Abstract
Rationale
Intermittent social defeat stress engenders persistent neuroadaptations and can result in later increased cocaine taking and seeking. However, there are individual differences in stress-escalated cocaine self-administration behavior, which may be a direct result of individual differences in the manner in which rats experience social defeat stress.
Objective
The present study dissected the discrete behavioral phases of social defeat and analyzed which behavioral characteristics may be predictive of subsequent cocaine self-administration.
Methods
Male Long-Evans rats underwent nine intermittent social defeat episodes over 21 days in a three-compartment apparatus permitting approach to and escape from a confrontation with an aggressive resident rat. Rats then self-administered intravenous cocaine, which culminated in a 24-h unlimited access “binge.” Behaviors during social defeat and cocaine self-administration were evaluated by principal component analysis (PCA).
Results
PCA revealed that the latency to enter the threatening environment was highly predictive of later cocaine self-administration during the 24-h binge. This behavior was not associated with other cocaine-predictive traits, such as reactivity to novelty in an open field, saccharin preference, and motor impulsivity. Additionally, there was no effect of latency to enter a threatening environment on physiological measures of stress, including plasma corticosterone and corticotropin releasing factor (CRF) in the extended amygdala. However, latency to enter the threatening environment was negatively correlated with brain-derived neurotropic factor (BDNF) and its receptor, tyrosine kinase B (TrkB) in the hippocampus.
Conclusion
These data suggest that latency to enter a threatening environment is a novel behavioral characteristic predictive of later cocaine self-administration.
Keywords: Social defeat stress, Cocaine self-administration, BDNF, Impulsivity, Behavior, Individual differences
Introduction
Stress is both a predisposing, triggering factor and consequence of drug taking. Individuals with high levels of previous stress and adverse life events are more likely to engage in drug use, initiate drug use at an earlier age, transition from occasional use to compulsive drug addiction more quickly, and relapse (Sinha 2001). Preclinical studies have largely paralleled these findings in both nonhuman primates and rodents. In particular, a model of intermittent social defeat stress in rats engenders long-lasting neuroadaptations, resulting in faster acquisition of cocaine self-administration, greater responding under progressive ratio schedules of reinforcement, increased cocaine self-administration during a 24-h unlimited access “binge,” and heightened context-induced reinstatement after abstinence (Covington and Miczek 2001; Holly et al. 2016; Miczek and Mutschler 1996; Miczek et al. 2011; Tidey and Miczek 1997).
However, despite substantial evidence linking stress and compulsive drug taking and seeking, not all individuals exposed to high levels of stress engage in drug use. Similarly, there is considerable variability in cocaine self-administration patterns and levels of consumption in rats exposed to models of intermittent social defeat stress (Covington and Miczek 2005; Covington et al. 2008; Kabbaj et al. 2001; Shimamoto et al. 2015). Understanding the behavioral sources and underlying mechanisms of individual differences in augmented cocaine self-administration following stress may lead to earlier and more effective therapeutic interventions for vulnerable individuals.
A possible underlying basis for this variability in drug taking is individual differences in behavior during social stress. Wood et al. (2010) found that active vs. passive coping during social defeat (operationally defined by latency to submit to the resident) was predictive of subsequent resilience or vulnerability, respectively, to maladaptive behavioral and physiological effects of stress. However, social defeat encompasses a myriad of behaviors, such as approach to a threatening situation, reaction to a fight encounter, escape behavior, and return to a safe environment (Koolhaas et al. 2010). How each of these distinct behaviors relates to subsequent drug self-administration has yet to be explored. In the current experiment, we dissected these phases of social confrontation and examined resulting effects on stress-associated physiological and behavioral measures. We designed an apparatus which allowed us to assess rats for their latencies to engage in each component of social defeat stress: entering a threatening zone, engaging in a confrontation, submitting to social defeat, escape, and return to a safe zone. After nine encounters, rats were tested for behavioral cross-sensitization to cocaine and catheterized for cocaine self-administration, which culminated in a 24-h binge. As other factors, such as reactivity to novelty, saccharin preference, impulsivity, and resistance to punishment have also been correlated with individual differences in cocaine self-administration (Belin et al. 2008; Carroll et al. 2002; Deroche-Gamonet et al. 2004; Piazza et al. 1989), we also examined whether these measures were associated with defeat-related behaviors.
Additionally, we evaluated whether behaviors during defeat were associated with neurophysiological differences in brain regions associated with stress and drug reward. Chronic stress downregulates hippocampal brain-derived neurotrophic factor (BDNF) transcription (Duclot and Kabbaj 2013; Haenisch et al. 2009; Komatsu et al. 2011; Patki et al. 2013; Zhang et al. 2015), with individual differences in chronic stress-induced changes in hippocampal BDNF and its receptor, tyrosine receptor kinase B (TrkB) (Duclot and Kabbaj 2013). Individual differences in the physiological response to stress may also contribute to individual differences in stress-escalated (as opposed to drug history-escalated) cocaine self-administration. Clinical work has shown that cortisol release in response to stress is positively correlated with positive feelings in response to amphetamine (Hamidovic et al. 2010). Corticotropin releasing factor (CRF) is a neuropeptide involved in the initiation of the hypothalamic-pituitary-adrenal axis stress response, but also has widespread extrahypothalamic sites of action (Swanson et al. 1983). In particular, CRF in the extended amygdala and mesocorticolimbic dopamine system has been strongly implicated in stress-induced psychiatric disorders, including drug addiction (Zorrilla et al. 2014). Therefore, we also examined whether hippocampal BDNF and TrkB transcript, CRF expression, or corticosterone levels were correlated with defeat behaviors.
