individuals have been conducted, although a number of laboratories are in the process of collecting such data. We consider only those studies that examined both youths (children and/or adolescents) and adults. Notably, only a few studies include all three age groups (children, adolescents, and adults), and these studies suggest that the adolescent iFC pattern reflects intermediary stages between children and adults. Overall, findings reflect robust transformations of the brain network organization from childhood into adulthood. These transformations can be organized along three themes: (a) local to distributed, (b) strength modifications, and (c) relationships among networks. Although these thematic categories are not necessarily mutually exclusive, this classification is helpful for its heuristic value (see also Figure 2).
Figure 2.
Graphic representations of the three types of ontogenic changes in functional neural connectivity described in the text. Networks grouped within the gray oval (last graph on right) represent a hierarchical grouping separate from the other network.
Local to Distributed Functional Integration
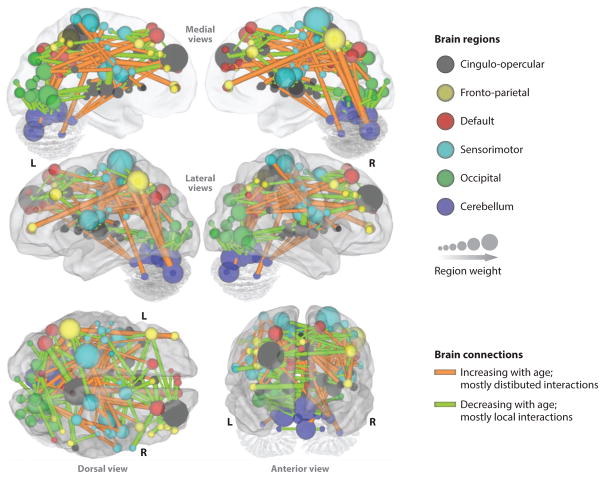
During prenatal development, neuronal communication consists largely of short-range links between regions, a pattern that forms local networks (Hoff et al. 2013). As development progresses, the abundance of short-range connections is tempered by an increasingly integrated system with long-range connections, such as those that link hemispheres or connect occipital or parietal with frontal or temporal lobes (Fair et al. 2007). Fair and colleagues (2009) suggest that the timing of this transition relies on the protracted axonal myelination, which greatly speeds information transfer across longer distances. As connections strengthen between networks, the brain function becomes more integrated, dynamic, and flexible (Hwang et al. 2013). Figure 3 represents the results of a multivariate pattern analysis of 238 scans of typically developing individuals, 7 to 30 years of age, conducted by Dosenbach et al. (2010). This figure illustrates a weakening of short-range connections (green lines) and a strengthening of long-range connections (orange lines) with age. This transition may represent a developmental shift of local to distributed function, which contributes to the increased efficiency of large-scale integration of information. Conversely, the smaller networks that are being integrated may become more refined, specialized, and restricted. More generally, the developmental shift from short-range to long-range connectivity together with the refinement of smaller networks is consistent with the many observations of greater focality of activation (i.e., stronger connectivity within discrete networks) in adults compared with children; this greater focality is associated with improved cognitive performance (for a review, see Rubia 2013). In addition to this general evolution in the global network architecture of the brain, strengths of connections within networks also undergo substantial modifications with development.
Figure 3.
A representation depicting the age-related changes in connections between brain regions on a surface rendering of the brain. Connections that increase with age are shown in orange; those that decrease with age are shown in green. The local interactions between brain regions seem to decrease ( green) with age, whereas a more distributed organization (orange) emerges with age. Also shown are the relative weights of various brain regions (160 ROIs), quantified by the weights of afferent and efferent connections of each region. The color-coding of these regions is based on six resting-state networks (e.g., cingulo-opercular in gray). Figure adapted from Dosenbach et al. (2010) and used with permission from the American Association for the Advancement of Science.
