Abstract
Rationale
Nicotine and alcohol co-use is highly prevalent, and as such, individuals experience the interoceptive effects of both substances together. Therefore, examining sensitivity to a compound nicotine and alcohol (N+A) interoceptive cue is critical to broaden our understanding of mechanisms that may contribute to nicotine and alcohol co-use.
Objectives
This work assessed the ability of a N+A interoceptive cue to gain control over goaltracking behavior and determined the effects of the α4β2 nicotinic partial agonist and smoking cessation compound varenicline on sensitivity to N+A.
Methods
Two groups of male Long-Evans rats were trained to discriminate N+A (0.4 mg/kg nicotine + 1 g/kg alcohol, IG) from water under two different training conditions using a Pavlovian drug discrimination task. The effects of varenicline (0, 1, 3 mg/kg, IP) administered alone and on sensitivity to N+A and the components were determined.
Results
Under both training conditions, N+A rapidly gained control over behavior, with a greater contribution of nicotine to the N+A compound cue. Varenicline fully substituted for the N+A training dose and varenicline (1 mg/kg) enhanced sensitivity to the lowest N+A dose (0.1N+0.1A). Given the high selectivity of varenicline for the α4β2 receptor, this finding suggests a functional role for α4β2 nicotinic acetylcholine receptors (nAChR) in modulating sensitivity to N+A.
Conclusions
The N+A compound cue is a unique cue that is modulated in part, by activity at the α4β2 nAChR. These findings advance understanding of the interoceptive effects of nicotine and alcohol in combination and may have implications in relation to their co-use.
Introduction
Combined nicotine and alcohol co-abuse constitutes a world-wide health risk. Considering the health risks of excessive alcohol use or smoking, it is not surprising that in combination, negative patient outcomes and mortality rates increase substantially (Hurt et al. 1996; Schmidt and Popham 1981). Moreover, given the frequency with which nicotine and alcohol are used in combination, it is important to consider that individuals often experience the interoceptive effects of both substances together (i.e., a unique compound interoceptive cue). Indeed, preclinical work using operant drug discrimination models has shown that a compound nicotine+alcohol interoceptive cue can serve as a discriminative stimulus to modulate behavior (Ford et al. 2013; Ford et al. 2012; Gauvin and Holloway 1993; Troisi et al. 2013).
In the present work, we sought to examine if a compound nicotine+alcohol interoceptive drug state could gain control of reward-seeking behavior using a Pavlovian drug discrimination procedure. Under such procedures, the drug state (e.g., nicotine+alcohol) sets the occasion for when the offset of an environmental stimulus (e.g., light) is followed by a reward (e.g., sucrose). On intervening vehicle training days, light offset is not followed by sucrose delivery. Consequently, the drug state comes to modulate behavior as evidenced by increased anticipatory reward-seeking during the cue light (i.e., head entries into a liquid receptacle; “goal-tracking”) on drug training sessions. These procedures have been used to characterize the interoceptive effects of several drugs including alcohol, amphetamine, caffeine, methamphetamine, and nicotine (Besheer et al. 2012; Murray et al. 2007; Palmatier et al. 2004; Palmatier et al. 2005; Reichel et al. 2007). Furthermore, the opposite association can also be trained, such that the drug state can signal the absence of reward (i.e., assessment of subject’s ability to withhold behavior). Together, these procedures provide Feature Positive (FP) and Feature Negative (FN) training conditions, respectively, producing a powerful complimentary assessment of drug-state control over behavior, as different behavioral and neurobiological mechanisms are likely recruited. For example, FP conditions are thought to be driven by a direct excitatory link to the conditioned stimulus, whereas FN conditions are thought to be driven by an activation of context-specific inhibitory processes (see Bouton 1998 for review). Therefore, the primary goal of the present work was to determine whether a nicotine+alcohol interoceptive cue could be trained to control discriminated goal-tracking behavior under FP and FN conditions in male Long Evans rats and the relative contribution of each component of the nicotine+alcohol cue under both training conditions. Based on the operant drug-discrimination literature, it was hypothesized that the alcohol component would have a greater control over goal-tracking behavior than the nicotine component (Ford et al. 2012).
In addition, there has been growing interest in the smoking cessation agent varenicline (VAR; Chantix), a partial agonist of α4β2 nicotinic acetylcholine receptors (nAChR), for the treatment of alcohol use disorders. The α4β2 receptor accounts for 90% of the high-affinity nicotine binding sites in the mammalian brain and is largely expressed in the prefrontal cortex, nucleus accumbens core and ventral tegmental area, brain regions important in associative learning and drug abuse (Chatterjee and Bartlett 2010; Colombo et al. 2013; Dineley et al. 2015; Everitt et al. 1999; Millar and Harkness 2008). Importantly, VAR has been shown to be significantly more selective for the α4β2 receptor compared to the α3β4, α1 and α7 receptors (Coe et al. 2005). In clinical studies, VAR has been shown to decrease alcohol consumption in heavy drinking smokers (Fucito et al. 2011; Mitchell et al. 2012). Therefore, another goal of the present work was to assess the effects of VAR pretreatment on: 1) sensitivity to the nicotine+alcohol interoceptive cue, 2) sensitivity to each component, and 3) to determine whether VAR has stimulus effects similar to the nicotine+alcohol cue when administered alone. Considering evidence that the alcohol component overshadows the nicotine component (Ford et al. 2012), and recent findings showing decreased sensitivity to alcohol following VAR pretreatment (Randall et al. 2015), it was hypothesized that VAR would decrease sensitivity to the compound cue and that this may be related specifically to decreased sensitivity to the alcohol component. Given that VAR has been shown to partially substitute for nicotine (i.e., have some nicotine-like effects) as assessed in operant discrimination procedures (LeSage et al. 2009; Paterson et al. 2010), it was hypothesized that similar results would be observed when VAR was administered alone and that VAR would potentiate sensitivity to the nicotine component.
Materials and Methods
Animals
Male Long Evans rats (Harlan Sprague Dawley, Indianapolis, IN) weighing 250-275 g upon arrival to the colony were individually housed in ventilated cages. Rats were handled and weighed daily for one week before training began. Rats were fed daily for the duration of the study such that weights maintained at approximately 325-340 g. Water was available continuously in the home cage. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine (DLAM) at UNC-Chapel Hill. All procedures were also carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines. UNC-Chapel Hill is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Pavlovian Drug Discrimination Training and Testing Procedures
Sucrose Access Training
Rats were trained using the same behavioral chambers and procedures were similar to those described in detail in (Besheer et al. 2012). Additionally, chambers were outfitted with infrared photobeams (that divided the chamber into 4 parallel zones) to measure locomotor activity during sessions (number of beam breaks). Briefly, rats had three 50-min sessions in which 26% sucrose (w/v) was randomly presented across the session to train rats to approach the liquid receptacle. The probability of sucrose presentation decreased from the first to the last session and by the last 10 min of the final session rats received ~0.75 sucrose presentations/min.
Discrimination Acquisition Training
Training sessions were 5 days per week (M-F) during which a nicotine (0.4 mg/kg) + alcohol (1 g/kg) mixture (N+A) or water was administered by intragastric gavage (IG) prior to the start of the sessions. Immediately following N+A or water administration the rats were placed in the chambers. During this time no cue lights were illuminated, no sucrose was presented and head entries into the liquid receptacle were not recorded. The 15-min session began after this 10-min delay. Therefore, based on the existing literature the 10 min delay represents a time point that likely corresponds to the ascending limb of plasma/blood and brain alcohol and nicotine levels following oral administration (Alsharari et al. 2014; Matta et al. 2007; Quertemont et al. 2003; Turner 1975). For the Feature Positive (FP) groups, on N+A sessions, the offset of each of the 15-sec cue light presentations was followed by sucrose presentation. On water sessions, no sucrose was delivered following the offset of the cue light presentations. For the Feature Negative (FN) groups, the reverse training occurred. That is, sucrose was presented following light presentations on water sessions, but not on N+A sessions. There were 10 cue light presentations (conditioned stimulus, CS) during each session. The onset of the first CS presentation varied from 45-75 s, and the inter-trial intervals (time from CS offset to the next CS onset) ranged from 30-105 s. Water and N+A training days varied on a double alternation schedule (W, W, N+A, N+A…). The training sessions for both groups continued until the following acquisition criteria were met for both the first and the average discrimination score: For FP rats, the average of the discrimination score from the preceding two N+A sessions had to be ≥150% of the average of the discrimination scores from the preceding two water sessions. The converse was true for the FN rats. Testing began once these criteria were met.