Methods
General methods
Subjects
Male Long-Evans rats (Charles River Laboratories, Wilmington, MA, n = 213) weighing 225–250 g upon arrival were individually housed in custom-built acrylic chambers (30.5 × 30.5 × 24.5 cm) lined with Cellu-Dri™ bedding (Shepherd Specialty Papers, Kalamazoo, MI) and provided food and water ad libitum. Rats were housed on a reverse light cycle (lights on 2000–0800 hours), with controlled temperature (21 ± 1 °C) and humidity (30–60 %). All behavioral testing was conducted during the dark phase, and all experimental procedures were approved by the Tufts Institutional Animal Care and Use Committee following the Guide for the Care and Use of Laboratory Animals (National Research Council 2011).
Experimental design
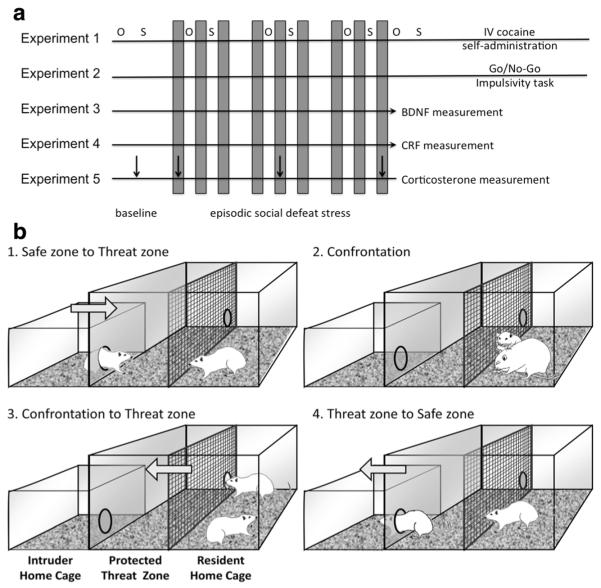
In experiment 1, rats were first tested for baseline saccharin preference and open field activity, after which they were exposed to social defeat stress or control handling, with weekly measurements of saccharin preference and locomotion in the open field. Saccharin preference and open field activity were tested again 1 week after the last social defeat. Rats were then assessed for locomotor cross-sensitization to cocaine, after which rats were implanted with intravenous catheters and trained to self-administer cocaine. In experiment 2, rats underwent social defeat or handling, followed by Go/No-Go testing for impulsivity. In experiment 3, rats were assessed for BDNF and TrkB messenger RNA (mRNA) expression in the prefrontal cortex and hippocampus after the last social defeat, while experiment 4 measured CRF protein expression in the nucleus accumbens, amygdala, and ventral tegmental area. Finally, experiment 5 measured plasma corticosterone after the first, fifth, and ninth defeat. Experimental design is shown in Fig. 1a.
Fig. 1.
Experimental and apparatus design. a In the first experiment, rats were tested for baseline open field activity (O) and saccharin drinking (S), which continued weekly throughout the course of intermittent social defeat stress (defeats represented by gray bars). After the last defeat, rats were catheterized and allowed to self-administer cocaine. In the second experiment, separate rats were tested for impulsivity in the Go/No-Go task after social defeat stress. Physiological measures were taken in the final three experiments, with one cohort assessed for BDNF and TrkB mRNA, another for extrahypothalamic CRF content, and a final cohort tested for corticosterone at baseline and after the first, fifth, and ninth defeat. b Three-chamber apparatus permitting entry and escape for the intruder. 1, The intruder voluntarily leaves the safe zone (home cage) and enters the threat zone. 2, After 5 min in the threat zone, the intruder is placed into the resident’s compartment for social defat. 3, After ten bites, 8 s supine, or 5 min, the porthole is opened and the intruder allowed to escape back to the threat zone. 4 After 5 min, the porthole to the safe zone is opened and the intruder allowed to return to its home cage
Social defeat stress
Upon arrival, rats were randomly assigned to two groups: defeat stressed or non-defeated control. Stressed rats were subjected to nine intermittent social defeat episodes over 21 days in a modified version of our previously described procedure (Covington and Miczek 2001), while non-defeated controls were briefly handled. Defeats occurred in a modified resident home cage (Fig. 1b) consisting of two compartments (the resident’s home compartment or “fight zone” and a neutral compartment or “threat zone” with clean bedding) connected by a porthole, with a second porthole allowing access to the experimental rat’s home cage (“safe zone”). See Electronic supplementary material for additional details.
During each defeat, the experimental intruder’s home cage was moved adjacent to the resident cage (Fig. 1b), the porthole opened, and the rat allowed up to 5 min to voluntarily move to the threat zone. After 5 min in the threat zone, the intruder was placed into the fight zone with the resident with the escape porthole closed until it showed 8 s continuous supine posture, received ten attack bites, or 5 min had elapsed. A screen was then used to separate the aggressive resident from the intruder and the escape porthole opened. The rat was allowed up to 5 min to voluntarily move from the fight zone to the threat zone. After 5 min in the threat zone, the safe zone porthole was opened and the intruder allowed up to 5 min to voluntarily return to its home cage. If an intruder did not move from one compartment to another after 5 min, the rat was placed into the adjacent compartment by the experimenter and the porthole closed.
Experiment 1: analysis of behaviors predictive of increased cocaine self-administration after social defeat
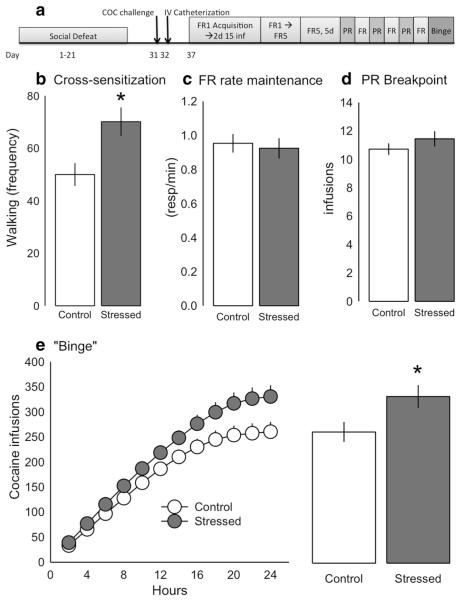
The aim of experiment 1 was to elucidate which behaviors during social stress were predictive of later augmented cocaine self-administration during the binge. Additionally, as individual differences in both saccharin drinking and open field locomotor activity have been associated with increased psychostimulant self-administration, we also investigated whether these factors were predictive of stress-escalated cocaine self-administration. Design for experiment 1 is shown in Fig. 1a, and a timeline shown in Fig. 2a.