Changing Network Strengths
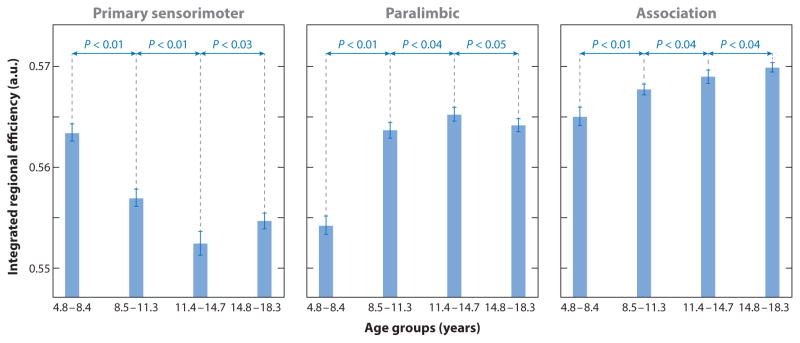
The general developmental trend of increasing long-range connections with decreasing short-range connections coincides with the strengthening of some specific networks and the weakening of others. Researchers have, for example, seeded nodes from different networks to investigate the integration of the default mode network (DMN) (Anderson et al. 2011, Fair et al. 2008), or the salience network (Uddin et al. 2011), or of thalamo-frontal communication (Fair et al. 2010). In a recent review, Sporns (2013) discussed the idea that networks observed during the resting state result from patterns of coactivation and common recruitment associated with cognitive processes and actions. In other words, the more frequently certain processes are used in daily life, the more these processes entrench themselves in networks that are detectable with resting-state analyses. Such an interpretation would extend the Hebbian theory that “neurons that fire together wire together” to the network level (Morris 1999), and it is critical for understanding changes during development, when experience-based plasticity is particularly high. This theory is supported by a study examining the different maturational trajectories across distinct functional networks (Figure 4) (Khundrakpam et al. 2013). Specifically, this study analyzed scans from 203 healthy subjects divided into four age groups. Figure 4 indicates that the sensorimotor network, which serves the most basic functions of an organism, matures the earliest, as evidenced by the highest efficiency level of this network in the youngest age group, whereas the highest efficiency level in the oldest group is found for the association-related network.
Figure 4.
Age-related differences in regional efficiency in three structural brain networks among four age groups (group 1, 4.8–8.4 years; group 2, 8.5–11.3 years; group 3, 11.4–14.7 years; group 4, 14.8–18.3 years) from a graph theory analysis of 203 individuals. Different developmental patterns of maturation (indexed by efficiency) among the three networks are depicted (e.g., the primary sensorimotor network evidences early maturation in the youngest group, whereas the association network in this group shows a protracted development). Figure adapted from Khundrakpam et al. (2013) and used with permission from Oxford University Press.
Although the experience-guided shaping of the functional brain organization is paramount during development, the genetically determined structural aspects of the formation of brain networks across development remain foundational to this organization. Clearly, the gradual shaping of brain function consists of a continuous interplay between hardwired capacities and experience. At present, most resting-state developmental investigations simply describe different types of changing neural network dynamics without links to causal mechanisms at molecular or genetic levels. The alteration of strengths of connections within and between networks introduces the next critical principle of network organization, which is based on the notion of modularity, and particularly hierarchical modularity (Meunier et al. 2010).
Network Interactions and Relationships
A brain network module is composed of processing units (nodes) that are closely interlinked by connections (edges) for the exchange of information (see Figure 2). Each module (i.e., a set of nodes interconnected by edges) supports a distinct function (e.g., color vision). A network can consist of one module or be composed of several modules. The principles under which these modules and networks work together are currently under investigation. One of the most robust but flexible and efficient schemes has invoked a hierarchical pattern in which networks themselves act modularly, nested within a hierarchical architecture. The shaping of such organization is guided by the integration of repeated experiences and a biological scaffolding, and the most critical time for the establishment of this organization is during development.
Several investigators have reported findings of ontogenic changes at the network level. Uddin and colleagues (2011) examined age-related changes of the relationships among three canonical resting-state networks: the frontoparietal central executive network, the DMN, and the salience network. The salience network (particularly the right fronto-insular cortex) has been hypothesized to facilitate the switch between the central executive network (externally oriented attention) and the DMN (internally oriented cognition) in response to cognitive demands. Using multimodal imaging methodologies (resting-state fMRI and diffusion tensor imaging tractography), Uddin et al. (2011) queried the extent to which this multinetwork model operant in adults gradually emerges from childhood. Findings revealed evidence of the strengthening of intra- and inter-network connectivity in adults compared to children. In agreement with previous work (Fair et al. 2009, Supekar et al. 2009), this study suggested that global brain organization appeared to be similar in both age groups but that significant sub-network reorganization takes place between childhood and adulthood, in line with an increased hierarchical modularity with age.