Testing
Individual test sessions were 2 min in duration (following the 10 min delay), with 1 light presentation that was followed by sucrose. For test sessions, onset of light presentation was randomized and varied from 45-105 seconds into the 2 minute test period. For Experiments 1 and 2, cumulative dosing procedures were used as we describe in (Besheer et al. 2012; 4 separate tests conducted in succession such that testing of the stated dose curve completed in ~48 min). For Experiment 3, single test sessions were used. Test sessions were interspersed with training sessions and if a rat did not meet the criteria for testing (i.e., acquisition criteria above), the rat remained in the home cage on that day. For all other experiments, testing occurred in a repeated measures (RM) design with all rats receiving all treatments in a randomized order.
Experiment 1. Characterization of the N+Alcohol compound cue under FP and FN conditions
Once acquisition criteria were met, rats in both groups (FP n=11, FN n=9) were tested on a cumulative N+A curve (0.1N+0.1A, 0.2N+0.3A, 0.4N+1A, 0.8N+1.7A mg/kg+g/kg, IG) to determine whether goal-tracking behavior was under the control of the interoceptive effects of the N+A drug state (i.e., confirm stimulus control). Following this test, sensitivity to nicotine alone (0.1, 0.2, 0.4, 0.8 mg/kg, IG), and then alcohol alone (0.1, 0.3, 1, 1.7 g/kg, IG) was assessed using the same cumulative dosing testing procedures.
Experiment 2. Effects of partial activation of α4β2 nAChR in the absence of the compound cue
In order to determine whether VAR has interoceptive effects similar to the N+A compound cue, rats in both groups (same rats from Experiment 1; FP n=11, FN n=9) received VAR alone (0, 1, 3 mg/kg, IP), 20 min prior to chamber placement (no IG treatment) for a 2-min test.
Experiment 3. Effects of partial activation of α4β2 nicotinic acetylcholine receptor (nAChR) on sensitivity to the N+A compound cue and its components
To determine whether VAR alters sensitivity to the N+A compound cue, another group N+A- trained rats (FP n=8, FN n=7) received VAR (0, 1 mg/kg, IP) 20 minutes prior to a cumulative N+A test (0.1N+0.1A, 0.2N+0.3A, 0.4N+1A, 0.8N+1.7A mg/kg+g/kg, IG), nicotine alone (0.1, 0.2, 0.4, 0.8 mg/kg, IG), and alcohol alone (0.1, 0.3, 1, 1.7 g/kg, IG).
Blood Alcohol Concentration (BAC) Analysis
To assess whether adding nicotine to the alcohol solution affected BAC, on a non-training day, a subset of animals from Experiment 1 (n=12; FP n=6, FN n=6) were pretreated with the N+A training dose (0.4 mg/kg nicotine + 1.0 g/kg alcohol; IG) or alcohol alone (1 g/kg, IG). Tail blood was collected 10 and 30 minutes later for BAC analysis. Plasma supernatant (5 μl) was analyzed for alcohol content using an Analox Alcohol Analyser (Model AM1, Analox Instruments USA Inc., Lunenburg, MA).
Drugs
Alcohol (95%, Pharmaco-AAPER, Shelbyville, KY) was diluted in distilled water to a concentration of 20% (v/v). For N+A treatments, nicotine tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in 20% alcohol. When nicotine was administered alone, it was dissolved in distilled water. All nicotine doses are expressed in base form and the pH of solutions was not adjusted as is common in studies using oral routes of nicotine administration (Alsharari et al. 2014; Aschhoff et al. 2000; Wilking et al. 2012). The N+A solution was administered IG, with volumes varied by weight to obtain the desired doses. Nicotine and alcohol doses were based on previous drug discrimination literature for each alone and in combination (Besheer et al. 2012; Charntikov et al. 2014; Ford et al. 2012; Gauvin and Holloway 1993; Pittenger and Bevins 2013; Pittenger et al. 2015; Polewan et al. 2013; Quertemont and Grant 2002; 2004; Quertemont et al. 2003; Troisi et al. 2013). Varenicline (Abcam Pharmaceuticals) dose and pretreatment interval were chosen based on previous studies (Ginsburg and Lamb 2013; Randall et al. 2015). VAR was dissolved in saline and injected intraperitoneally (IP) at a volume of 1 ml/kg.
Statistical Analyses
Given that the FP and FN groups have opposite training experience, these groups were analyzed separately. The number of head entries into the liquid receptacle was recorded in 15-s intervals throughout the training and testing sessions. The discrimination score was calculated by subtracting the number of head entries that occurred in the 15 sec before light onset (i.e., pre-CS) from the head entries that occurred during the 15-s light CS (Besheer et al. 2012; Murray et al. 2007; Palmatier et al. 2004; Palmatier et al. 2005). The first head entry discrimination score (i.e. prior to feedback from sucrose delivery) was used as the primary dependent variable. In order to provide a measure of general head entry activity (in addition to cue-related activity), head entry rate (head entries/min) was analyzed for all sessions. Locomotor rate (beam breaks/min) was analyzed for the entire session and served as a measure of non-specific motor activity. For acquisition training (Experiment 1), two-way repeated measures (RM) analysis of variance (ANOVA) was used to analyze discrimination scores between N+A and water sessions. For the cumulative substitution tests, to confirm that the training dose induced similar discrimination performance to that of training sessions, a paired samples t-test was used to compare the discrimination score from the training dose (0.4N+1A) at the test to the average of the 2 N+A sessions prior to testing (i.e., baseline). RM ANOVA was used to assess differences in discrimination score across each dose in the curve, with post-hoc analysis (Tukey) used to compare doses to the training dose within the curve. For locomotor rate and head entry rate, RM ANOVA was used to assess differences across each dose in the curve with post-hoc analysis (Tukey) used to compare doses to the lowest (i.e., subthreshold) dose. Full substitution for the N+A training dose was determined when the discrimination score at a given dose did not differ from discrimination score at the N+A training dose in the curve. For VAR discrete dosing (Experiment 2), RM ANOVA was used to assess effects of VAR doses on discrimination score. For Experiment 3, two-way RM ANOVA with VAR dose and cumulative dose as factors was used to assess effects of VAR pretreatment on each group separately (FP or FN). Similar to Experiment 1, paired-samples t-test was used to determine whether the training dose in the curve following saline treatment was significantly different from the preceding baseline sessions. For BAC analysis, paired samples t-test was used to determine whether N+A (0.4N+1.0A) was different from alcohol alone (1 g/kg) at either time point. The area under the curve was also determined and compared by t-test. Significance was declared at p ≤ 0.05.