Fig. 2.
Social defeat stress induces behavioral cross-sensitization to cocaine and escalated cocaine self-administration during a 24-h “binge.” a Timeline for the cocaine self-administration methods. Animals were challenged with cocaine (10 mg/kg, ip) 10 days after the last social defeat to assess for behavioral cross-sensitization and were catheterized for intravenous cocaine self-administration the following day. After 5 days recovery, animals were initially allowed to self-administer cocaine (0.75 mg kg−1 infusion−1) on a fixed ratio 1 (FR1) schedule until they obtained the maximal 15 infusions in two consecutive days. The FR schedule was then gradually increased to FR5, followed by 5 days FR5 maintenance. Three progressive ratio (PR) sessions were then alternated with three FR sessions across 6 days, and the experiment culminated in a 24-h binge. b Stressed rats (n = 39) had a significantly greater walking frequency in response to cocaine compared with non-stressed controls (n = 43), indicative of behavioral cross-sensitization. c There were no differences between stressed and control rats in mean response rate (responses/min) across the last three FR5 maintenance sessions d nor were there any differences in median breakpoint (defined as infusions self-administered) across the three PR sessions. e Stressed rats self-administered significantly more cocaine across the 24-h binge compared with controls. Data shown are mean ± SEM; *p < 0.01 vs. control
Saccharin drinking and open field testing
Rats were randomly assigned to stressed (n = 39) or control (n = 43) groups and were tested for 0.02 % saccharin preference in a weekly 1-h two bottle choice procedure. Rats were also tested weekly for locomotor activity in an open field. Rats were removed from their homecage during the dark phase and placed in the center of a large open field (71 × 45 × 45 cm). After 30 min habituation, a novel object was placed into the arena and one 5-min video sample was recorded for novel object exploration. Total distance traveled throughout the habituation and novel object exploration was measured by Ethovision. Baseline measurements were performed 1 week prior to the social stress phase. Further testing occurred once per week throughout the stress period and 1 week after stress exposure (for additional details, see Electronic supplementary material).
Locomotor cross-sensitization to cocaine
Ten days after the final social defeat episode (day 31), rats were tested for cross-sensitization to acute cocaine challenge (Boyson et al. 2014; Covington and Miczek 2001).
Intravenous cocaine self-administration
Methods for cocaine self-administration have been previously described (Boyson et al. 2014; Holly et al. 2016). Timeline for self-administration methods is shown in Fig. 2a and additional details in Electronic supplementary material.
Rats were permanently implanted with an indwelling catheter (Silastic® silicon tubing, ID 0.63 mm, OD 1.17 mm) into the right jugular vein under ketamine (100 mg/kg) and xylazine (6 mg/kg) anesthesia. Rats were allowed to recover for at least 5 days, then were moved from their home cage to permanent housing inside cocaine self-administration chambers.
Rats were initially allowed to self-administer cocaine (0.75 mg kg−1 infusion−1, administered 0.15 ml/kg at 0.018 ml/s) without priming or autoshaping on a fixed ratio 1 (FR1) schedule of reinforcement, followed by a 30-s timeout. Each daily session terminated after 15 infusions or 5 h. Once animals demonstrated reliable, stable responding at FR1, operationally defined as two consecutive days of maximal responding (15 infusions), the schedule was gradually advanced to FR5 to ensure rats were reliably performing for cocaine. Rats were maintained on a limited access FR5 schedule for at least five consecutive days. Rats were then subjected to three progressive ratio (PR) sessions (0.3 mg kg−1 infusion−1), alternated with three FR5 sessions (0.75 mg kg−1 infusion−1) across 6 days. The day after the final FR5 maintenance session, rats were given unlimited access to cocaine (0.3 mg kg−1 infusion−1) for 24 h.
Experiment 2: Go/No-Go task
The aim of experiment 2 was to assess whether individual differences in impulsivity could explain the individual differences in behavior during social defeat stress. A separate cohort of rats underwent social defeat (n = 21) or control handling (n = 11) as described above and was subsequently tested using a modified version of the Go/No-Go task (Helms et al. 2008).
Each session of the Go/No-Go task ended after 60 trials. For each trial of the Go/No-Go task, there was a variable precue period (9–24 s), signaled by the house light, and responses were recorded but not reinforced. During the initial training phase, the precue period was followed by a 30-s “Go” cue (constant light above the Go hole). When the Go cue was displayed, the first nosepoke response resulted in the delivery of 0.07 ml 0.02 % saccharin. After rats successfully completed >30 trials within 1 h for four consecutive sessions, the cue duration was reduced from 30 to 10 s. After completion of >30 trials within 1 h for four consecutive sessions at 10 s, the cue light was reduced to 5 s.
After stable responding was again demonstrated, the “No-Go” cue (flashing cue light of a different color above the opposite nosepoke) was introduced on five consecutive sessions. When the No-Go cue was presented, rats must inhibit nosepoke responding during the cue. A nosepoke response (false alarm) terminated the trial and no saccharin was delivered. Go and No-Go trials were randomly ordered, with Go trials occurring 75 % of the time. After either trial type, a 10-s intertrial period occurred with all lights off and no reward available.
Experiment 3: BDNF and TrkB mRNA measurement
The aim of experiment 3 was to determine whether altered BDNF signaling in the hippocampus or prefrontal cortex (PFC) was an underlying mechanism behind individual differences in social defeat behavior. One hour after the last defeat, a separate group of rats (n = 20 control, n = 20 stressed) were anesthetized with isofluorane and rapidly decapitated for quantification of BDNF and TrkB mRNA through quantitative reverse transcription polymerase chain reaction (qRT-PCR, see Electronic supplementary material).