Accordingly, another study found that children had lower levels of global brain hierarchical organization in comparison with young adults, who in turn possessed more long-range functional connections, which facilitate functional integration across the whole brain (Supekar et al. 2009). The reduction in short-range connectivity with age also suggests the emergence of specialized networks. Another study (Stevens et al. 2009) measured “causal density,” a metric of network function that reflects the degree of mutual causal interactions between networks, perhaps a measure of how tightly the network functions in isolation. Findings revealed a reduction in this measure with age, which was interpreted as evidence for a more efficient and flexible function of these networks. The Stevens et al. (2009) study also reported a decrease in the number of within- and between-network effective connections, a result that was construed as reflecting greater independence of these networks from one another. As noted by the authors, the complexity of the methodology, including the number of assumptions required to conduct these analyses, limits the interpretation of the results. More generally, the results represent the current state of this field, which requires highly sophisticated network computational approaches that need to be tested systematically before being fully exploited to understand the function and development of brain systems.
Summary
Three categories of change occur in iFC from childhood into adulthood. These local to distal, connectivity strength, and network organization changes are all different facets of the same general trajectory of the brain organization to become more flexible, efficient, and specialized. We are still at the beginning of this exciting new line of discovery about the brain function at the systems level. Great hopes accompany this line of research, which holds promise to significantly contribute to our understanding of disease mechanisms and to open new research directions for other neuroscience fields, such as the molecular and genetic domains. Given that most mental disorders are now considered neurodevelopmental, it is important to assign a high priority to neurodevelopmental research to understand vulnerability to disease states. In the next section, we provide an example of the disease formation that is the most common in adolescence, drug addiction.
RELEVANCE FOR ADOLESCENCE VULNERABILITY TO ADDICTION
Substance use is a critical contributor to health problems among youths and constitutes a major public health concern (NIAD 2013). In addition, adults who abuse substances typically begin substance use in adolescence (Chambers et al. 2003). These observations highlight the notion that adolescence is a critical time of vulnerability for the development of substance abuse. Ontogenetic changes in functional brain connectivity can provide insight into the mechanisms underlying this susceptibility.
As discussed above, functional neural networks mature over the course of development, and the networks that mediate higher cognitive processes are among the last to mature (Supekar et al. 2009). We identified three major categories of changes. The first category refers to functional changes from local to more distributed connectivity patterns. This type of change would permit greater processing efficiency and behavioral flexibility during adolescent development. Such behavioral improvement might facilitate exploratory behaviors on the part of the adolescents, diversifying their life experience. Although these behaviors are the norm and are socially encouraged during this transition period, potentially dangerous explorations, such as substance use, could strengthen specific networks (e.g., reward and salience) that could reinforce substance use–related behaviors, leading to abuse and then addiction.
Indeed, the molding and entrenchment of brain networks by experience, expected to be particularly strong during adolescence, is what the second category of changes across development shows: the modification of network strengths. Developmental findings seem to indicate a strengthening of connections within the default mode network in adults (Anderson et al. 2011, Fair et al. 2008). Such molding of networks is likely influenced by experience, and particularly by exposure to substances of abuse. Specifically, resting-state neuroimaging studies of animal models of addiction, which permit researchers to control critical levels and contextual parameters of substance exposure and to measure detailed underlying biological mechanisms, provide helpful clues to guide human research (e.g., van der Marel et al. 2014).
Finally, the third category of changes highlights the organization and formation of hierarchical networks. Efficient systems design is often hierarchical, and as such a developed neural hierarchy may also result in greater behavioral flexibility (alluded to above) and behavioral and cognitive control. Related to control, the prominence of the limbic networks (bottom-up processing) in adolescents might contribute to impulsivity and increased emotionality, potentiated by less efficient inhibitory controls from the attention and executive networks (top-down processing), a pattern of network connectivity that is consistent with the neural systems models of adolescent motivated behavior (Casey et al. 2011, Ernst & Fudge 2009). A lack of proper interaction of networks, possibly related to asynchrony in the timing of network developmental trajectories, might also result in greater vulnerability to addictive behaviors, such as substance abuse.
CONCLUSION
After first describing the various approaches to studying functional brain connectivity, each targeting unique questions, we extracted three categories of developmental changes across childhood into adulthood based on resting-state functional brain connectivity. A critical aspect of these changes is the issue of whether experience contributes to a great extent to the shaping and strength of brain networks. A few large-scale longitudinal studies, which include resting-state fMRI together with intense sample characterization, are under way and will soon provide answers to this question. Connectivity tools and analyses are continuously being developed and refined. However, for more specific goals, such as understanding the neural basis of adolescent behavior and of vulnerabilities or psychopathologies such as addiction, additional studies will be necessary, including the use of resting-state fMRI in animal models of adolescence and addiction to inform research in humans.