Results
Experiment 1. Characterization of the N+A compound cue under FP and FN conditions
Acquisition of discrimination behavior
FP group
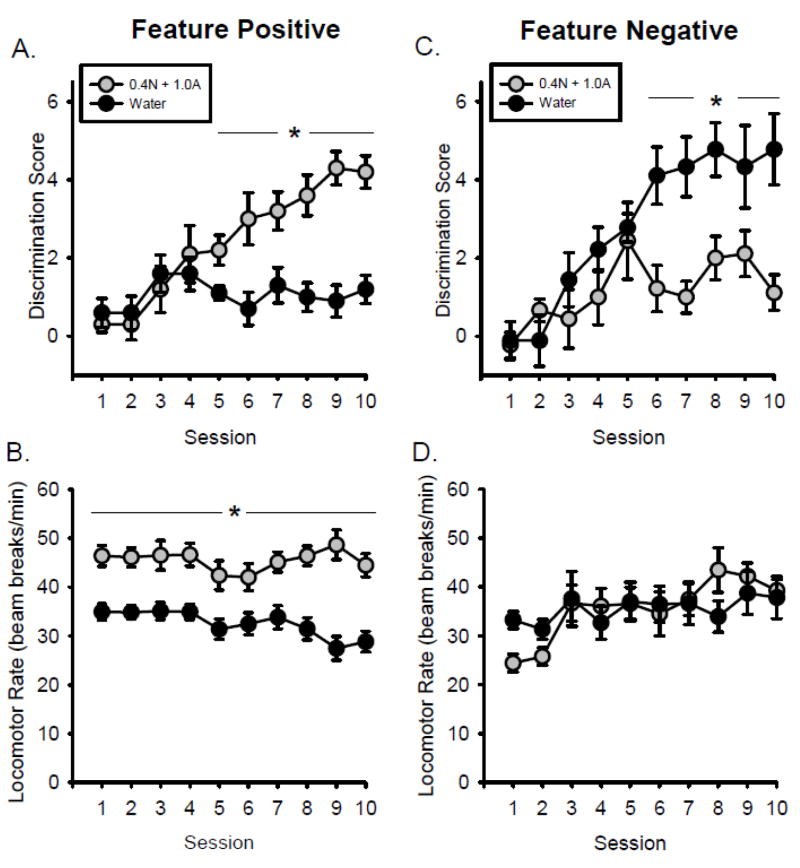
Acquisition of the discrimination in the FP group is illustrated in Figure 1A. Two-way RM ANOVA using treatment and session as factors found that discrimination scores were affected by treatment condition (F[1,10] = 30.435, p < 0.01), session (F[9,90] = 4.489, p < 0.01) and there was a treatment by session interaction in discrimination score (F[9,90] = 5.438, p < 0.01). By the fifth session (i.e., 10 training days), rats showed significantly greater discrimination scores on N+A sessions compared to water sessions (Figure 1A), indicating acquisition of the discrimination. There was a significant effect of session (F[9,90] = 2.461, p < 0.05) and a day by treatment interaction (F[9,90] = 2.556, p < 0.01) on locomotor rate, with higher locomotor rates on N+A compared to water sessions (Figure 1B). Furthermore, there was a main effect of session (F[9,90] = 14.093, p < 0.01) and a session by treatment interaction (F[9,90] = 6.964, p < 0.01), with higher head entry rates on N+A than water on sessions 2-10 (Table 1). Only the first ten sessions are shown for acquisition as all rats achieved testing criteria within that time.
Figure 1.
Acquisition of nicotine+alcohol discrimination. Mean(±S.E.M.) discrimination score (head entries during the 15-s light CS minus head entries during 15 seconds before light onset, A) and locomotor rate (beam breaks per minute, B) in the Feature Positive group. Mean(±S.E.M.) discrimination score (C) and locomotor rate (D) in the feature negative group. *- denotes significant differences between N+A and water session (p < 0.05).
Table 1.
Mean (+S.E.M.)7/18/2016 head entries/min for the first ten acquisition sessions.
| Session | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feature Positive | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|
|
||||||||||
| N+A | 10.73±1.38 | 12.23±1.02* | 15.28±1.12* | 16.61±1.37* | 13.43±0.85* | 10.23±0.5* | 11.13±0.85* | 9.49±0.71* | 8.6±0.85* | 8.69±0.7* |
| Water | 9.66±0.82 | 9.25±0.66 | 8.59±0.55 | 6.98±0.63 | 7.90±0.67 | 7.96±0.53 | 7.81±0.86 | 6.43±0.65 | 6.23±0.3 | 4.7±0.47 |
| Feature Negative | ||||||||||
| N+A | 2.87±0.39* | 1.98±0.31* | 3.92±0.55* | 3.61±0.63* | 3.46±0.64* | 3.41±0.52* | 3.46±0.44* | 3.30±0.6* | 3.10±0.42* | 3.08±0.4* |
| Water | 4.89±0.6 | 6.44±0.85 | 8.65±1.04 | 9.46±1.04 | 10.72±0.79 | 9.47±0.62 | 7.46±0.33 | 7.68±0.57 | 9.10±0.75 | 8.54±0.37 |
denotes significant difference (p < 0.05) from water. All rats met criteria for testing within ten sessions
FN Group
Acquisition of the discrimination in the FN group is illustrated in Figure 1C. Two- way RM ANOVA using treatment and session as factors showed a significant effect of treatment (F[1,8] = 12.535, p < 0.01), session (F[9,72] = 8.784, p < 0.01) and a treatment by session interaction (F[9,72] = 3.904, p < 0.01). By the sixth session, rats showed significantly greater discrimination scores on water compared to N+A sessions (Figure 1C), indicating acquisition of the discrimination. As shown in Figure 1D, locomotor rate was not different between N+A and water sessions. In addition, ANOVA showed a significant effect of session (F[9,72] = 7.979, p < 0.01) and a session by treatment interaction (F[9,72] = 4.031, p < 0.01), with greater head entry rate on water than N+A sessions on all sessions (Table 1). Only the first ten sessions are shown as all rats achieved testing criteria within that time.
Confirmation of Stimulus Control and expression of sensitivity to components
FP Group
N+A compound substitution
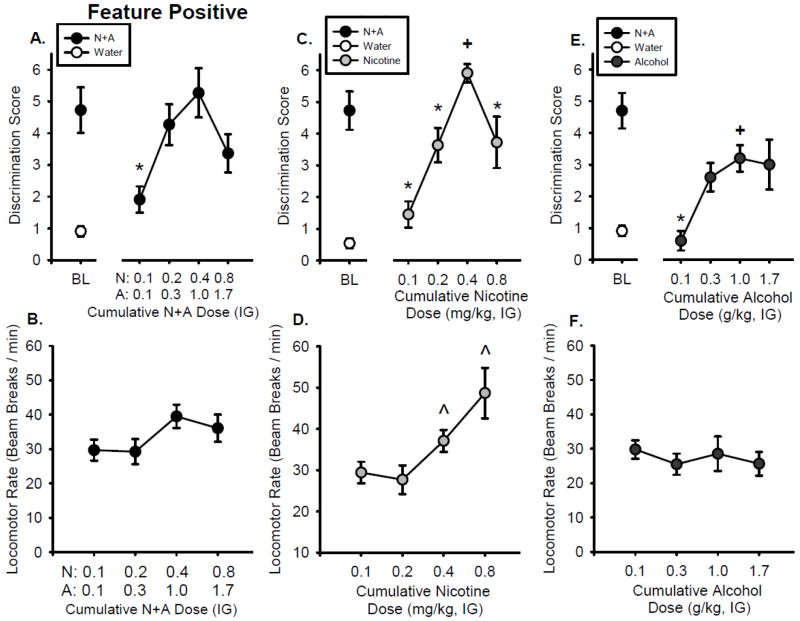
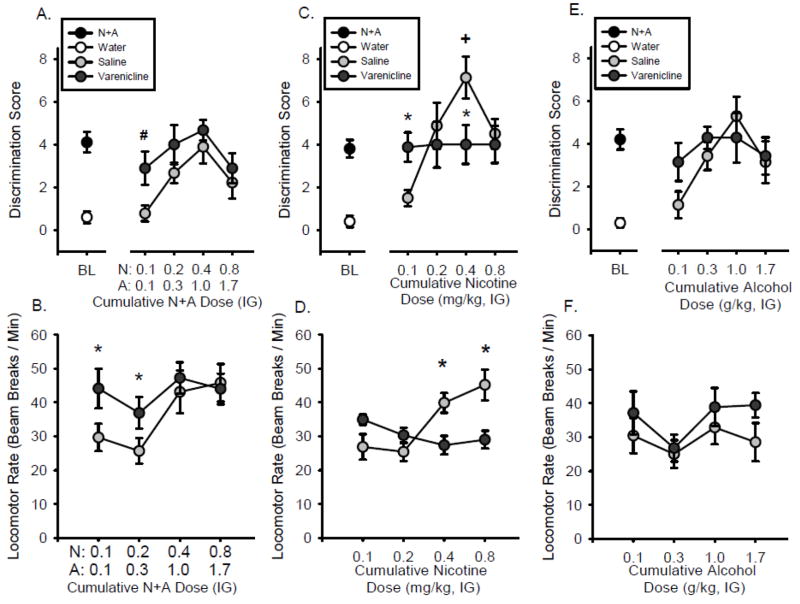
As shown in Figure 2A, during the substitution test, the discrimination score of the training dose (0.4N+1.0A) did not differ from baseline (i.e., BL, left of axis break), indicating appropriate performance and sensitivity to the training dose under the cumulative dosing procedures. The discrimination score was significantly affected by N+A dose combination (F[3,30] = 6.512, p < 0.01), with all doses substituting for the training dose except the sub-threshold dose combination (0.1N+0.1A; p<0.05), confirming N+A stimulus control. Locomotor rate was not significantly affected by N+A dose (Fig. 2B). ANOVA showed that head entry rate significantly increased as N+A dose increased (F[3,30] = 3.46, p < 0.05), with higher head entry rate following the training dose than the lowest dose (Table 2).