Experiment 4: CRF measurement
The aim of experiment 4 was to evaluate whether individual differences in social defeat behavior resulted in altered CRF expression in limbic regions associated with stress and reward. A separate cohort of rats (n = 12 control, n = 12 stressed) was rapidly decapitated under isofluorane anesthesia 1 h after the final defeat to measure extrahypothalamic CRF content using a commercially available enzyme immune assay kit (EIA; Peninsula Laboratories, San Carlos, CA, see Electronic supplementary material).
Experiment 5: corticosterone measurement
Finally, experiment 5 aimed to determine whether individual differences in stress-escalated cocaine self-administration could be simply explained by differences in the physiological response to social defeat. A separate cohort of rats (control n = 16, stressed n = 18) was used to measure plasma corticosterone at baseline and 20 min following social defeat on days 1, 10, and 21. Blood was collected via tail vein puncture and centrifuged to obtain plasma. Corticosterone concentration (ng/ml) was determined using a commercially available EIA kit (Arbor Assays Detect X, Ann Arbor, MI). Standard curve range was 78.125–10000 pg/ml.
Statistical analysis
A principle component factor analysis was used to determine the relationship between specific behaviors during social defeat stress and subsequent cocaine self-administration (SAS, SAS Institute). To assess behaviors during aggressive encounters, the average latencies for the slow and fast groups were collapsed across the nine encounters and then were followed by a one-way analysis of variance (ANOVA). A two-way (group × time) repeated measures ANOVA was used to assess differences in body weight, saccharin drinking, and locomotor activity. Locomotor sensitization and IV cocaine self-administration were assessed by a one-way ANOVA. All post-hoc tests were analyzed by Holm-Sidak corrections for multiple comparisons. In experiment 2, a two-way (group × time) repeated measures ANOVA was used to assess precue, go hits, and false alarms (Sigma Plot version 11.0, Systat Software). All post-hoc tests were analyzed with Holm-Sidak corrections for multiple comparisons. In experiments 3–5, one-way ANOVAs were used to assess total BDNF and TrkB receptor mRNA and CRF content, while a two-way (group × time) repeated measures ANOVA was used to determine corticosterone secretion. A linear regression was used for correlational analyses of BDNF and average latency to enter into a threat zone as well as TrkB receptor mRNA and average latency to enter into a threat zone. All post-hoc tests were analyzed by Holm-Sidak corrections for multiple comparisons.
Results
Experiment 1: analysis of behaviors predictive of increased cocaine self-administration after social defeat
Rats with a history of social defeat stress showed behavioral cross-sensitization to cocaine, as measured by walking frequency (Student’s t = −2.826, df = 83, p = 0.006, Fig. 2b) and self-administered significantly more cocaine under 24 h unlimited access binge condition (Student’s t = −2.328, df = 72, p = 0.023, Fig. 2e). FR response rate (Fig. 2c) and PR breakpoint (Fig. 2d) were not affected by stress history.
Principal component analysis: latency to enter threat zone is predictive of stress-escalated cocaine self-administration
We next assessed whether any behaviors during social defeat (latency to enter the threat zone, fight duration, latency to leave fight, return to safe zone) were predictive of subsequent cocaine self-administration behavior (progressive ratio breakpoint, rate of fixed ratio maintenance, total binge infusions, binge duration). Principal component analysis (PCA) revealed two factors with an eigenvalue >1 (Fig. 3a). Five behavioral elements loaded onto factor 1; four of these behaviors were related to cocaine self-administration, and only one was related to the social defeat procedure (r = −0.45–0.90). The three other social defeat-related behaviors loaded onto separate factor with minimal cross-loading (r = 0.41–0.84). Based on the results from the PCA, latency to enter the threat zone was then used as a predictive factor to assess all subsequent behavioral and neurophysiological results. Differences in latency to enter the threatening zone emerged by the second defeat (Fig. 3b). To enhance contrast between groups, the upper and lower tails of the distribution for the latency measure were used to separate socially defeated rats, with the lower 33rd percentile termed “fast,” and upper 33rd percentile termed “slow.” The middle 33 % were removed from all further analyses (Fig. 3c).
Fig. 3.
Principle component analysis (PCA) reveals latency to enter threat zone is correlated with cocaine self-administration behavior. a Two factors were extracted from the PCA; factor 1, on the x-axis, accounts for 50.1 % of the total variance, while factor 2, on the y-axis, accounts for 30.8 % of the total variance. Latency to enter the threat zone was the only behavioral component of social defeat that loaded onto the factor with cocaine self-administration variables. b Latency to enter the threat zone significantly differed between “fast” (black, n = 13) and “slow” (gray, n = 13) rats across the nine social defeats, and group differences emerged by the second defeat. c Mean latency to enter the threat zone across nine social defeat encounters was a continuous distribution, and with the upper 1/3 (n = 13) of the distribution considered slow and the lower 1/3 (n = 13) of the distribution considered fast. Data shown are mean ± SEM. ***p < 0.001
Individual differences in latency to enter threat zone did not affect other defeat behavior
The fast rats had significantly lower latencies to enter the threat zone compared with the slow rats (Student’s t = 14.328, df = 24, p < 0.001), however, these groups did not differ in fight duration, latency to escape the fight, or latency to return to the safe zone (Table 1). Furthermore, there were no significant differences between fast and slow rats on specific behaviors before, during, or after the fight period (see Electronic supplementary material).
Table 1.