We end this review with reflections on the future of functional brain connectivity. From a methodology perspective, we exclusively addressed in this review fMRI connectivity without considering other modalities of data collection, such as diffusion tensor imaging tractography, magnetoencephalography, or interventional techniques, such as transmagnetic stimulation. The judicious combination of these modalities with connectivity fMRI, which has already begun to be implemented (Brookes et al. 2011, de Pasquale et al. 2010), could significantly improve the anatomical, neural (e.g., temporal resolution with magnetoencephalography) and functional (e.g., causal manipulation with transmagnetic stimulation) interpretability of connectivity fMRI findings. Conceptually, these techniques will help us test neural mechanisms, including neural systems models, to gain insight into how the brain develops and functions in health. Clinically, the potential of connectivity fMRI for applications is huge. We see three main prospects for connectivity fMRI: to provide (a) markers for diseases, (b) paths to investigate disease mechanisms, and (c) predictors of clinical course.
Acknowledgments
The authors thank Andrew John Ogilvie Davis for his assistance in the final preparation of this manuscript.
Footnotes
This is a work of the U.S. Government and is not subject to copyright protection in the United States.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Monique Ernst, Email: ernstm@mail.nih.gov.
Salvatore Torrisi, Email: sam.torrisi@nih.gov.
Nicholas Balderston, Email: nicholas.balderston@nih.gov.
Christian Grillon, Email: grillonc@mail.nih.gov.
Elizabeth A. Hale, Email: elizabeth.hale@nih.gov.
LITERATURE CITED
- Adkins D. When is puberty too early? Durham, NC: Duke Univ. Health Syst; 2013. http://www.dukemedicine.org/blog/when-puberty-too-early. [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connectivity. 2011;1:147–57. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child Adolesc Psychiatr Clin N Am. 2006;15:919–37. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. Am Psychol. 1999;54:317–26. doi: 10.1037//0003-066x.54.5.317. [DOI] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLOS ONE. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc B. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blanton RE, Levitt JG, Thompson PM, Narr KL, Capetillo-Cunliffe L, et al. Mapping cortical asymmetry and complexity patterns in normal children. Psychiatry Res. 2001;107:29–43. doi: 10.1016/s0925-4927(01)00091-9. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–20. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Woolrich M, Luckhoo H, Price D, Hale JR, et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci USA. 2011;108:16783–88. doi: 10.1073/pnas.1112685108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones? Evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Jones RM, Somerville LH. Braking and accelerating of the adolescent brain. J Res Adolesc. 2011;21:21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012;33:1914–28. doi: 10.1002/hbm.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. NeuroImage. 2005;26:141–48. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLOS Biol. 2008;6:2683–97. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci USA. 2010;107:6040–45. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Hale E, O’Connell K. Response to commentaries regarding the Triadic Systems Model perspective. Brain Cogn. 2014;89:122–26. doi: 10.1016/j.bandc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: insights from functional neuroimaging research. Dev Neurobiol. 2008;68:729–43. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci USA. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–78. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Kahan J, Biswal B, Razi A. A DCM for resting state FMRI. NeuroImage. 2014;94:396–407. doi: 10.1016/j.neuroimage.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Dev Psychol. 2004;40:177–90. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–63. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Ernst M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents. J Addict Med. 2009;3:47–54. doi: 10.1097/ADM.0b013e31819ca788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff GEA, Van Den Heuvel M, Benders MJNL, Kersbergen KJ, de Vries LS. On development of functional brain connectivity in the young brain. Front Hum Neurosci. 2013;7:650. doi: 10.3389/fnhum.2013.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Hallquist MN, Luna B. The development of hub architecture in the human functional brain network. Cereb Cortex. 2013;23:2380–93. doi: 10.1093/cercor/bhs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state fMRI, with artifact detection and removal. NeuroImage. 2010;52:571–82. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J, Foltynie T. Understanding DCM: ten simple rules for the clinician. NeuroImage. 2013;83:542–49. doi: 10.1016/j.neuroimage.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, et al. Youth risk behavior surveillance—United States, 2013. MMWR Surveill Summ. 2014;63(SS-3) [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Khundrakpam BS, Reid A, Brauer J, Carbonell F, Lewis J, et al. Developmental changes in organization of structural brain networks. Cereb Cortex. 2013;23:2072–85. doi: 10.1093/cercor/bhs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–73. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Mantini D, Zhang Z, Pan Z, Ding J, et al. Evaluating the effective connectivity of resting state networks using conditional Granger causality. Biol Cybern. 