Figure 2.
Substitution curves for the Feature Positive group. Mean(±S.E.M.) discrimination score (head entries during the 15-s light CS minus head entries during 15 seconds before light onset, A) and locomotor rate (beam breaks per minute, B) for N+A substitution test. Mean(±S.E.M.) discrimination score (C) and locomotor rate (D) for nicotine only substitution test. Mean(±S.E.M.) discrimination score (E) and locomotor rate (F) for the alcohol only substitution test. *-denotes significant difference from training dose in the curve (p < 0.05). +-denotes significant difference from N+A baseline (p < 0.05). ˆ- denotes significant difference from lowest dose for locomotor rate (p < 0.05)
Table 2.
Mean (±S.E.M.) head entries/min for each experiment.
| Experiment 1 | ||||||||
| N+A Substitution | 0.1N+0.1A | 0.2N+0.3A | 0.4N+1.0A | 0.8N+1.7A | ||||
|
|
||||||||
| FP | 3.13±0.73 | 5.13±0.72 | 6.00±0.90* | 4.63±0.68 | ||||
| FN | 4.70±0.70 | 4.90±0.76 | 4.25±0.90 | 4.75±1.00 | ||||
| Nicotine Substitution | 0.1N | 0.2N | 0.4N | 0.8N | ||||
|
|
||||||||
| FP | 4.10±0.66 | 5.22±0.64 | 7.27±0.72* | 3.95±0.52 | ||||
| FN | 4.40±0.45 | 4.10±1.26 | 4.05±0.57 | 3.60±0.66 | ||||
| Alcohol Substitution | 0.1A | 0.3A | 1.0A | 1.7A | ||||
|
|
||||||||
| FP | 2.95±0.47 | 3.10±0.54 | 3.45±0.53 | 2.90±0.43 | ||||
| FN | 4.05±0.36 | 2.90±0.62 | 3.65±0.55 | 3.60±0.55 | ||||
| Experiment 2 | ||||||||
| Var Alone | Saline | 1.0 Var | 3.0 Var | |||||
|
|
||||||||
| FP | 3.12±0.48 | 5.12±0.78 | 3.87±0.76 | |||||
| FN | 3.62±0.33 | 3.68±0.70 | 3.87±0.86 | |||||
| Experiment 3 | ||||||||
| Var + N+A Substitution | Saline | Var (1.0) | ||||||
| 0.1N+0.1A | 0.2N+0.3A | 0.4N+1.0A | 0.8N+1.7A | 0.1N+0.1A | 0.2N+0.3A | 0.4N+1.0A | 0.8N+1.7A | |
|
|
|
|||||||
| FP | 2.33±0.72 | 4.05±0.85 | 5.22±0.82 | 2.44±0.46 | 3.66±0.70 | 4.50±0.70 | 4.72±0.45 | 2.77±0.52 |
| FN | 3.41±0.56 | 4.25±0.80 | 4.66±1.05 | 2.58±0.66 | 3.50±0.46 | 3.41±1.10 | 3.66±0.84 | 3.66±0.73 |
| Var + Nicotine Substitution | Saline | Var (1.0) | ||||||
| 0.1N | 0.2N | 0.4N | 0.8N | 0.1N | 0.2N | 0.4N | 0.8N | |
|
|
|
|||||||
| FP | 2.81±0.64 | 7.31±2.39 | 8.12±1.32 | 4.56±0.40 | 4.68±0.90 | 5.68±1.15 | 4.81±0.58 | 3.75±0.63 |
| FN | 4.35±0.68 | 4.78±0.82 | 6.21±0.91 | 3.64±0.45 | 3.57±0.50 | 4.57±0.76 | 4.57±0.38 | 5.14±0.97 |
| Var + Alcohol Substitution | Saline | Var (1.0) | ||||||
| 0.1A | 0.3A | 1.0A | 1.7A | 0.1A | 0.3A | 1.0A | 1.7A | |
|
|
|
|||||||
| FP | 2.21±0.30 | 3.57±0.42 | 4.14±0.53 | 2.78±0.65 | 5.07±1.50 | 4.57±1.02 | 4.42±0.55 | 3.42±0.36 |
| FN | 4.43±0.50 | 4.87±0.47 | 4.68±0.66 | 3.56±0.43 | 4.93±0.73 | 6.31±1.07 | 5.12±0.88 | 5.12±0.57 |
denotes significant difference (p < 0.05) from lowest dose or saline.
Nicotine Substitution
As shown in Figure 2C, the discrimination score following the nicotine component of the training dose (0.4 mg/kg nicotine) was significantly higher than the N+A baseline (p<0.05), suggesting that the nicotine component may act as a more salient cue than the N+A training dose. Analysis of the discrimination score showed an inverted u-shaped function (F[3,30] = 11.630, p < 0.01), with each nicotine dose significantly lower than the training dose component (p<0.05). Nicotine significantly increased locomotor rate (F[3,30] = 5.915, p < 0.05), with 0.4 and 0.8 mg/kg nicotine significantly higher than the lowest dose (0.1 mg/kg). Similarly, head entry rate significantly increased as the nicotine dose increased (F[3,30] = 6.14, p < 0.01), with higher head entry rates at 0.4 mg/kg nicotine than the lowest dose (Table 2).
FP Alcohol Substitution
Figure 2E shows that the discrimination score for the alcohol component of the training dose (1.0 g/kg alcohol) was significantly lower than the N+A baseline, suggesting that alcohol alone does not substitute for the N+A training dose. However, there was a significant increase in discrimination score with increasing alcohol doses (F[3,27] = 6.043, p < 0.05), with a significantly lower discrimination score at the 0.1 mg/kg dose relative to the alcohol component (1 g/kg) of the N+A training dose. There were no significant effects of alcohol dose on locomotor rate (Figure 2F) or head entry rate (Table 2).