Latencies of behaviors in “slow” (bottom 33 %, n = 13) and “fast” (top 33 %, n = 13) rats
| Slow | Fast | |
|---|---|---|
| Latency to leave home cage prior to fight (s) | 269.6 ± 5.4 | 75.9 ± 12.2* |
| Fight duration (s) | 131.8 ± 17.6 | 126.0 ± 20.7 |
| Latency to escape fight (s) | 32.8 ± 9.4 | 31.4 ± 4.0 |
| Latency to return to home cage after fight (s) | 74.0 ± 9.9 | 72.2 ± 18.0 |
All values are means ± SEM
p < 0.001 slow vs. fast
Individual differences in latency to enter the threat zone do not affect body weight gain, saccharin preference, and open field activity
Body weight
Overall, in regard to body weight (g, Fig. 4a), there was a significant main effect of week (two-way repeated measures ANOVA F4, 264 = 516.630, p < 0.001), with a significant main effect of group (control, fast, and slow, F2, 66 = 3.874, p = 0.026) and week × group interaction (F8, 264 = 5.689, p < 0.001). Post-hoc analyses revealed no significant group differences in body weight at baseline. When collapsed across all weeks tested (baseline, weeks 1–3 of stress, and 1 week post-stress), fast rats weighed significantly less than both controls (Holm-Sidak t = 2.575, p = 0.036) and slow stressed rats (Holm-Sidak t = 2.425, p = 0.036). However, when data were normalized to percent of baseline for each rat, there were no significant differences in weight gain across groups (Fig. 4b).
Fig. 4.
Latency to enter the threat zone does not affect body weight gain, saccharin preference, or open field activity. a Body weight (g) increased in all groups from baseline (BL), through the 3 weeks of social defeat stress, to 1 week post-stress (PS), although overall, “fast” (black circles, n = 13) rats weighed significantly less than “slow” (gray circles, n = 13) and control (white circles, n = 43) rats (*p < 0.05). b Percent change in body weight from baseline (%BL) did not differ between groups. c Saccharin intake (mg/kg body weight) increased from BL to the 1 week PS in all groups, but no differences in saccharin consumption between groups were observed. d Saccharin preference (%, saccharin intake/(saccharin intake + water intake) also did not differ between groups. e On the first day of baseline open field testing, distance traveled (cm) in 5 min bins decreased across the 30-min habituation period but did not differ between groups. Insertion of a novel object (N) into the open field caused increased locomotor activity, but distance traveled did not differ between groups. f Average locomotor activity at BL, during each of the 3 weeks of stress, or PS did not differ across groups. Data shown are mean ± SEM
Saccharin drinking
There were no significant differences between fast- and slow-defeated rats and non-stressed controls in saccharin preference (Fig. 4d). However, there was a significant main effect of day for total saccharin intake (two-way repeated measures ANOVA F1, 62 = 24.118, p < 0.001, Fig. 4c), suggesting all groups increased total consumption across time.
Open field
There was a significant main effect of time on total locomotor activity in an open field during the first session of testing (“baseline”, two-way repeated measures ANOVA F5, 283 = 124.673, p < 0.001), but no effect of group (Fig. 4e). There was a significant main effect of day on total distance traveled across the five weeks of testing (Two-way repeated measures ANOVA F4, 231 = 3.350, p = 0.011; Fig. 4f), suggesting no locomotor activity deficits in slow rats.
Individual differences in latency to enter the threat zone affect cocaine self-administration
Cross-sensitization to cocaine
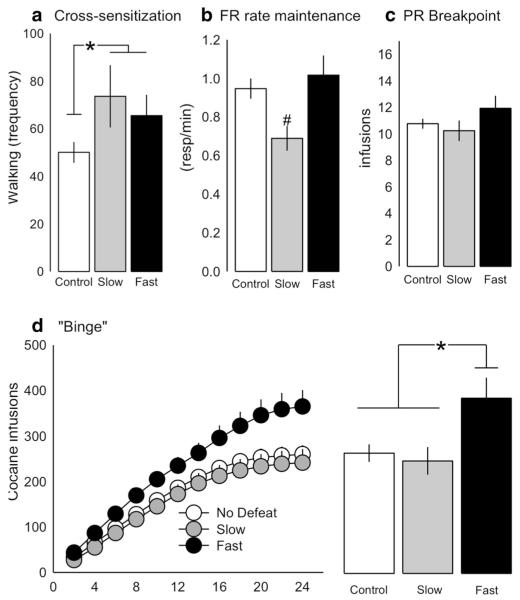
Escapable intermittent social defeat stress increased locomotor activity in response to an acute cocaine challenge. There was a significant main effect of group (control, fast, and slow, one-way ANOVA F2, 64 = 3.846, p = 0.026, Fig. 5a), with stressed groups exhibiting significantly increased walking frequency in response to cocaine compared with controls (Holm-Sidak t = 2.715, p = 0.008).
Fig. 5.
Latency to enter the threat zone was predictive of cocaine self-administration. a Both “fast” (n = 13) and “slow” (n = 13) rats showed stress-induced cross-sensitization to cocaine compared with controls (n = 43) as assessed by walking frequency after cocaine challenge (10 mg/kg, ip). b Rate of cocaine self-administration (responses/min) during fixed ratio (FR) was lower in slow rats compared with fast rats. c There was no effect of group on breakpoint (number of infusions obtained) in a progressive ratio (PR) schedule of reinforcement. d Cumulative cocaine infusions self-administered across a 24-h period was significantly lower in slow rats compared with fast rats (left) with fast rats self-administering significantly more total cocaine infusions compared with both slow rats and non-defeated controls (right). Data shown are mean ± SEM. *p < 0.05 vs. non-defeated controls; #p < 0.05 vs. “fast” rats
Acquisition/maintenance
There were no significant differences in acquisition among the fast, slow, and control groups. However, once rats acquired self-administration, there was a significant main effect of group on the average rate of cocaine self-administration during the last 3 days of maintenance (one-way ANOVA F2, 65 = 3.896, p = 0.025). Post-hoc analyses revealed a significantly lower response rate in the slow-defeated rats compared with the fast-defeated rats (Holm-Sidak t = 2.544, p = 0.039; Fig. 5b) and non-defeated controls (Holm-Sidak t = 2.475, p = 0.032).