2010;102:57–69. doi: 10.1007/s00422-009-0350-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, Erfurth K, Muller K, Turner R. Critical comments on dynamic causal modelling. NeuroImage. 2012;59:2322–29. doi: 10.1016/j.neuroimage.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Mädler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26:874–88. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Hansen LK, Sejnowsk TJ. Independent component analysis of functional MRI: What is signal and what is noise? Curr Opin Neurobiol. 2003;13:620–29. doi: 10.1016/j.conb.2003.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Lambiotte R, Bullmore ET. Modular and hierarchically modular organization of brain networks. Front Neurosci. 2010;4:200. doi: 10.3389/fnins.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Hebb DO. The Organization of Behavior. Wiley; New York: 1999. [DOI] [PubMed] [Google Scholar]; Brain Res Bull. 1949;50:437. doi: 10.1016/s0361-9230(99)00182-3. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol. 1998;79:1193–209. doi: 10.1152/jn.1998.79.3.1193. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. NeuroImage. 2013;80:349–59. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natl. Inst. Drug Abuse (NIAD) Monitoring the Future Survey: Overview of Findings 2013. Bethesda, MD: NIDA; 2013. http://www.drugabuse.gov/monitoring-future-survey-overview-findings-2013. [Google Scholar]
- O’Muircheartaigh J, Dean DC, 3rd, Dirks H, Waskiewicz N, Lehman K, et al. Interactions between white matter asymmetry and language during neurodevelopment. J Neurosci. 2013;33:16170–77. doi: 10.1523/JNEUROSCI.1463-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, et al. Comparing families of dynamic causal models. PLOS Comput Biol. 2010;6:e1000709. doi: 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. NeuroImage. 2004;22:1157–72. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C. Six problems for causal inference from fMRI. NeuroImage. 2010;49:1545–58. doi: 10.1016/j.neuroimage.2009.08.065. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Grefkes C. State-dependent differences between functional and effective connectivity of the human cortical motor system. NeuroImage. 2013;67:237–46. doi: 10.1016/j.neuroimage.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents versus adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 2013;37:976–91. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage. 2005;25:230–42. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–99. e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2013;22:719–31. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DH, Balderston NL, Helmstetter FJ. Resting-state connectivity of the amygdala is altered following Pavlovian fear conditioning. Front Hum Neurosci. 2012;6:242. doi: 10.3389/fnhum.2012.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu MR, Johnson KA. Network assemblies in the functional brain. Curr Opin Neurol. 2012;25:384–91. doi: 10.1097/WCO.0b013e328355a8e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Halari R, Giampetro V, Brammer M, Rubia K. Developmental effects of reward on sustained attention networks. NeuroImage. 2011a;56:1693–704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–45. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, et al. Network modelling methods for fMRI. NeuroImage. 2011b;54:875–91. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage. 2005;26:1164–73. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Snyder AN, Bockbrader MA, Hoffa AM, Dzemidzic MA, Talavage TM, et al. Psychometrically matched tasks evaluating differential fMRI activation during form and motion processing. Neuropsychology. 2011;25:622–33. doi: 10.1037/a0022984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15:247–62. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Wollard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–78. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. NeuroImage. 2010;49:3099–109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–66. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogatz SH. Exploring complex networks. Nature. 2001;410:268–76. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLOS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Dassanayake MT, Shen S, Katkuri Y, Alexis M, et al. Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013;5:173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Moody TD, Vizueta N, Thomason ME, Monti MM, et al. Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord. 2013a;15:156–66. doi: 10.1111/bdi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi SJ, Lieberman MD, Bookheimer SY, Altshuler LL. Advancing understanding of affect labeling with dynamic causal modeling. NeuroImage. 2013b;82:481–88. doi: 10.1016/j.neuroimage.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–89. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- van der Marel K, Klomp A, Meerhoff GF, Schipper P, Lucassen PJ, et al. Long-term oral methylphenidate treatment in adolescent and adult rats: differential effects on brain morphology and function. Neuropsychopharmacology. 2014;39:263–73. doi: 10.1038/npp.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JT, Ferguson MA, Nielsen JA, Anderson JS. BOLD Granger causality reflects vascular anatomy. PLOS ONE. 2013;8:e84279. doi: 10.1371/journal.pone.0084279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–13. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]