FN Group
N+A compound substitution
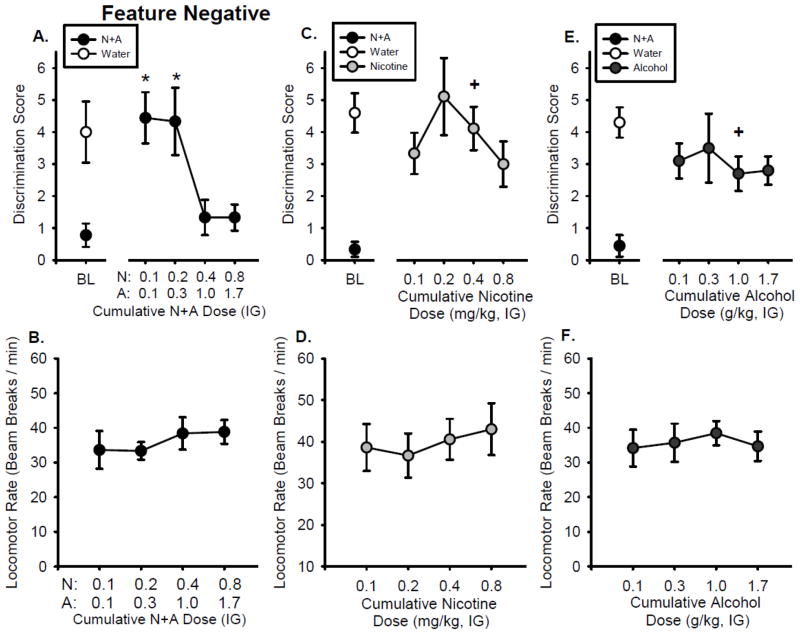
As shown in Figure 3A, the discrimination score following the training dose (0.4N+1A) did not differ from baseline (left of axis break). The discrimination score decreased as the N+A dose combination increased (F[3,24] = 6.02, p < 0.05), confirming stimulus control. The 0.1N+0.1A and 0.2N+0.3A doses did not substitute for the N+A training dose, showing significantly higher discrimination scores than the training dose. Locomotor rate and head entry rate were not affected by N+A dose (Figure 3B, Table 2).
Figure 3.
Substitution curves for the Feature Negative group. Mean(±S.E.M.) discrimination score (head entries during the 15-s light CS minus head entries during 15 seconds before light onset, A) and locomotor rate (beam breaks per minute, B) for N+A substitution test. Mean(±S.E.M.) discrimination score (C) and locomotor rate (D) on the nicotine only substitution test. Mean(±S.E.M.) discrimination score (E) and locomotor rate (F) on the alcohol only substitution test. *-denotes significant difference from training dose in the curve. +-denotes significant difference from N+A baseline.
Nicotine Substitution
As shown in Figure 3C, the discrimination score following the nicotine component (0.4 mg/kg) of the compound training dose was significantly higher than the N+A baseline (p < 0.05) suggesting that the nicotine component alone does not substitute for the N+A training dose under these conditions. As shown in Figure 3C, there were no significant effects of nicotine dose on discrimination score, locomotor rate or head entry rate (Figure 3D, Table 2).
Alcohol Substitution
Figure 3E shows that the discrimination score following the alcohol component (1.0 g/kg) of the compound training dose was significantly higher than the N+A baseline (p < 0.05), suggesting that the alcohol component alone does not substitute for the N+A training dose. There was no significant effect of alcohol dose on discrimination score (Figure 3E), locomotor rate or head entry rate (Figure 3F, Table 2).
Experiment 2. Effects of partial activation of α4β2 nAChR in the absence of the compound cue
FP Group
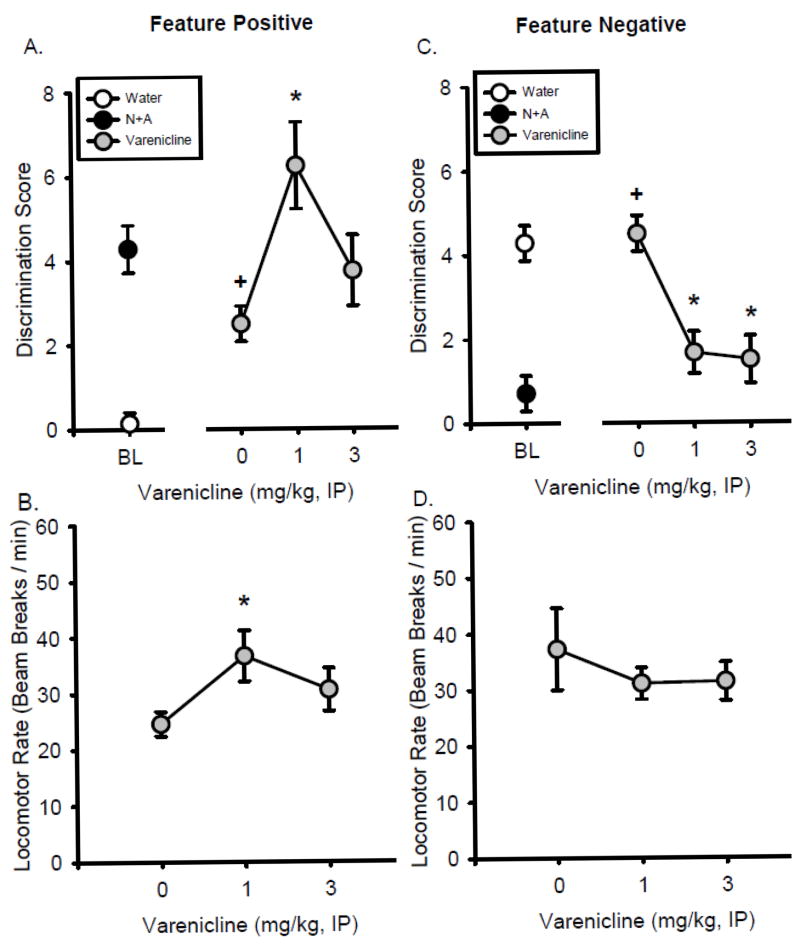
As shown in Figure 4A, VAR administered alone (in the absence of the N+A compound cue) increased discrimination score (F[2,14] = 3.74, p < 0.05), with a higher discrimination score at 1.0 mg/kg VAR relative to saline (p<0.05). Furthermore, the discrimination score following 1 mg/kg VAR was not different from the N+A baseline (left of axis break) indicating that VAR had interoceptive effects similar to the N+A training dose (i.e., full substitution). In addition, as shown in Figure 4B, locomotor rate was significantly increased by VAR alone (F[2,14] = 3.99, p < 0.05) at the 1.0 mg/kg dose. There were no significant effects of VAR alone on head entry rate (Table 2).
Figure 4.
Effects of varenicline alone on rats trained to discriminate N+A. Mean (±S.E.M.) discrimination score (head entries during the 15-s light CS minus head entries during 15 seconds before light onset, A) and locomotor rate (beam breaks per minute, B) following varenicline injection in the feature positive group. Mean(±S.E.M.) discrimination score (C) and locomotor rate (D) following varenicline injection in the feature negative group. *-denotes significantly different from saline (p < 0.05). +-denotes significantly different from N+A baseline (p < 0.05).
FN Group
VAR administered alone decreased discrimination score (F[2,10] = 10.882, p < 0.01), with significant decreases at both doses relative to saline (p<0.05; Figure 4C). Moreover, the discrimination score following both doses was not different from N+A baseline (i.e., full substitution), again indicating that VAR had interoceptive effects similar to the N+A compound. VAR did not affect locomotor rate (Figure 4D) or head entry rate (Table 2).
Experiment 3. Effects of partial activation of α4β2 nicotinic acetylcholine receptor (nAChR) on sensitivity to the N+A compound cue and its components
FP Group
Effects of VAR on N+A substitution curve
Following saline pretreatment, the discrimination score at the N+A training dose was not different from the N+A baseline (Figure 5A), similar to the findings from Experiment 1 (Figure 2A). As shown in Figure 5A, RM ANOVA found a significant main effect of N+A dose on discrimination score (F[3,24] = 3.880, p < 0.05) and VAR dose (F[1,24] = 6.497, p < 0.05), however there was no VAR by N+A dose interaction. Given that Experiment 2 found that this dose of VAR (1 mg/kg) alone produced interoceptive effects similar to the N+A compound cue, planned comparisons were conducted to examine whether VAR potentiated sensitivity to N+A. Indeed, at the lowest N+A dose combination (0.1N+0.1A) the discrimination score was significantly higher following VAR relative to saline pretreatment (p < 0.05). In addition, as shown in Figure 5B, there was a significant main effect of VAR dose (F[1,24] = 5.196, p < 0.05), N+A dose (F[3,24] = 4.278, p < 0.05), and a significant VAR by N+A dose interaction on locomotor rate (F[3,24]= 3.270, p < 0.05), with increased locomotor rate following VAR treatment relative to saline at the 0.1N+0.1A and 0.2N+0.3A dose combinations (p < 0.05). Head entry rate was not affected by VAR (Table 2).