Progressive ratio
There were no significant differences among the fast, slow, or non-defeated control rats on measures of motivation as assessed by total cocaine infusions (breakpoints) under a PR schedule of reinforcement (Fig. 5c).
Twenty-four-hour binge
There was a significant main effect of group (one-way ANOVA F2, 62 = 5.117, p = 0.009) on the number of cocaine infusions self-administered during a 24-h continuous access binge. Post-hoc analyses fast rats self-administered significantly more infusions than both the slow-defeated rats and non-defeated controls (Holm-Sidak t = 2.766, p = 0.015; t = 2.965, p = 0.013, respectively; Fig. 5d).
Experiment 2: Go/No-Go task
Individual differences in latency to enter the threat zone do not affect impulsivity as measured by the Go/No-Go task
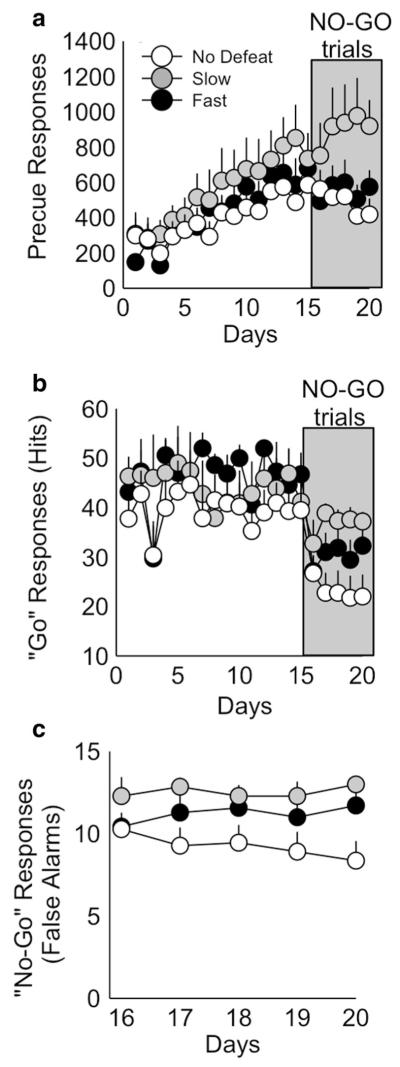
No significant differences between the fast, slow, and non-defeated control groups were found in any of the impulsive-like measures in the Go/No-Go task (precue responses, hits, and false alarms), suggesting that fast latency to enter the threat zone is not indicative of motor impulsivity (Fig. 6).
Fig. 6.
Latency to enter the threat zone did not significantly affect impulsivity as measured by the Go/No-Go task. a Precue responses during training (days 1–15) as well as No-Go testing (shaded box, days 16–20) for fast (black circles, n = 7), slow (gray circles, n = 7), and non-defeated controls (white circles, n = 11) did not significantly differ. b Go “hits” during the training phase (days 1–15) and Go/No-Go phase (days 16–20, gray box), did not significantly differ between groups. c False alarms during the Go/No-Go phase (days 16–20) also did not differ between groups. Data shown are mean ± SEM
Experiment 3: BDNF and TrkB mRNA measurement
Individual differences in latency to enter the threat zone is associated with altered hippocampal BDNF and TrkB mRNA transcription
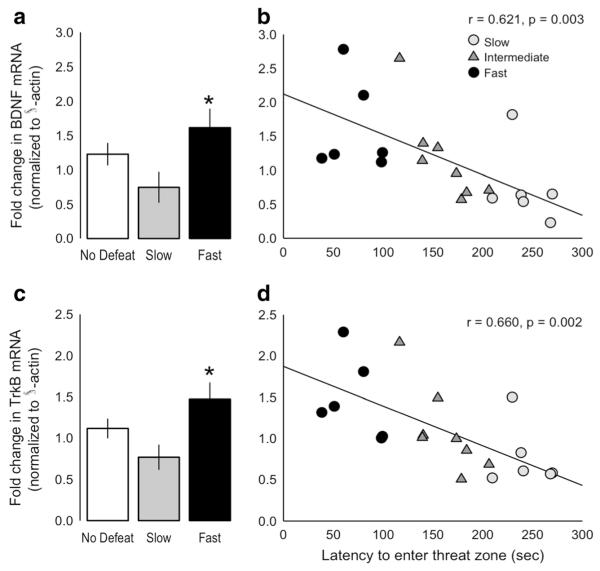
There was no significant effect of group on BDNF or TrkB mRNA in the prefrontal cortex, but there was a significant effect of group on BDNF and TrkB mRNA within the hippocampus. Hippocampal BDNF was significantly affected by group (one-way ANOVA F2, 29 = 4.178, p = 0.025), with fast rats expressing significantly more BDNF mRNA in the hippocampus relative to slow rats (p = 0.023). Similarly, hippocampal TrkB mRNA was affected by group (F2, 29 = 3.534, p = 0.042), with fast rats again expressing significantly more hippocampal TrkB mRNA relative to slow rats (p = 0.037). There was a significant negative correlation between average latency to enter the threat zone and hippocampal BDNF (r = 0.621, p = 0.003) and TrkB (0.660, p = 0.002) mRNA (Fig. 7).
Fig. 7.
Latency to enter the threat zone was associated with altered hippocampal BDNF and TrkB mRNA. a “Fast” (n = 7, black) rats showed significantly greater hippocampal BDNF mRNA expression compared with “slow” (n = 7, gray) rats and non-defeated controls (n = 20, white), b resulting in a significant negative correlation between latency to enter the threat zone and hippocampal BDNF mRNA (intermediate 1/3 (n = 7) of defeated rats shown as gray triangles). c Similarly, fast rats showed significantly greater hippocampal TrkB mRNA expression compared with slow rats, d which also resulted in a significant negative correlation between latency to enter threat zone and hippocampal TrkB mRNA. Data shown are mean ± SEM. *p < 0.05 vs. slow
Experiments 4 and 5: CRF and corticosterone measurement
Individual differences in latency to enter the threat zone do not affect the physiological stress response
Corticosterone
Social stress resulted in a group × day interaction in plasma corticosterone 20 min after defeat (F3, 94 = 5.93, p < 0.001). This difference appears to be a result of increased corticosterone in stressed rats limited to Day 1. Fast and slow rats did not differ in the corticosterone response to stress (Fig. 8a).
Fig. 8.
Latency to enter the threat zone was not associated with altered physiological responses to stress. a Plasma corticosterone (CORT) did not differ between groups at baseline (BL), and while there was a significant effect of stress on the first day of defeat (D1), there was no difference between “slow” (n = 6, gray) and “fast” (n = 6, black) groups (control n = 16, white). There was no effect of stress on plasma corticosterone on day 10 or 21. b Total CRF content (pg/mg protein) in the nucleus accumbens (NAc), amygdala (AMY), and ventral tegmental area (VTA) also did not differ between groups (control n = 12, fast n = 4, slow n = 4). Data shown are mean ± SEM
Extrahypothalamic CRF
There was no effect of group on CRF content in the nucleus accumbens (NAc), amygdala, or ventral tegmental area (VTA). However, there was a main effect of brain region regardless of group (two-way repeated measures ANOVA F2, 33 = 22.262, p < 0.001), with significantly more CRF in the amygdala and VTA compared with the NAc (Holm-Sidak t = 6.488, p < 0.001; t = 4.580, p < 0.001, respectively; Fig. 8b).
Discussion
Through dissecting the cluster of behaviors involved in social defeat stress, we have shown that latency to enter into a threatening zone is highly predictive of the subsequent development of stress-escalated cocaine self-administration. Rats with a short latency to enter a threatening environment (“fast” group) self-administer cocaine at a higher rate and accumulate more cocaine infusions during a 24-h binge compared with rats with a long latency to enter the threatening environment (“slow” group). Importantly, separating animals based upon this measure did not result in significant differences in saccharin preference, open field locomotor activity, or motor impulsivity, all of which have been linked with increased rates of psychostimulant self-administration (Belin et al. 2008; Carroll et al. 2002; Piazza et al. 1989). This indicates that latency to enter a threatening environment is a distinct individual difference in behavior related to escalated self-administration. Furthermore, differences in cocaine self-administration between fast and slow rats are not the result of altered behavioral (immobility, reaction to attack bite) or physiological (CRF, corticosterone) responses to social defeat stress. Finally, we demonstrate that fast and slow rats show altered BDNF and TrkB transcription in the hippocampus, which may be an underlying mechanism for the persistent neuroadaptations engendered by social defeat stress which promote escalated cocaine self-administration.
Individual differences in factors that promote enhanced cocaine self-administration in rodents have received considerable attention. Of note, high preference for and locomotor reactivity to a novel environment, preference for sucrose, and impulsivity have all been associated with increased psychostimulant self-administration (Belin et al. 2008; Carroll et al. 2002; Piazza et al. 1989). The current series of experiments complement this growing literature, indicating that a consistent decision to rapidly and voluntarily enter into a threatening social environment, despite known consequences, is also highly predictive of intensified cocaine self-administration. Differences between rats fast and slow to approach social threat emerge by the second social defeat encounter and are sustained through the 21 days of social stress. Importantly, this maladaptive choice by some stressed rats is distinct from other previously studied innate traits associated with subsequent maladaptive behavior.
As a motor behavior, rapid approach to social threat could be function of high-responder (HR) and low-responder (LR) rats, which has been established as a highly predictive trait for faster acquisition of psychostimulant self-administration (Kabbaj et al. 2001; Piazza et al. 1989). Innate traits, such as HR or increased locomotor behavior in a novel open field environment, are highly associated with acquisition rates of cocaine self-administration, although there have been no reported effects on other measures of psychostimulant self-administration (Bardo et al. 2013; Blanchard et al. 2009; Piazza et al. 1989). Although the current study used different methods from previous HR/LR experiments, fast and slow rats showed no significant differences in reactivity to a novel open field and novel object exploration, as assessed by total distance traveled. This suggests that latency to enter a threatening environment may be a distinct trait from HR and LR phenotypes, although future work should assess this in a circular arena using methods initially promoted by Piazza et al. (1989). Importantly, however, differences in general locomotor behavior and locomotor activity in response to a novel object cannot explain differences in latency to enter the threat zone exhibited by fast and slow rats.
An alternative explanation for the behavioral differences observed between fast and slow rats is individual differences in motor impulsivity, also established as a predisposing factor for the transition from initial cocaine self-administration to escalated, compulsive self-administration (Belin et al. 2008; Murray et al. 2014). However, following social defeat stress, fast and slow rats did not differ in impulsivity from non-stressed controls in the Go/No-Go task. In contrast to motoric impulsivity, fast and slow rats may be exhibiting increased and decreased levels of social impulsivity, as exhibited by consistent low or high latency to approach a novel stimulus animal despite possible negative consequences.
Furthermore, we have demonstrated that fast and slow rats do not differ in two physiological measures of responsivity to social defeat stress, namely corticosterone secretion and extrahypothalamic CRF concentration. Although these physiological measures are not necessarily direct correlates of the amount of stress the animals experience and the single time point measurement cannot rule out differences in HPA axis negative feedback, they indicate that fast and slow rats may generally experience the same level of stress. This is significant because it precludes the possibility that fast rats self-administer more cocaine due to an increased physiological stress response and points to the important role of other factors, such as decision-making, altered cocaine responsivity, or altered hippocampal BDNF/TrkB signaling.