Figure 5.
Effects of varenicline in substitution curves for the Feature Positive group. Mean(+S.E.M.) discrimination score (head entries during the 15-s light CS minus head entries during 15 seconds before light onset, A) and locomotor rate (beam breaks per minute, B) for the N+A substitution test following varenicline (1 mg/kg) or saline pretreatment. Mean(±S.E.M.) discrimination score (C) and locomotor rate (D) for the nicotine only substitution test following varenicline or saline pretreatment. Mean(±S.E.M.) discrimination score (E) and locomotor rate (F) of the alcohol only substitution test following varenicline or saline pretreatment. *-denotes significant difference between varenicline and saline pretreatments (p < 0.05). +-denotes significant difference between training dose in the curve and N+A baseline (p < 0.05). #-denotes significant difference with planned comparison between varenicline and saline pretreatments (p < 0.05).
Effects of VAR on nicotine substitution curve
Following saline, the discrimination score at the nicotine component (0.4 mg/kg) of the N+A training dose was significantly higher than the N+A baseline (p < 0.05; Figure 5C), consistent with the data pattern observed in Figure 2C. As shown in Figure 5C, two-way RM ANOVA found a significant main effect of nicotine dose (F[3,21] = 5.423, p < 0.05) and a VAR by nicotine dose interaction (F[3,21] = 4.051, p < 0.05) on discrimination score, with significantly higher discrimination scores than saline at the lowest nicotine dose (0.1 mg/kg), but significantly lower at the training dose component (0.4 mg/kg) compared to saline (p < 0.05). These findings indicate that VAR enhanced the effects of the lower nicotine doses and blocked the effects of the higher nicotine doses. There was a significant main effect of VAR dose (F[1,21] = 7.842, p < 0.05), nicotine dose (F[3,21] = 4.431, p < 0.05) and interaction (F[3,21] = 9.994, p < 0.05) on locomotor rate, with increased locomotor rate at the lowest nicotine dose and decreased locomotor rate at the training and highest nicotine dose relative to vehicle (p < 0.05; Figure 5D). VAR did not alter head entry rate (Table 2).
Effects of VAR on alcohol substitution curve
Following saline, the discrimination score following 1 g/kg alcohol was not significantly different from N+A baseline (Figure 5E). VAR had no effects on alcohol discrimination score (Figure 5E). Therefore, given that under saline conditions alcohol alone had effects similar to the N+A training dose, VAR did not alter sensitivity to the alcohol component alone. Locomotor rate (Figure 5F) and head entry rate (Table 2) were also not affected by VAR.
FN Group
Effects of VAR on substitution curves
Under saline conditions, discrimination score following the N+A training dose was not different from N+A baseline, again confirming stimulus control (Table 4). However, nicotine and alcohol alone were significantly higher than the N+A baselines (p < 0.05, Table 4). There were no significant effects of VAR on discrimination scores, locomotor rate or head rate at any dose combination of N+A, nicotine alone or alcohol alone, suggesting that VAR does not affect processes related to the FN discrimination (Table 2, 4).
Table 4.
Effects of varenicline on sensitivity to N+A, nicotine alone and alcohol alone in the Feature Negative group. Mean(±S.E.M.) discrimination score (head entries during 15 second light stimulus minus head entries before) and locomotor rate (beam breaks / min) following pretreatment with varenicline.
| Varenicline Dose (mg/kg IP) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | |||||||
|
|
||||||||
| N+A (mg/kg + g/kg) | 0.1N+0.1A | 0.2N+0.3A | 0.4N+1A | 0.8N+1.7A | 0.1N+0.1A | 0.2N+0.3A | 0.4N+1A | 0.8N+1.7A |
|
|
||||||||
| Discrimination Score | 3.0±0.45 | 2.84±0.91 | 1.33±0.62 | 0.5±0.76 | 4.0±0.45 | 3.5±1.06 | 3.0±0.73 | 2.33±0.72 |
| Locomotor Rate | 41.42±7.42 | 34.58±7.67 | 48.0±7.10 | 50.0±3.92 | 52.92±7.0 | 26.92±4.83 | 41.08±6.67 | 45.0±5.34 |
|
|
||||||||
| Nicotine (mg/kg) | 0.1N | 0.2N | 0.4N | 0.8N | 0.1N | 0.2N | 0.4N | 0.8N |
|
|
||||||||
| Discrimination Score | 3.0±0.62 | 3.57±1.25 | 3.14±0.80 | 2.86±1.0 | 2.43±0.78 | 3.57±0.84 | 4.14±0.77 | 3.57±0.65 |
| Locomotor Rate | 39.5±7.34 | 25.36±2.34 | 23.36±5.26 | 40.64±9.61 | 33.43±3.12 | 23.57±2.64 | 27.93±3.95 | 23.21±4.0 |
|
|
||||||||
| Alcohol (g/kg) | 0.1A | 0.3A | 1A | 1.7A | 0.1A | 0.3A | 1A | 1.7A |
|
|
||||||||
| Discrimination Score | 4.75±0.62 | 4.88±1.0 | 3.25±0.94 | 1.5±0.6 | 3.38±0.78 | 4.75±1.24 | 3.63±0.87 | 3.13±1.04 |
| Locomotor Rate | 36.94±4.4 | 30.13±4.52 | 43.06±4.56 | 39.81±4.33 | 40.36±5.6 | 29.56±5.38 | 37.38±6.08 | 37.81±1.95 |
Blood Alcohol Concentration (BAC) Analysis
There was no significant difference in BAC or area under the curve between rats treated with N+A or alcohol alone at either time point (Table 3), suggesting that under these conditions and time points that are relevant for the present work, addition of nicotine to the alcohol solution does not interfere with alcohol blood levels.
Table 3.
Mean (±S.E.M.) mg/dl blood alcohol concentration (BAC) and area under the curve (AUC) following N+A (0.4 mg/kg N + 1 g/kg A) or alcohol alone (1 g/kg), IG.
| BAC (mg/dl) | 10 min | 30 min |
|
|
||
| 0.4N+1A | 28.28±2.59 | 41.21±2.90 |
| 1A | 27.48±4.33 | 47.03±6.26 |
|
| ||
| AUC | ||
| 0.4N+1A | 695.00±80.14 | |
| 1A | 745.16+74.10 | |
Discussion
Nicotine and alcohol co-abuse is a complex and costly public health issue. Understanding this problem from a behavioral perspective is crucial to developing more effective treatments. To this end, the current experiments have numerous important findings. First, oral administration of N+A effectively served as an interoceptive compound cue as evidenced by modulation of discriminated goal-tracking behavior. Second, this N+A drug state represents a unique interoceptive cue from each of the components (i.e., nicotine or alcohol alone). Third, the nicotinic partial agonist VAR produces interoceptive effects similar to the N+A compound cue as demonstrated by substitution for the N+A compound cue when administered alone.
A unique feature of the current experiments is that the nicotine was delivered orally as a cocktail with the alcohol. To our knowledge, this is the first drug discrimination study to train a N+A compound cue by an oral route of administration. The selection of this route of administration was related to feasibility given that in our previous work, alcohol is administered IG (Besheer et al. 2012; Jaramillo et al. 2015; Randall et al. 2015) and previous drug discrimination work has shown that nicotine administered IG shows stimulus equivalence to nicotine administered IP or SC (Craft and Howard 1988). While there is evidence that oral coadministration of nicotine (≥0.5 mg/kg) and alcohol (4 g/kg) significantly decreases BAC in adult rats (Parnell et al. 2006), here, we show that under the time parameters and doses relevant to the present work, IG administration of both does not affect BAC (Table 3). Therefore, differences in the relative contribution of each component to the N+A drug state is likely not related to nicotine interfering with alcohol levels, however, it will be important to determine if under our training conditions nicotine levels are affected by alcohol.