Latency to enter a threatening zone was positively correlated with hippocampal BDNF and TrkB (Fig. 5). This is consistent with other work demonstrating individual differences are regulated by hippocampal BDNF signaling. Greater hippocampal BDNF is associated with the promotion of resilience to anhedonia-like symptoms induced by chronic stress (Bergstrom et al. 2008; Duclot and Kabbaj 2013; Taliaz et al. 2011). Duclot and Kabbaj (2013) report that altered BDNF signaling is a key mechanism underlying individual differences in vulnerability to chronic stress; enhanced hippocampal BDNF signaling promotes resilience, whereas disruption promotes vulnerability to chronic social defeat-induced anhedonia-like behavior.
In the present series of experiments, as opposed to investigating individual differences in anhedonic-like behavior after chronic stress, we focused on individual differences in hedonic-like behavior (i.e., cocaine self-administration) following intermittent social defeat, which does not induce an anhedonic-like phenotype (Miczek et al. 2011). We demonstrate that BDNF and TrkB transcription is altered within the slow group, resilient to the hedonic-promoting effects of intermittent social defeat, but remains equivalent to controls in the fast or vulnerable group. BDNF/TrkB signaling may be functioning similarly to promote resilience to the divergent behavioral effects of chronic and intermittent stress. BDNF facilitates synaptic plasticity and enhances postsynaptic responsivity (Cordeira et al. 2010; Fortin et al. 2012; Madara and Levine 2008; Park and Poo 2013), so enhanced hippocampal BDNF/TrkB signaling observed in “resilient” rats may promote synaptic and behavioral adaptations after stress. We initially investigated hippocampal BDNF due to the well-described role of hippocampal plasticity in response to stress and notable glucocorticoid sensitivity (McEwen 1999), but it will be important to evaluate BDNF and TrkB in other limbic brain regions associated with drug self-administration, particularly the VTA and NAc. Additionally, in the current experimental design, it is unclear whether BDNF signaling is causal or resultant of individual differences in defeat, as individual differences in latency to enter the threatening zone by definition cannot be ascertained until after defeat experience.
This is the first study to our knowledge to thoroughly explore the full repertoire of specific behaviors involved in social defeat stress and link them with later cocaine self-administration. Understanding behavioral indices of stress associated with later maladaptive behaviors, as well as neurobiological differences between vulnerable and resilient subjects, may lead to more targeted and effective therapeutic interventions for stress-related disorders, such as addiction or depression. Ultimately, we propose that individual differences in latency to enter into a threatening environment are highly predictive of subsequent stress-related maladaptive behavior. Future work will continue to explore how this particular aspect of social defeat stress plays a role in escalated cocaine self-administration.
Supplementary Material
Footnotes
Compliance with ethical standards
Conflicts of interest All authors declare no conflicts of interest.
Christopher O. Boyson and Elizabeth N. Holly are co-first authors.
Electronic supplementary material The online version of this article (doi:10.1007/s00213-016-4363-1) contains supplementary material, which is available to authorized users.
References
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom A, Jayatissa MN, Mork A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression; an in situ hybridization study. Brain Res. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–54. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Holly EN, Shimamoto A, Albrechet-Souza L, Weiner LA, DeBold JF, Miczek KA. Social stress and CRF-dopamine interactions in the VTA: role in long-term escalation of cocaine self-administration. J Neurosci. 2014;34:6659–67. doi: 10.1523/JNEUROSCI.3942-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–41. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–98. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology (Berl) 2005;183:331–40. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–16. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Duclot F, Kabbaj M. Individual differences in novelty seeking predict subsequent vulnerability to social defeat through a differential epigenetic regulation of brain-derived neurotrophic factor expression. J Neurosci. 2013;33:11048–60. doi: 10.1523/JNEUROSCI.0199-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Srivastava T, Dwarakanath D, Pierre P, Nygaard S, Derkach VA, Soderling TR. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J Neurosci. 2012;32:8127–37. doi: 10.1523/JNEUROSCI.6034-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Bilkei-Gorzo A, Caron MG, Bonisch H. Knockout of the norepinephrine transporter and pharmacologically diverse anti-depressants prevent behavioral and brain neurotrophin alterations in two chronic stress models of depression. J Neurochem. 2009;111:403–16. doi: 10.1111/j.1471-4159.2009.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Childs E, Conrad M, King A, de Wit H. Stress-induced changes in mood and cortisol release predict mood effects of amphetamine. Drug Alcohol Depend. 2010;109:175–80. doi: 10.1016/j.drugalcdep.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Gubner NR, Wilhelm CJ, Mitchell SH, Grandy DK. D4 receptor deficiency in mice has limited effects on impulsivity and novelty seeking. Pharmacol Biochem Behav. 2008;90:387–93. doi: 10.1016/j.pbb.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA. Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci. 2016;36:4093–105. doi: 10.1523/JNEUROSCI.2232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H. Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology (Berl) 2001;158:382–7. doi: 10.1007/s002130100918. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Ohara A, Sasaki K, Abe H, Hattori H, Hall FS, Uhl GR, Sora I. Decreased response to social defeat stress in mu-opioid-receptor knockout mice. Pharmacol Biochem Behav. 2011;99:676–82. doi: 10.1016/j.pbb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–21. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurophysiol. 2008;100:3175–84. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH. Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology (Berl) 1996;128:256–64. doi: 10.1007/s002130050133. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–57. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Dilleen R, Pelloux Y, Economidou D, Dalley JW, Belin D, Everitt BJ. Increased impulsivity retards the transition to dorsolateral striatal dopamine control of cocaine seeking. Biol Psychiatry. 2014;76:15–22. doi: 10.1016/j.biopsych.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. 8th edn The National Academies Press; Washington: 2011. [Google Scholar]
- Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Shimamoto A, Holly EN, Boyson CO, DeBold JF, Miczek KA. Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats. Psychopharmacology (Berl) 2015;232:825–34. doi: 10.1007/s00213-014-3725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–83. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–12. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Han M, Hashimoto K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015;232:4325–35. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–44. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.