The initial series of experiments demonstrated that a N+A compound interoceptive cue could be trained to modulate behavior using a Pavlovian drug discrimination procedure. That is, the N+A drug cue effectively engendered goal-tracking behavior in rats with FP training (Fig 1A) and withholding of goal-tracking behavior in rats with FN training (Fig 1C). Interestingly, the FP discrimination was acquired slightly faster and less variable than the FN discrimination, consistent with previous suggestions that a FP discrimination is easier to learn than a FN discrimination (Hearst 1987). Furthermore, locomotor rate was generally dependent on training condition. That is, in the FP group, locomotor rate was greater on N+A relative to water sessions, whereas in the FN group locomotor rate did not differ between N+A and water sessions. A possible explanation is that the combination of the excitatory properties of the cue light CS in the FP group, coupled with the psychomotor stimulating properties of nicotine results in an overall locomotor enhancement that is not observed in the FN group due to the inhibitory relationship of the cue light CS. Indeed, previous studies suggest that the inhibitory relationship between the drug state and CS in a FN association competes with the psychomotor stimulant properties of test compounds such as methamphetamine or cocaine, causing an overall decrease in activity (Pinkston and Branch 2003; Reichel et al. 2007). In addition, it is unlikely that sensitization to the locomotor effects of nicotine is a factor as locomotor rate in the FN group was not affected.
Characterization of each component of the N+A compound cue showed that in the FP group, discrimination scores were potentiated following the nicotine component of the training dose (0.4 mg/kg) relative to the N+A training dose baseline, whereas discrimination scores following the alcohol component of the training dose (1 g/kg) failed to reach the N+A baseline level. This suggests that the nicotine component may play a greater role in the interoceptive effects of the N+A compound cue (i.e., nicotine effects may “overshadow” the alcohol effects). This suggestion is consistent with work showing that nicotine evokes greater control over behavior than alcohol in rats trained to discriminate a N+A compound cue (Troisi et al. 2013), but in contrast to work showing that alcohol “overshadows” the nicotine component in mice trained to discrimination a N+A compound cue (Ford et al. 2012). Moreover, the lack of alcohol substitution for the N+A training dose is consistent with other work using a similar N+A training dose to the present study (administered IP), but in that study nicotine failed to fully substitute for the N+A training dose which is in contrast to our results (Gauvin and Holloway 1993). This array of findings highlights the complex nature of the N+A interoceptive cue and differences across studies could be attributed to variables such as strain or species - which may have differential sensitivity to nicotine and/or alcohol, behavioral training procedures, route of drug administration - which may be related to differences in ascending/descending limbs of drug levels, and importantly, the N+A training dose combinations used.
An important feature of the current experiments is the use of both FP and FN training conditions. Given that these drug-conditioned associations likely rely on different behavioral/neural mechanisms, these two training procedures allow for a multidimensional assessment of the ability of the drug state to excite or inhibit behavior. For example, in contrast to the data pattern in the FP group, in the FN group, nicotine did not substitute for the N+A training dose. Further, in the FN group, alcohol also did not substitute which is consistent with the findings of the FP group (Fig. 2 and 3). This would suggest that the cue light CS is generally excitatory in both the FP and FN groups. The exception is when the CS is paired with the specific training condition (N+A) in the FN group, upon which inhibitory processes outweigh the excitatory influence of the CS. Together, these findings suggest that the N+A compound represents a unique interoceptive cue from either component on its own. This assertion is supported by another study in which extinction of the components of a N+A interoceptive cue did not extinguish discrimination following re-exposure to the N+A cue (Troisi et al. 2013).
Considering the growing interest in using VAR for the treatment of AUDs, it is important to assess whether sensitivity to the N+A compound cue or its components is altered by VAR pretreatment. In both the FP and FN groups, VAR fully substituted for the N+A compound cue (i.e., VAR has stimulus effects similar to the N+A compound cue; Figure 4). These results are consistent with previous nicotine studies that show VAR substitutes for nicotine and generalizes to nicotine if extinguished using a Pavlovian goal-tracking procedure (Reichel et al. 2010), and partially substitutes for nicotine in operant drug discrimination studies (LeSage et al. 2009; Paterson et al. 2010). Interestingly, previous operant drug discrimination work from our lab has shown that VAR does not substitute for alcohol (Randall et al. 2015). This finding lends further support to the idea that the nicotine component may play a greater role in the N+A cue. Lastly, although there was a significant VAR-induced increase in locomotor rate in the FP group, there were no significant effects on head entry rate in either group, demonstrating that behavior specifically related to the light cue was enhanced.
Consistent with VAR having N+A-like effects, VAR enhanced sensitivity to the lowest N+A dose (0.1N+0.1A) in the FP group (Figure 5A). Interestingly, when VAR was administered with nicotine alone, discrimination scores were similar across all nicotine doses (i.e., potentiation at the lowest dose (0.1N) and suppressed at the training dose (0.4N) relative to saline pretreatment; Figure 5C). Given the partial agonist properties of VAR, this outcome is not entirely unexpected. VAR induces a reduced but sustained level of intrinsic activity at nAChRs compared to nicotine while also preventing nicotine from binding and producing greater intrinsic effects (Coe et al. 2005). In contrast to the FP group, in the FN group, VAR pretreatment did not affect sensitivity to the N+A compound or the nicotine or alcohol components (Table 4). This finding suggests that VAR may not affect behavioral mechanisms related to the FN association. That is, VAR may only exert effects on excitatory processes related to the FP association, as opposed to the inhibitory processes active during that FN association.
Taken together, these VAR findings point to a possible convergent pathway by which nAChRs (specifically α4β2) modulate sensitivity to a N+A drug state. In addition to nicotine- induced activity at nAChRs, alcohol can also affect nAChRs activity. For example, alcohol has been shown to enhance the binding affinity of acetylcholine for the nAChR (Ei-Fakahany et al. 1983; Forman et al. 1989). Moreover, alcohol can directly modulate nAChR ligand-gated cation channel activity (K+/Na+), increasing duration of the open state (Wu et al. 1994). Additionally, α4β2 receptors are highly expressed in nucleus accumbens core and medial prefrontal cortex (Klink et al. 2001; Zoli et al. 2002), brain regions important for goal-directed, reinforcement- seeking behavior (see Everitt et al., 1999 for review). These receptors also play an important role in modulating the reinforcing properties of nicotine (Crawley et al. 1997; Tapper et al. 2004) and potentially alcohol, (see Chatterjee and Bartlett, 2010 for review). These effects are thought to be due, in part, to nAChR-mediated effects on mesolimbic dopamine release. Indeed, both alcohol and nicotine alone increase forebrain dopamine (DA) release (Di Chiara and Imperato 1985; Ericson et al. 2009; Tizabi et al. 2002) and N+A increases DA release to a greater degree than each alone (Doyon et al. 2013; Tizabi et al. 2007). It will be interesting for future experiments to investigate brain regional involvement of nAChRs, the potential role of DA, and the neural circuitry underlying sensitivity to a N+A drug state.
An important aspect of understanding drug use and relapse are the numerous cues, both external and internal (i.e., interoceptive), with which drug use and drug effects become associated. As such, these conditioned cues can come to gain motivational value such that they can play an important role in promoting drug taking and seeking behaviors. Therefore, examining and characterizing interoceptive drug cues are critical to broadening our understanding of addiction-related processes. In summary, the nicotine+alcohol compound cue represents a salient stimulus, that once associated with reward (i.e., sucrose) can come to entrain discriminated goal-tracking behavior. This work represents the first study to characterize the N+A compound cue utilizing a Pavlovian drug discrimination procedure. In addition, partial activation of nAChRs using VAR produces behavior similar to that of the N+A compound cue, suggesting that VAR produces interoceptive effects similar to that of the N+A cue. Taken together, it will be important for future work to consider how interoceptive effects of a N+A compound cue (or nicotine and alcohol alone) may influence ongoing co-use as measured by self-administration (or self-administration of each alone), relapse-like behavior or the effects of extinguishing the N+A cue or its components on these behaviors.
Acknowledgments
This work was supported, in part, by funds from the National Institutes of Health AA019682 (JB), AA07573, and by the Bowles Center for Alcohol Studies.
References
- Alsharari SD, Siu EC, Tyndale RF, Damaj MI. Pharmacokinetic and pharmacodynamics studies of nicotine after oral administration in mice: effects of methoxsalen, a CYP2A5/6 inhibitor. Nicotine Tob Res. 2014;16:18–25. doi: 10.1093/ntr/ntt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschhoff S, Schroff KC, Wildenauer DB, Righter E. Nicotine consumption of several mouse strains using a two bottle choice paradigm. J Exp Anim Sci. 2000;40:171–177. [Google Scholar]
- Besheer J, Fisher KR, Durant B. Assessment of the interoceptive effects of alcohol in rats using short-term training procedures. Alcohol. 2012;46:747–55. doi: 10.1016/j.alcohol.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton MEN JB. Mechanisms of Feature-Positive and Feature-Negative Discrimination Learning in an Appetitive Conditioning Paradigm. In: Schmajuk NAH PC, editor. Occasion Setting: Associative Learning and Cognition in Animals. American Psychological Association; Washington DC: 1998. pp. 69–113. [Google Scholar]
- Charntikov S, deWit NR, Bevins RA. Interoceptive conditioning with nicotine using extinction and re-extinction to assess stimulus similarity with bupropion. Neuropharmacology. 2014;86:181–91. doi: 10.1016/j.neuropharm.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors as pharmacotherapeutic targets for the treatment of alcohol use disorders. CNS Neurol Disord Drug Targets. 2010;9:60–76. doi: 10.2174/187152710790966597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Colombo SF, Mazzo F, Pistillo F, Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol. 2013;86:1063–73. doi: 10.1016/j.bcp.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Craft RM, Howard JL. Cue properties of oral and transdermal nicotine in the rat. Psychopharmacology (Berl) 1988;96:281–4. doi: 10.1007/BF00216050. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–2. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36:96–108. doi: 10.1016/j.tips.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol. 2013;86:1181–93. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ei-Fakahany EF, Miller ER, Abbassy MA, Eldefrawi AT, Eldefrawi ME. Alcohol modulation of drug binding to the channel sites of the nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 1983;224:289–96. [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329:225–30. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- Ford MM, Davis NL, McCracken AD, Grant KA. Contribution of NMDA glutamate and nicotinic acetylcholine receptor mechanisms in the discrimination of ethanol-nicotine mixtures. Behav Pharmacol. 2013;24:617–22. doi: 10.1097/FBP.0b013e3283654216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, McCracken AD, Davis NL, Ryabinin AE, Grant KA. Discrimination of ethanol-nicotine drug mixtures in mice: dual interactive mechanisms of overshadowing and potentiation. Psychopharmacology (Berl) 2012;224:537–48. doi: 10.1007/s00213-012-2781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SA, Righi DL, Miller KW. Ethanol increases agonist affinity for nicotinic receptors from Torpedo. Biochim Biophys Acta. 1989;987:95–103. doi: 10.1016/0005-2736(89)90459-8. [DOI] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–63. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Holloway FA. The discriminative stimulus properties of an ethanol-nicotine mixture in rats. J Psychopharmacol. 1993;7:52–62. doi: 10.1177/026988119300700109. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Effects of varenicline on ethanol- and food-maintained responding in a concurrent access procedure. Alcohol Clin Exp Res. 2013;37:1228–33. doi: 10.1111/acer.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E. Extinction reveals stimulus control: latent learning of feature-negative discriminations in pigeons. J Exp Psychol Anim Behav Process. 1987;13:52–64. [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. Jama. 1996;275:1097–103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Frisbee S, Fisher KR, Besheer J. Activation of mGluR2/3 following stress hormone exposure restores sensitivity to alcohol in rats. Alcohol. 2015;49:525–32. doi: 10.1016/j.alcohol.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–63. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, Corrigall WA. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacol Biochem Behav. 2009;91:461–7. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Millar NS, Harkness PC. Assembly and trafficking of nicotinic acetylcholine receptors (Review) Mol Membr Biol. 2008;25:279–92. doi: 10.1080/09687680802035675. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Li C, Palmatier MI, Bevins RA. The interoceptive Pavlovian stimulus effects of caffeine. Pharmacol Biochem Behav. 2007;86:838–46. doi: 10.1016/j.pbb.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–94. [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a pavlovian appetitive discrimination task in rats. Neuropsychopharmacology. 2005;30:731–41. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- Parnell SE, West JR, Chen WJ. Nicotine decreases blood alcohol concentrations in adult rats: a phenomenon potentially related to gastric function. Alcohol Clin Exp Res. 2006;30:1408–13. doi: 10.1111/j.1530-0277.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Min W, Hackett A, Lowe D, Hanania T, Caldarone B, Ghavami A. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1455–64. doi: 10.1016/j.pnpbp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Pinkston JW, Branch MN. Sensitization to cocaine in pigeons: interaction with an operant contingency. Exp Clin Psychopharmacol. 2003;11:102–9. doi: 10.1037//1064-1297.11.1.102. [DOI] [PubMed] [Google Scholar]
- Pittenger ST, Bevins RA. Interoceptive conditioning with a nicotine stimulus is susceptible to reinforcer devaluation. Behav Neurosci. 2013;127:465–73. doi: 10.1037/a0032691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Zeplin LC, Dwoskin LP, Bevins RA. The effect of switching pharmacological intervention during extinction on nicotine-evoked conditioned responding in rats. Psychopharmacology (Berl) 2015;232:4347–58. doi: 10.1007/s00213-015-4067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polewan RJ, Savala SA, Bevins RA. Interoceptive conditioning with the nicotine stimulus: extinction learning as a method for assessing stimulus similarity across doses. Behav Pharmacol. 2013;24:45–54. doi: 10.1097/FBP.0b013e32835d5278. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Grant KA. Role of acetaldehyde in the discriminative stimulus effects of ethanol. Alcohol Clin Exp Res. 2002;26:812–7. [PubMed] [Google Scholar]
- Quertemont E, Grant KA. Discriminative stimulus effects of ethanol: lack of interaction with taurine. Behav Pharmacol. 2004;15:495–501. doi: 10.1097/00008877-200411000-00005. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Green HL, Grant KA. Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology (Berl) 2003;168:262–70. doi: 10.1007/s00213-003-1437-7. [DOI] [PubMed] [Google Scholar]
- Randall PA, Jaramillo AA, Frisbee S, Besheer J. The role of varenicline on alcohol-primed self-administration and seeking behavior in rats. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58:1237–45. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Wilkinson JL, Bevins RA. Methamphetamine functions as a positive and negative drug feature in a Pavlovian appetitive discrimination task. Behav Pharmacol. 2007;18:755–65. doi: 10.1097/FBP.0b013e3282f14efc. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Popham RE. Alcohol consumption and ischemic heart disease: some evidence from population studies. Br J Addict. 1981;76:407–17. doi: 10.1111/j.1360-0443.1981.tb03239.x. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–32. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–6. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–9. [PubMed] [Google Scholar]
- Troisi JR, 2nd, Dooley TF, 2nd, Craig EM. The discriminative stimulus effects of a nicotine-ethanol compound in rats: Extinction with the parts differs from the whole. Behav Neurosci. 2013;127:899–912. doi: 10.1037/a0034824. [DOI] [PubMed] [Google Scholar]
- Turner DM. Influence of route of administration on metabolism of [14C]nicotine in four species. Xenobiotica. 1975;5:553–61. doi: 10.3109/00498257509056125. [DOI] [PubMed] [Google Scholar]
- Wilking JA, Hesterberg KG, Nguyen VH, Cyboron AP, Hua AY, Stitzel JA. Comparison of nicotine oral consumption and baseline anxiety measures in adolescent and adult C57BL/6J and C3H/Ibg mice. Behav Brain Res. 2012;233:280–7. doi: 10.1016/j.bbr.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Tonner PH, Miller KW. Ethanol stabilizes the open channel state of the Torpedo nicotinic acetylcholine receptor. Mol Pharmacol. 1994;45:102–8. [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–9. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]